
The efficacy and safety of immune checkpoint inhibitors combined with chemotherapy or anti-angiogenic therapy as a second-line or later treatment option for advanced non-small cell lung cancer: a retrospective comparative cohort study
Introduction
Lung cancer is the second most prevalent (11.4%) and deadliest (18%) cancer worldwide; non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer cases, with most patients already in the middle and advanced stages when diagnosed (1). Precision therapy for lung cancer has rapidly progressed, and immune checkpoint inhibitors (ICIs) have drawn considerable attention and have greatly improved lung cancer prognosis. Several clinical studies have demonstrated that programmed cell death 1 (PD-1) and programmed cell death ligand 1 (PD-L1) inhibitors, such as pembrolizumab (2,3), nivolumab (4-8), and atezolizumab (9,10), outperformed docetaxel in the survival and prognosis of advanced NSCLC patients without prior therapy and thus have been approved by the Food and Drug Administration (FDA) as second-line treatments for advanced NSCLC. However, the objective response rate (ORR) in patients receiving ICI monotherapy is low (~15%), and only a limited proportion of advanced patients demonstrate long-term benefits from second-line immunotherapy. There is an urgent need to increase the response population of second-line immunotherapy, and ICI combination therapy may be a possible option. However, the evidence for choosing ICI combination therapy as a second-line treatment for NSCLC is insufficient.
Several trials have explored new modalities of combined immunotherapy as the first-line treatment for advanced NSCLC. They have demonstrated that chemotherapy combined with immunotherapy is superior to standard chemotherapy alone (11-21). In the second-line setting, the PROLUNG study found that pembrolizumab plus docetaxel substantially improved ORR and progression-free survival (PFS) with manageable toxicity than docetaxel alone in patients with previously treated advanced NSCLC (22). Anti-angiogenic agents can synergistically enhance the efficacy of ICIs by regulating the tumour immune microenvironment (23,24). Some clinical trials also suggested that ICI combined with anti-angiogenic therapy demonstrated encouraging clinical activity and a manageable safety profile in previously treated patients with advanced NSCLC (25-27). Therefore, it is necessary to determine whether ICI combination therapy improves prognosis of previously treated advanced NSCLC patients compared with ICI monotherapy. This study aimed to explore the efficacy and safety of combined ICI/chemotherapy or ICI/anti-angiogenic therapy as second-line or later treatment options for advanced NSCLC patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-697/rc).
Methods
Patient enrolment and study design
This was a retrospective comparative cohort study. Patients with lung cancer treated with immunotherapy at the Hunan Cancer Hospital between April 2016 and August 2021 were screened retrospectively. The last follow-up and data collection were by March 2022. The inclusion criteria were as follows: (I) patients aged 18–75 years; (II) patients who scored 0–2 on the Eastern Cooperative Oncology Group performance status (ECOG PS); (III) patients histologically or cytologically diagnosed with unresectable stage III/IV NSCLC; (IV) patients without epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) fusions; (V) patients who have received ICI monotherapy or ICI therapy combined with chemotherapy/anti-angiogenic therapy as second-line or later treatment; (VI) patients with at least one evaluable target lesion (except those with uncontrolled brain metastases) according to Response Evaluation Criteria In Solid Tumours (RECIST, version 1.1); (VII) patients who had undergone at least one efficacy evaluation during treatment. The exclusion criteria were as follows: (I) patients pathologically diagnosed with small-cell lung cancer (SCLC) mixed with NSCLC; (II) patients previously treated with a PD-1 inhibitor, PD-L1 inhibitor, or any other antibody or drug targeting an immune checkpoint; (III) patients with other concurrent malignancy. Detailed clinical characteristics including sex, age, ECOG PS, smoking history, TNM stage, histology, metastasis sites, driver mutation status, PD-L1 expression level and therapeutic regimens were collected. The outcome variables assessed in the study were ORR, disease control rate (DCR), PFS, overall survival (OS) and adverse events (AEs). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Hunan Cancer Hospital (No. 202257) and individual consent for this retrospective analysis was waived.
Efficacy and safety evaluation
Patients were followed up with computed tomography (CT) or magnetic resonance imaging (MRI) every six weeks during the treatment cycles. Disease assessments were performed according to RECIST, version 1.1. PFS was defined as the duration from the start of the ICI therapy to the onset of disease progression or any-cause of death (whichever occurred first). If no disease progression or death occurred, the date of the last imaging examination was used. OS was defined as the duration from the start of ICI therapy to any-cause death or date of the last follow-up if no death occurred. Treatment-related AEs were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE, version 5.0).
Statistical analyses
All statistical analyses were performed using R statistical software (version 3.6.1; The R Foundation of Statistical Computing, Vienna, Austria). Two-tailed tests were performed at a significance level of α=0.05, with P<0.05 indicative of statistical significance. Inter-group comparisons of count data were performed using the chi-square test or Fisher’s exact test. The relationship between the variables and survival was assessed using Kaplan-Meier curves and the log-rank test‘s inter-group differences in survival were assessed. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% confidence interval (CI) to determine the differences in survival. Multivariate Cox regression analysis was used to determine the independent prognostic factors for improved PFS and OS.
