Lung cancer stem cells—characteristics, phenotype
Introduction
Lung cancer is considered to be a pandemic with major financial and social consequences (1). It is estimated to be responsible for nearly 1.59 million deaths worldwide (19.4% of all cancers in total) (2,3). In the last years, there is growing evidence that solid tumors are composed of cells with different biological properties. Despite advances in definitive local and/or systemic treatment, lung cancer has the remarkable ability to recur suggesting that minimal residual disease contains a cell population that sustains its capability to grow despite treatment. This enormous capacity for self-renewal and regeneration is a biological function typical for normal somatic stem cells therefore this particular cell population within tumors is termed ‘cancer stem cells’ (CSCs) (4,5). In this review we give an overview of lung CSCs characteristics and different phenotypes.
The CSC hypothesis
The CSC hypothesis dates back to the mid-19th century. Rudolph Virchow and Julius Cohnheim were the first to identify histological similarities between developing foetuses and certain types of cancers such as teratomas (6,7). This led them to the hypothesis that cancer cells were the results of activation of embryonic tissue remnants rather than occurring spontaneously. They suggested that cancer is a hierarchically heterogeneous cell population that is dominated and sustained by a very small subpopulation of cancer cells with the property of self-renewal and differentiation (6,7).
On this background, Bonnet and Dick were the first to isolate a subpopulation of CD34+ CD38– acute myeloid leukemia (AML) cells that were capable of initiating hematopoietic malignancy in transplanted mice from human patients with AML. These cells possessed the capacity to self-renew, proliferate, and differentiate (8). Following this major scientific breakthrough, proposed CSCs are thought to be responsible for cancer initiation, progression, metastasis, recurrence, and drug resistance and several attempts have been made to isolate them from various organ sites including the lung (9-14).
In light of the CSC model, normal stem cells can be considered as the primary tumorigenic cells endowed with some properties typical of malignant cells such as the activation of survival pathways and the ability of indefinite proliferation. This is a favourable background for oncogenic mutations to occur and in this case the finely regulated growth potential of normal stem cells can turn into the uncontrolled growth of cancer cells. These cells do not only have the ability of expanding in vitro as tumor spheres but they can also reproduce the original tumor when transplanted in immunodeficient mice (9). Therefore, the CSC are able to expand the CSC pool and also differentiate to give rise to heterogeneous, non-tumourigenic cells that make up the bulk of the tumor and ultimately define the histological type of the cancer. Furthermore, stems cells express anti-apoptotic and drug resistant proteins at high levels and this can justify the fact that systemic treatment can be effective in treating the majority of lung tumors but remains ineffective against metastasis and disease recurrence. Should the CSC hypothesis be confirmed, then their therapeutic targeting has the potential to delay or prevent disease progression and recurrence.
Lung stem cell characteristics and their potential implication in lung cancer
The actual origin of CSC is currently a matter of debate however the most popular hypothesis is that they originate from normal tissue-specific stem cells in their tissues of origin (15,16). Tissues with rapid proliferation (e.g., intestinal epithelium) are likely to present the typical stem cell hierarchy where stem cells produce progenitor cells giving rise to fully differentiated cells. However, the identification of the origin of stem cells in the lungs poses a challenge as the lung epithelium is quiescent with the tracheal and bronchiolar epithelia having a proliferative fraction of merely 0.06–1.3% (17). In addition to that, it has been questioned whether slowly renewing tissues can host stem cells (18,19). All the above in combination with cellular heterogeneity and complex structure of the lung justify the slow pace in lung CSC research.
Anatomical sites
Trachea and main bronchi
The tracheal epithelium consists mainly of columnar and mucus secreting goblet cells. Proliferative fraction is fairly low unless pollutant or pathogen-induced epithelial injuries trigger a rapid proliferation in surviving cells to repair the tissue (17,20). In this case, lung cells, with the ability to differentiate, start proliferating and act as progenitor cells that can generate several different cell types (21). Cell proliferation starts 48–72 h post injury and the epithelium is regenerated in 2–4 weeks (22).
Initial attempts to purify and characterize airway epithelial stem cells were made by Schoch et al. (23) who studied mouse models and identified increased keratin five promoter activity in a subset of tracheal epithelial basal cells that presented stem cell-like behavior and formed colonies.
Hajj et al. studied the same group of cells in human tracheas and showed that the epithelial basal cells can differentiate and proliferate to form a fully differentiated mucociliary and functional airway epithelium (24). Similar activity has been identified by in vitro studies where human bronchial basal cells are capable of self-renewal and differentiation as confirmed by the formation of heterogeneous spheres (25,26).
Consequently, basal cells are a stem cell population and any imbalance between their proliferation and differentiation can lead either to basal cell hyperplasia or epithelial hypoplasia. These changes can contribute to squamous cell metaplasia or dysplasia that are precursors of squamous cell lung carcinoma (26,27).
Bronchioles and alveoli
Non-ciliated Clara cells are located in the bronchiolar and alveolar epithelium and their aim is to protect it. Their isolation is challenging and therefore most of the available data come from in vitro studies. They were initially suggested as stem/progenitor cells as it was observed they could self-renew and differentiate into ciliated cells after exposure to oxidant induced damage (28). However, their role as progenitor cells has been disputed after the use of a new biomarker [Clara cell secretory protein (CCSP)] identified murine lung CCSP-expressing cells that presented with a stem cell-like behavior; expression of stem cell markers CD44, CD133, and Sox2; bronchosphere colony formation; and self-renewal capacity (29). These cells were variant Clara cells, type A cells, OCT4-expressing stem cells, and bronchioalveolar stem cells (29-32).