Results
Baseline patient characteristics
In total, 697 patients who underwent immunotherapy between April 2016 and August 2021 were screened in a procedure. Of the 697 patients, 371 underwent first-line immunotherapy, 112 were diagnosed with SCLC, and 69 did not have complete treatment records were excluded from the study. A total of 145 patients were ultimately enrolled in this study (Figure 1), consisting of 63 patients in the ICI monotherapy group. The remaining 82 (ICI combination therapy group) patients were divided into the ICI/chemotherapy combination (ICI + C) group (n=57) and the ICI/anti-angiogenic therapy combination (ICI + A) group (n=25).
Table 1 shows the baseline clinical characteristics of the three study groups. The baseline was comparable among the three subgroups. In the ICI monotherapy group, 84.1% were male (53/63), with 30.2% (19/63) of the patients aged ≥65 years. In the ICI + C group, 77.2% were male (44/57), with 28.1% (16/57) of the patients aged ≥65 years. The ICI + A group included 19 males (19/25, 76.0%), of whom 28.0% (7/25) were aged ≥65 years. The patients in each group mostly scored 0–1 on the ECOG PS. In the ICI, ICI + C, and ICI + A groups, respectively, the smoking rate was 81.0% (51/63), 71.9% (41/57), and 68.0% (17/25), while the proportion of patients in clinical stage IV was 88.9% (56/63), 84.2% (48/57), and 84.0% (21/25). Data relating to driver mutation and PD-L1 expression were also collected from each group, and no significant inter-group differences were detected. There was no significant difference in baseline metastatic site and first-line treatment among the three groups. Most patients in the three groups received second-line therapy, and few received third-line or higher treatment. Among the chemotherapeutic agents selected for the ICI + C group, docetaxel, nab-paclitaxel, gemcitabine, and pemetrexed were administered in 13 (22.8%), 30 (52.6%), 7 (12.3%), and 7 (12.3%) cases, respectively. In the ICI + A group, anlotinib and bevacizumab were administered in 20 (80.0%) and 5 (20.0%) cases, respectively.
Table 1
| Characteristics | ICI Monotherapy (N=63) | ICI + C (N=57) | ICI + A (N=25) | P |
|---|---|---|---|---|
| Sex, n (%) | 0.548 | |||
| Male | 53 (84.1) | 44 (77.2) | 19 (76.0) | |
| Female | 10 (15.9) | 13 (22.8) | 6 (24.0) | |
| Age (years), n (%) | 0.962 | |||
| <65 | 44 (69.8) | 41 (71.9) | 18 (72.0) | |
| ≥65 | 19 (30.2) | 16 (28.1) | 7 (28.0) | |
| ECOG PS, n (%) | 1 | |||
| 0–1 | 59 (93.7) | 54 (94.7) | 24 (96.0) | |
| 2 | 4 (6.3) | 3 (5.3) | 1 (4.0) | |
| Smoking histology, n (%) | 0.343 | |||
| Ever smoker | 51 (81.0) | 41 (71.9) | 17 (68.0) | |
| Never smoker | 12 (19.0) | 16 (28.1) | 8 (32.0) | |
| TNM stage, n (%) | 0.695 | |||
| III | 7 (11.1) | 9 (15.8) | 4 (16.0) | |
| IV | 56 (88.9) | 48 (84.2) | 21 (84.0) | |
| Histology, n (%) | 0.231 | |||
| Squamous | 33 (52.4) | 21 (36.8) | 11 (44.0) | |
| Non-squamous | 30 (47.6) | 36 (63.2) | 14 (56.0) | |
| Metastatic site, n (%) | ||||
| Lung | 19 (30.2) | 19 (33.3) | 10 (40.0) | 0.675 |
| Pleura | 12 (19.0) | 8 (14.0) | 3 (12.0) | 0.720 |
| Liver | 8 (12.7) | 5 (8.8) | 3 (12.0) | 0.776 |
| Bone | 17 (27.0) | 19 (33.3) | 7 (28.0) | 0.734 |
| Brain | 10 (15.9) | 11 (19.3) | 4 (16.0) | 0.918 |
| Driver mutation, n (%) | 0.909 | |||
| KRAS | 4 (6.3) | 6 (10.5) | 2 (8.0) | |
| BRAF | 2 (3.2) | 1 (1.8) | 0 (0) | |
| None | 57 (90.5) | 50 (87.7) | 23 (92.0) | |
| PD-L1 expression, n (%) | 0.992 | |||
| ≥50% | 4 (6.3) | 3 (5.3) | 1 (4.0) | |
| ≥1%, <50% | 7 (11.1) | 7 (12.3) | 3 (12.0) | |
| <1% | 14 (22.2) | 10 (17.5) | 6 (24.0) | |
| Unknown | 38 (60.3) | 37 (64.9) | 15 (60.0) | |
| First-line treatment, n (%) | 0.932 | |||
| Platinum-based chemotherapy | 44 (69.8) | 38 (66.7) | 17 (68.0) | |
| Platinum-based chemotherapy plus bevacizumab | 19 (30.2) | 19 (33.3) | 8 (32.0) | |
| Line of ICI therapy, n (%) | 0.749 | |||
| 2 | 52 (82.5) | 47 (82.5) | 19 (76.0) | |
| ≥3 | 11 (17.5) | 10 (17.5) | 6 (24.0) | |
| Selection of ICI, n (%) | 0.954 | |||
| Pembrolizumab | 15 (23.8) | 13 (22.8) | 6 (24.0) | |
| Nivolumab | 27 (42.9) | 21 (36.9) | 8 (32.0) | |
| Sintilimab | 12 (19.0) | 10 (17.5) | 5 (20.0) | |
| Camrelizumab | 8 (12.7) | 11 (19.3) | 5 (20.0) | |
| Toripalimab | 1 (1.6) | 2 (3.5) | 1 (4.0) |
ICI, immune checkpoint inhibitor; ICI + C, ICI plus chemotherapy; ICI + A, ICI plus anti-angiogenic therapy; ECOG, Eastern Cooperative Oncology Group performance status.
Efficacy
ORR and DCR
ORR and DCR were significantly higher in the ICI combination therapy group than in the ICI monotherapy group (ORR: 29.3% vs. 11.1%, P=0.008 and DCR: 85.4% vs. 61.9%, P=0.001). For patients who received second-line treatment, ORR and DCR remained significantly higher in the ICI combination therapy group than in the ICI monotherapy group (ORR: 31.7% vs. 11.5%, P=0.01 and DCR: 85.7% vs. 61.5%, P=0.003) (Table 2).
Table 2
| Items | ICI monotherapy (%) | ICI combination therapy (%) | P |
|---|---|---|---|
| ORR | |||
| Total | 11.1 | 29.3 | 0.008 |
| Second-line | 11.5 | 31.7 | 0.01 |
| DCR | |||
| Total | 61.9 | 85.4 | 0.001 |
| Second-line | 61.5 | 85.7 | 0.003 |
ORR, objective response rate; DCR, disease control rate; ICI, immune checkpoint inhibitor.
Moreover, when the differences in ORR and DCR between the three groups were evaluated, ORR and DCR were significantly higher in the ICI + C group than in the ICI monotherapy group (ORR: 31.6% vs. 11.1%, P=0.006; DCR: 84.2% vs. 61.9%, P=0.006). For patients who received second-line treatment, ORR and DCR remained higher in the ICI + C combination group than in the ICI monotherapy group (ORR: 31.9% vs. 11.5%, P=0.013; DCR: 85.1% vs. 61.5%, P=0.009) (Table 3). DCR was significantly higher in the ICI + A group than in the ICI monotherapy group (88.0% vs. 61.9%, P=0.021), while ORR was not significantly different (24.0% vs. 11.1%, P=0.181). For patients who received second-line treatment, ORR and DCR were not significantly different between the ICI + A therapy and ICI monotherapy groups (ORR: 31.3% vs. 11.5%, P=0.113; DCR: 87.5% vs. 61.5%, P=0.069) (Table 4). Additionally, ORR and DCR were not significantly different between the ICI + C and ICI + A groups (ORR: 31.6% vs. 24.0%, P=0.487; DCR: 84.2% vs. 88.0%, P=0.748) (Table 5).
Table 3
| Items | ICI monotherapy (%) | ICI + C (%) | P |
|---|---|---|---|
| ORR | |||
| Total | 11.1 | 31.6 | 0.006 |
| Second-line | 11.5 | 31.9 | 0.013 |
| DCR | |||
| Total | 61.9 | 84.2 | 0.006 |
| Second-line | 61.5 | 85.1 | 0.009 |
ORR, objective response rate; DCR, disease control rate; ICI + C, ICI plus chemotherapy; ICI, immune checkpoint inhibitor.
Table 4
| Items | ICI monotherapy (%) | ICI + A (%) | P |
|---|---|---|---|
| ORR | |||
| Total | 11.1 | 24.0 | 0.181 |
| Second-line | 11.5 | 31.3 | 0.113 |
| DCR | |||
| Total | 61.9 | 88.0 | 0.021 |
| Second-line | 61.5 | 87.5 | 0.069 |
ORR, objective response rate; DCR, disease control rate; ICI + A, ICI plus anti-angiogenic therapy; ICI, immune checkpoint inhibitor.
Table 5
| Items | ICI + C (%) | ICI + A (%) | P |
|---|---|---|---|
| ORR | |||
| Total | 31.6 | 24.0 | 0.487 |
| Second-line | 31.9 | 31.3 | 1.000 |
| DCR | |||
| Total | 84.2 | 88.0 | 0.748 |
| Second-line | 85.1 | 87.5 | 1.000 |
ORR, objective response rate; DCR, disease control rate; ICI + C, ICI plus chemotherapy; ICI + A, ICI plus anti-angiogenic therapy; ICI, immune checkpoint inhibitor.
PFS and OS
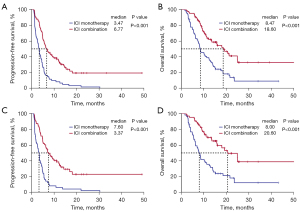
Median survival follow-up time was 20.83 months (95% CI: 17.49–24.17 months). PFS was significantly longer in the ICI combination therapy groups than in the ICI monotherapy group (mPFS: 6.77 vs. 3.47 months, P<0.001; HR =0.37, 95% CI: 0.26–0.53, P<0.001) (Figure 2A). OS was also significantly longer in the ICI combination therapy groups than in the ICI monotherapy group (mOS: 18.60 vs. 8.47 months, P<0.001; HR =0.41, 95% CI: 0.27–0.62, P<0.001) (Figure 2B). For patients who received a second-line treatment, PFS and OS remained significantly longer in the ICI combination therapy groups than in the ICI monotherapy group (mPFS: 7.60 vs. 3.37 months, P<0.001; HR =0.33, 95% CI: 0.22–0.50, P<0.001; mOS: 20.60 vs. 8.00 months, P<0.001; HR =0.35, 95% CI: 0.22–0.57, P<0.001) (Figure 2C,2D).
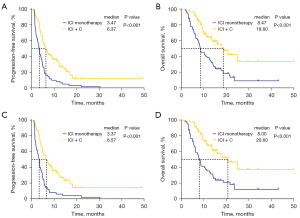
Moreover, PFS and OS were significantly longer in the ICI + C group than in the ICI monotherapy group (mPFS: 6.37 vs. 3.47 months, P<0.001; HR =0.42, 95% CI: 0.29–0.63, P<0.001; mOS: 18.60 vs. 8.47 months, P<0.001; HR =0.40, 95% CI: 0.25–0.64, P<0.001) (Figure 3A,3B). For patients who received a second-line treatment, PFS and OS were also longer in the ICI + C group than in the ICI monotherapy group (mPFS: 6.57 vs. 3.37 months, P<0.001; HR =0.39, 95% CI: 0.25–0.60, P<0.001; mOS: 20.60 vs. 8.00 months, P<0.001; HR =0.36, 95% CI: 0.21–0.62, P<0.001) (Figure 3C,3D).
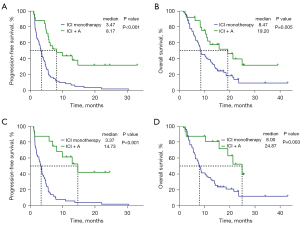
Furthermore, PFS and OS were significantly longer in the ICI + A group than in the ICI monotherapy group (mPFS: 8.17 vs. 3.47 months, P<0.001; HR =0.30, 95% CI: 0.17–0.53, P<0.001; mOS: 19.20 vs. 8.47 months, P=0.005; HR =0.44, 95% CI: 0.24–0.80, P=0.007) (Figure 4A,4B). For patients who received a second-line treatment, PFS and OS were also longer in the ICI + A group than in the ICI monotherapy group (mPFS: 14.73 vs. 3.37 months, P<0.001; HR =0.19, 95% CI: 0.09–0.42, P<0.001; mOS: 24.87 vs. 8.00 months, P=0.003; HR =0.31, 95% CI: 0.14–0.70, P=0.005) (Figure 4C,4D).
In contrast, PFS and OS were not significantly different between the ICI + C group and the ICI + A group (Figure S1A,S1B), with PFS of 6.37 vs. 8.17 months (HR =1.46, 95% CI: 0.82–2.60, P=0.19) and OS of 18.60 vs. 19.20 months (HR =0.95, 95% CI: 0.49–1.83, P=0.88).
Multivariate Cox regression analysis, including sex, age, ECOG PS score, smoking history, clinical stage, pathological type, treatment line, and therapeutic regimen, further confirmed that ICI combination therapy (PFS: HR =0.35, 95% CI: 0.24–0.51, P<0.001, Table S1; OS: HR =0.39, 95% CI: 0.26–0.60, P<0.001, Table S2), ICI + C (PFS: HR =0.38, 95% CI: 0.25–0.57, P<0.001, Table S3; OS: HR =0.38, 95% CI: 0.24–0.62, P<0.001, Table S4) and ICI + A (PFS: HR =0.30, 95% CI: 0.17–0.53, P<0.001, Table S5; OS: HR =0.43, 95% CI: 0.23–0.80, P=0.008, Table S6) were independent prognostic factors for improved PFS and OS compared to ICI monotherapy, respectively.
Safety
The number of patients with treatment-related AEs was 42 (66.6%), 44 (77.2%), and 17 (68.0%) in the ICI monotherapy, ICI + C and ICI + A groups, respectively, while the incidence of grade ≥3 treatment-related AEs in the same groups was 9.5%, 12.3%, and 8.0%, respectively. ICI combination therapy did not significantly increase the incidence of treatment-related AEs compared to ICI monotherapy (Table 6).
Table 6
| Events | Grade | ICI monotherapy (N=63) | ICI + C (N=57) | ICI + A (N=25) | P |
|---|---|---|---|---|---|
| Treatment-related AE, n (%) | Any grade | 42 (66.6) | 44 (77.2) | 17 (68.0) | 0.418 |
| Grade 3–5 | 6 (9.5) | 7 (12.3) | 2 (8.0) | 0.877 | |
| Treatment-related irAE, n (%) | Any grade | 23 (36.5) | 15 (26.3) | 8 (32.0) | 0.488 |
| Grade 3–5 | 3 (4.8) | 3 (5.3) | 1 (4.0) | 1.000 | |
| Nausea, n (%) | Any grade | 2 (3.2) | 3 (5.3) | 1 (4.0) | 0.864 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Vomiting, n (%) | Any grade | 2 (3.2) | 3 (5.3) | 1 (4.0) | 0.864 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Anemia, n (%) | Any grade | 5 (7.9) | 5 (8.8) | 2 (8.0) | 1.000 |
| Grade 3–5 | 2 (3.2) | 1 (1.8) | 1 (4.0) | 0.824 | |
| Leukopenia, n (%) | Any grade | 3 (4.8) | 7 (12.3) | 1 (4.0) | 0.317 |
| Grade 3–5 | 0 (0) | 2 (3.5) | 0 (0) | 0.469 | |
| Neutropenia, n (%) | Any grade | 1 (1.6) | 5 (8.8) | 1 (4.0) | 0.153 |
| Grade 3–5 | 0 (0) | 1 (1.8) | 0 (0) | 0.566 | |
| Thrombocytopenia, n (%) | Any grade | 2 (3.2) | 3 (5.3) | 1 (4.0) | 0.864 |
| Grade 3–5 | 0 (0) | 1 (1.8) | 0 (0) | 0.566 | |
| ALT/AST level increase, n (%) | Any grade | 4 (6.3) | 3 (5.3) | 2 (8.0) | 0.903 |
| Grade 3–5 | 1 (1.6) | 1 (1.8) | 0 (0) | 1.000 | |
| irAEs | |||||
| Rash, n (%) | Any grade | 5 (7.9) | 3 (5.3) | 2 (8.0) | 0.755 |
| Grade 3–5 | 1 (1.6) | 0 (0) | 0 (0) | 1.000 | |
| Hyperthyroidism, n (%) | Any grade | 2 (3.2) | 1 (1.8) | 1 (4.0) | 0.824 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Hypothyroidism, n (%) | Any grade | 3 (4.8) | 2 (3.5) | 1 (4.0) | 1.000 |
| Grade 3–5 | 1 (1.6) | 0 (0) | 0 (0) | 1.000 | |
| Hypopituitarism, n (%) | Any grade | 2 (3.2) | 1 (1.8) | 1 (4.0) | 0.824 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Pneumonia, n (%) | Any grade | 6 (9.5) | 4 (7.0) | 2 (8.0) | 0.922 |
| Grade 3–5 | 1 (1.6) | 1 (1.8) | 1 (4.0) | 0.574 | |
| Myocarditis, n (%) | Any grade | 2 (3.2) | 2 (3.5) | 1 (4.0) | 1.000 |
| Grade 3-5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Amylase level increase, n (%) | Any grade | 1 (1.6) | 1 (1.8) | 0 (0) | 1.000 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 | |
| Lipase level increase, n (%) | Any grade | 2 (3.2) | 1 (1.8) | 0 (0) | 1.000 |
| Grade 3–5 | 0 (0) | 0 (0) | 0 (0) | 1.000 |
ICI + C, ICI plus chemotherapy; ICI + A, ICI plus anti-angiogenic therapy; AE, adverse event; irAE, immune-related adverse event; ALT, alanine aminotransferase; AST, aspartate transaminase; ICI, immune checkpoint inhibitor.
The incidence of immune-related AEs (irAEs) was 36.5% (23/63) in the ICI monotherapy group, 26.3% (15/57) in the ICI + C group, and 32.0% (8/25) in the ICI + A group, with the ICI + C group showing the lowest incidence. Most patients only developed grade 1–2 irAEs, while 4.8%, 5.3%, and 4.0% of patients in the three groups developed grade ≥3 treatment-related irAEs, respectively, without treatment discontinuation due to irAEs.
Discussion
This study explored the efficacy and safety of combined ICI/chemotherapy or ICI/anti-angiogenic therapy as second-line or later treatment for advanced NSCLC. ICI combination therapy exhibited good overall efficacy and safety; the ICI/chemotherapy combination or ICI/anti-angiogenic therapy combination may be a novel second-line or later treatment for NSCLC patients who have not received prior immunotherapy.
On comparing the different combination therapy regimens, the following observations were made: (I) compared to ICI monotherapy, ICI/chemotherapy led to a significantly higher ORR and DCR, and ICI/anti-angiogenic therapy also had a favorable DCR; (II) ICI/chemotherapy and ICI/anti-angiogenic therapy were independent prognostic factors for improved PFS and OS compared to ICI monotherapy, respectively.
Immunotherapy has greatly improved the prognosis of driver gene-negative advanced NSCLC. Some regimens of ICI monotherapy (28-34), combined ICI/chemotherapy (11-21), ICI therapy combined with chemotherapy and anti-angiogenic therapy (35,36), dual immunotherapy combinations (37,38), and dual immunotherapy combined with chemotherapy (39) have been approved by the FDA and/or National Medical Products Administration for the first-line treatment of advanced NSCLC. Nevertheless, many patients in China fail to receive first-line immunotherapy. In contrast, some phase III trials (2-10) have confirmed the effectiveness of ICI monotherapy as a second-line for advanced NSCLC. Although second-line treatment with ICI monotherapy improved the survival and prognosis of advanced NSCLC patients, the ORR in the population receiving ICI monotherapy remained low (~15%), and only a limited proportion of patients with advanced NSCLC derived longer-term benefit from second-line immunotherapy. To further improve the efficacy of second-line immunotherapy, several studies have explored combined immunotherapy regimens.
Zhang et al. showed that PD-1 inhibitors combined with chemotherapy and/or bevacizumab may be favorable for advanced NSCLC patients with prior therapy. The study included 22 patients who received combined immunotherapy and 33 treated with monotherapy group. That study reported significant difference in DCR (95.5% vs. 46.7%, P<0.001) and PFS (mPFS: 7.5 vs. 3.3 months, HR =0.28, P<0.001), and no significant difference in ORR (31.8% vs. 10.0%, P=0.075) between the two groups (40). In our study, the pooled patients who received ICI immunotherapy combined with either chemotherapy or anti-angiogenic therapy showed a significantly improved ORR, DCR and PFS compared to those who received ICI immunotherapy alone. Moreover, the combined ICI therapy significantly prolonged OS.
In addition, Arrieta et al. reported in a phase II clinical trial that the ORR and PFS were significantly better in the combined Docetaxel/Pembrolizumab group than in the chemotherapy-only group (ORR: 42.5% vs. 15.8%, P=0.01; mPFS: 9.5 vs. 3.9 months, HR =0.24, P<0.001) for advanced NSCLC in the second-line setting (41). Similarly, Mao et al. showed a significantly improved ORR (35.5% vs. 15.7%, P=0.039), PFS (mPFS: 5.6 vs. 2.5 months, P=0.013), and OS (mOS: NE vs. 12.6 months, P=0.038) in another study that included NSCLC patients treated with combined ICI/chemotherapy compared to ICI monotherapy as second-line or later treatment (42). Moreover, a retrospective study found that the ORR, depth of response and PFS of ICIs plus chemotherapy were better than ICI monotherapy and ICIs plus anti-angiogenic therapy in patients with previously treated advanced NSCLC (43). In our study, the ICI/chemotherapy group showed a significantly higher ORR and DCR and a longer PFS and OS than the ICI monotherapy group. Meanwhile, there was no significant difference between the ICI/chemotherapy group and ICI/anti-angiogenic therapy group.
Introducing new drugs and combinations has also shown intriguing findings that favor using immunotherapy in combination. A recent phase I/II clinical trial evaluated the combination of camrelizumab/apatinib as the second-line for advanced squamous NSCLC and reported an ORR, DCR, mPFS, and mOS of 30.8%, 95%, 5.9 months, and 12.8 months, respectively; these data are superior to those presented by the registration studies of monotherapy with ICIs in the second line (44). In our study, the ICI/anti-angiogenic therapy group showed a significantly higher DCR and a longer PFS and OS than the ICI monotherapy group.
No new safety issues were observed in our analysis, consistent with previous studies (40-44). In the combined ICI/chemotherapy group, the AEs with an increased incidence were mostly related to hematologic toxicity, while the incidence of treatment-related irAEs did not increase. The ICI combination therapy did not significantly increase the incidence of treatment-related AEs compared to ICI monotherapy, suggesting that ICI combination therapy was well-tolerated.
This study has several limitations. First, the sample size of this study was relatively small, especially in the ICI/anti-angiogenic therapy combination group, which might affect the results. Second, this was a retrospective comparative cohort study, leading to inevitable selection bias. Third, the treatment regimen in this study involved five different ICIs, four different chemotherapeutic agents and two different anti-angiogenic agents, which may have a confounding effect on the efficacy.
Conclusions
Combined ICI/chemotherapy or ICI/anti-angiogenic therapy is well tolerated and has higher efficacy than ICI monotherapy as a second-line or later treatment option for advanced NSCLC patients without prior immunotherapy, thereby providing a potentially superior treatment strategy. However, our findings require verification by prospective randomized controlled trials.
Acknowledgments
We sincerely thank all the patients who participated in this research and the investigators and project team who worked on the study. The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This research was funded by the Wu Jieping Medical Foundation (No. 320.6750.19088-11, to LW), the Health Research Foundation of Chinese Society of Clinical Oncology (No. Y-2019Genecast-024, to LW), the Hunan Cancer Hospital Climb plan (No. ZX2020005-5, to LW), and the Hunan Provincial Natural Science Foundation of China (No. 2021JJ30430, to LW).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-697/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-697/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-697/coif). LW reports that this research was funded by the Wu Jieping Medical Foundation (No. 320.6750.19088-11), the Health Research Foundation of Chinese Society of Clinical Oncology (No. Y-2019Genecast-024), the Hunan Cancer Hospital Climb plan (No. ZX2020005-5), and the Hunan Provincial Natural Science Foundation of China (No. 2021JJ30430). LW also received personal fees from AstraZeneca, Roche, Bristol-Myers Squibb, MSD, Pfizer, Lilly, Boehringer Ingelheim, Merck, Innovent, and Hengrui, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional ethics board of Hunan Cancer Hospital (No. 202257) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Herbst RS, Garon EB, Kim DW, et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J Thorac Oncol 2021;16:1718-32. [Crossref] [PubMed]
- Wu YL, Lu S, Cheng Y, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol 2019;14:867-75. [Crossref] [PubMed]
- Font EF, Gettinger SN, Burgio MA, et al. Three-Year Follow-Up From CheckMate 017/057: Nivolumab Versus Docetaxel in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC). Ann Oncol 2017;28:v462. [Crossref]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Gettinger S, Vokes EE, et al. Five-Year Outcomes From the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non-Small-Cell Lung Cancer. J Clin Oncol 2021;39:723-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Mazieres J, Rittmeyer A, Gadgeel S, et al. Atezolizumab Versus Docetaxel in Pretreated Patients With NSCLC: Final Results From the Randomized Phase 2 POPLAR and Phase 3 OAK Clinical Trials. J Thorac Oncol 2021;16:140-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Gray J, Rodríguez-Abreu D, Powell SF, et al. FP13. 02 Pembrolizumab+ Pemetrexed-Platinum vs Pemetrexed-Platinum for Metastatic NSCLC: 4-Year Follow-Up From KEYNOTE-189. J Thorac Oncol 2021;16:S224. [Crossref]
- Gadgeel S, Rodríguez-Abreu D, Speranza G, et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:1505-17. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Paz-Ares L, Vicente D, Tafreshi A, et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients With Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J Thorac Oncol 2020;15:1657-69. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): a randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med 2021;9:305-14. [Crossref] [PubMed]
- Zhou C, Chen G, Huang Y, et al. P79. 02 Updated OS and Time to Second Progression With First-Line Camrelizumab Plus Chemo vs Chemo for Advanced Non-Squamous NSCLC. J Thorac Oncol 2021;16:S645-S646. [Crossref]
- Yang Y, Wang Z, Fang J, et al. Efficacy and Safety of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC: a Randomized, Double-Blind, Phase 3 Study (Oncology pRogram by InnovENT anti-PD-1-11). J Thorac Oncol 2020;15:1636-46. [Crossref] [PubMed]
- Zhou C, Wu L, Fan Y, et al. LBA56 ORIENT-12: Sintilimab Plus Gemcitabine and Platinum (GP) as First-Line (1L) Treatment for Locally Advanced or Metastatic Squamous Non-Small-Cell Lung Cancer (sqNSCLC). Ann Oncol 2020;31:S1186. [Crossref]
- Lu S, Yu Y, Yu X, et al. 1263P Tislelizumab+ Chemotherapy vs Chemotherapy Alone as First-Line Treatment for Locally Advanced/Metastatic Nonsquamous NSCLC. Ann Oncol 2020;31:S816-S817. [Crossref]
- Wang J, Yu X, Lu S, et al. Phase III Study of Tislelizumab Plus Chemotherapy vs Chemotherapy Alone as First-Line (1L) Treatment for Advanced Squamous Non-Small Cell Lung Cancer (sq NSCLC). J Clin Oncol 2020;38:abstr 9554.
- Arrieta O, Barrón F, Ramírez-Tirado LA, et al. Efficacy and Safety of Pembrolizumab Plus Docetaxel vs Docetaxel Alone in Patients With Previously Treated Advanced Non-Small Cell Lung Cancer: The PROLUNG Phase 2 Randomized Clinical Trial. JAMA Oncol 2020;6:856-64. [Crossref] [PubMed]
- Huang Y, Kim BYS, Chan CK, et al. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol 2018;18:195-203. [Crossref] [PubMed]
- Liang H, Wang M. Prospect of immunotherapy combined with anti-angiogenic agents in patients with advanced non-small cell lung cancer. Cancer Manag Res 2019;11:7707-19. [Crossref] [PubMed]
- Brose MS, Vogelzang NJ, DiSimone C, et al. A phase Ib/II trial of lenvatinib plus pembrolizumab in non-small cell lung cancer. J Clin Oncol 2019;37:16. [Crossref]
- Herbst RS, Arkenau HT, Santana-Davila R, et al. Ramucirumab plus pembrolizumab in patients with previously treated advanced non-small-cell lung cancer, gastro-oesophageal cancer, or urothelial carcinomas (JVDF): a multicohort, non-randomised, open-label, phase 1a/b trial. Lancet Oncol 2019;20:1109-23. [Crossref] [PubMed]
- Reckamp KL, Redman MW, Dragnev KH, et al. Overall Survival from A Phase II Randomized Study of Ramucirumab Plus Pembrolizumab Versus Standard of Care for Advanced Non–Small Cell Lung Cancer Previously Treated with Immunotherapy: Lung-MAP Nonmatched Substudy S1800A. J Clin Oncol 2022;40:abstr 9004
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. LBA51 KEYNOTE-024 5-Year OS Update: First-Line (1L) Pembrolizumab (Pembro) vs Platinum-Based Chemotherapy (Chemo) in Patients (pts) With Metastatic NSCLC and PD-L1 Tumour Proportion Score (TPS) ≥50%. Ann Oncol 2020;31:S1181-S1182. [Crossref]
- Cho BC, Wu Y, Lopes G, et al. FP13. 04 KEYNOTE-042 3-Year Survival Update: 1L Pembrolizumab vs Platinum-Based Chemotherapy for PD-L1+ Locally Advanced/Metastatic NSCLC. J Thorac Oncol 2021;16:S225-S226. [Crossref]
- Wu YL, Zhang L, Fan Y, et al. Randomized clinical trial of pembrolizumab vs chemotherapy for previously untreated Chinese patients with PD-L1-positive locally advanced or metastatic non-small-cell lung cancer: KEYNOTE-042 China Study. Int J Cancer 2021;148:2313-20. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Herbst R, De Marinis F, Giaccone G, et al. FP13. 03 IMpower110: Updated OS Analysis of Atezolizumab vs Platinum-Based Chemotherapy as First-Line Treatment in PD-L1–Selected NSCLC. J Thorac Oncol 2021;16:S224-S225. [Crossref]
- Carbone DP, Reck M, Paz-Ares L, et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2415-26. [Crossref] [PubMed]
- Socinski MA, Mok TS, Nishio M, et al. IMpower150 Final Analysis: Efficacy of Atezolizumab (atezo) + Bevacizumab (bev) and Chemotherapy in First-line (1L) Metastatic Nonsquamous (nsq) Non-small Cell Lung Cancer (NSCLC) Across Key Subgroups. Cancer Res 2020;80-abstract CT216. [Crossref]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Ramalingam SS, Ciuleanu TE, Pluzanski A, et al. Nivolumab + Ipilimumab Versus Platinum-Doublet Chemotherapy as First-Line Treatment for Advanced Non-Small Cell Lung Cancer: Three-Year Update From CheckMate 227 Part 1. J Clin Oncol 2020;38: abstr 9500.
- Boyer M, Şendur MAN, Rodríguez-Abreu D, et al. Pembrolizumab Plus Ipilimumab or Placebo for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%: Randomized, Double-Blind Phase III KEYNOTE-598 Study. J Clin Oncol 2021;39:2327-38. [Crossref] [PubMed]
- Reck M, Ciuleanu TE, Dols MC, et al. Nivolumab (NIVO)+ Ipilimumab (IPI)+ 2 Cycles of Platinum-Doublet Chemotherapy (Chemo) vs 4 Cycles Chemo as First-Line (1L) Treatment (tx) for Stage IV/Recurrent Non-Small Cell Lung Cancer (NSCLC): CheckMate 9LA. J Clin Oncol 2020;38:abstr 9501.
- Zhang F, Huang D, Li T, et al. Anti-PD-1 Therapy plus Chemotherapy and/or Bevacizumab as Second Line or later Treatment for Patients with Advanced Non-Small Cell Lung Cancer. J Cancer 2020;11:741-9. [Crossref] [PubMed]
- Arrieta OG, Barrón F, Carmona A, et al. MA11. 03 Pembrolizumab Plus Docetaxel Increases Progression-Free Survival Compared With Docetaxel Alone in Previously Treated Advanced Non-Small Cell Lung Cancer Patients. J Thorac Oncol 2019;14:S291. [Crossref]
- Mao S, Zhou F, Liu Y, et al. ICI plus chemotherapy prolonged survival over ICI alone in patients with previously treated advanced NSCLC. Cancer Immunol Immunother 2022;71:219-28. [Crossref] [PubMed]
- Zhang T, Yang X, Zhao J, et al. The application of combined immune checkpoint inhibitor modalities in previously treated non-small cell lung cancer patients and the associations thereof with the lung immune prognostic index. Front Oncol 2021;11:690093. [Crossref] [PubMed]
- Gao G, Wang Y, Ren S, et al. 1267P Efficacy of Camrelizumab (SHR-1210) Plus Apatinib as Second-Line Treatment for Advanced Squamous NSCLC. Ann Oncol 2020;31. [Crossref]
(English Language Editor: D. Fitzgerald)