Both variant Clara cells and nearby pulmonary neuroendocrine cells (PNECs) have been shown to proliferate and participate in regeneration of rodent epithelium following naphthalene exposure whereas by acting independently they do not seem to present both properties (30-32). It is of note that small cell lung cancer (SCLC) predominately localizes to the midlevel bronchioles and is associated with primitive neuroendocrine features, such as expression of the neuropeptide calcitonin-gene related peptide (CGRP) (30). This resembles the behavior of PNECs and potentiates that PNECs may be the origin of SCLC.
Bronchioalveolar stem cells are located in the bronchioalveolar duct junction, the branch point between a terminal bronchiole and the alveolar space. They can express both CCSP and type II cell markers (SPC-alveolar type 2 cell specific marker pro-surfactant apoprotein-c) therefore reflecting stem cell behavior for both bronchiolar and alveolar epithelium (33).
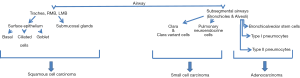
Jackson et al. (34) suggested that tumors triggered by the activation of oncogenic K-Ras derive from cells in the bronchioalveolar duct junction that express CCA (Clara cell specific marker) and SPC. Kim et al. explored this further and showed that these double positive stained cells (bronchioalveolar stem cells) are quiescent in normal lung homeostasis but proliferate after causing lung injury with naphthalene and bleomycin treatment (33). Bronchioalveolar stem cells have the ability to self-renew and differentiate and they would constitute good targets for deleterious mutations therefore having the potential to transform into CSCs. Indeed, serial section analysis has showed that Lox-K-Ras adenomas frequently developed near terminal bronchioles. The identification of bronchioalveolar stem cells at the bronchioalveolar duct junction hints that bronchioalveolar stem cells are potential cancer originating stem cells. Figure 1 shows possible sites of tumor initiation and the possible stem/progenitor cell each type of cancer originates from. Squamous cell lung cancer which is usually centrally located is proposed to develop from transformed basal cells. SCLC which usually occurs in the midlevel bronchioles is proposed to develop from malignant PNECs while adenocarcinomas (ACs) are proposed to derive from transformed bronchioalveolar stem cells as they occur in the distal airways.
Kajstura et al. (35) has recently provided evidence suggesting the existence of human lung stem cells. In his in vivo mouse model, clonal human lung stem cells divided asymmetrically and generated bronchioles, alveoli, and pulmonary vessels of various dimensions. Furthermore, he obtained human lung stem cells from regenerated lung tissue that were able to self-renew and create lung parenchyma. The newly regenerated pulmonary structures were immuno-histochemicaly identified by the recognition of human sex chromosomes and human transcripts of epithelial and vascular genes within the regenerated mouse lung (36,37). The detection of human X chromosome in regenerated type II alveolar epithelial cells, suggested the capacity of the human lung stem cells to terminally differentiate in vivo into functional, highly specialized cells that secrete SPC which is a critical determinant of alveolar function and lung performance.
Lung CSC markers and phenotypes
Biomarkers have been widely used in stem cell research to identify and classify tumourigenic and non tumourigenic cell types. These cells are passaged through various assays to determine tumourigenicity and expression of biomarkers; one such method is the detection of side-population (SP) phenotypes (38,39).
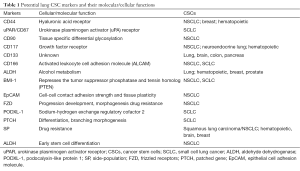
SP phenotyping is based on the differential ability of the cancer cells to efflux Hoechst 33342 dye, as imparted by the ATP-binding cassette family of transporter proteins present on the cellular membrane (39) However, the Hoechst dye is toxic to the cells and also some stromal cells may present dye exclusion properties and consequently this can be a challenging process (40). Therefore, identification and validation of lung CSCs has been obstructed by the complexity of the disease, diverse cellular behavior to the identification process and incomplete understanding of the hierarchical structure of lung epithelial stem cells (7). The most commonly used lung CSC markers are CD44 and CD133. Table 1 provides an overview of currently used lung CSC markers.
Full table
CD44
CD44 is a transmembrane glycoprotein that binds hyaluronic acid, an abundant polysaccharide in stem cell (41), to facilitate adhesion, differentiation, homing, and migration within normal and CSC. CD44 is considered to be a key player in identifying CSCs as it regulates adhesion, differentiation, homing, and migration.
In lung cancer models, increased CD44 expression was initially reported in activated type II pneumocytes, squamous metaplasia, and NSCLC cells suggesting it may play a role in disease progression (42).
Leung et al. (43) reported that a subpopulation of CD44+ NSCLC cells were capable of spheroid body formation and in vivo tumor initiation. CD44+ cells expressed the pluripotency genes OCT4, NANOG and SOX2 while these markers were lost in CD44-cells. It is of note that CD44+ cells were cisplatin-resistant and CD44 expression was associated with squamous cell carcinoma (SCC) but unexpectedly, a longer survival was observed in CD44-expressing ACs. CD44 is a potential CSC marker of NSCLC cell lines.
However, Wang et al. (44) confirmed and extended the above findings by showing that the CD44+ potential of sphere formation and mesenchymal morphology is also present in SCLC and large cell carcinoma (LCC). The team chose to study primary cell lines rather than surgical specimens as the latter could be restrictive due to the low number of cells available from surgical specimens. Therefore, they studied cell lines derived from four representative lung cancer subtypes (SCLC, LCC, SCC and AC). In this study, co-expression of CD90 further narrowed down the putative stem cell population as spheroid-forming cells were mainly found within the CD44+ CD90+ sub-population which also revealed mesenchymal morphology, increased expression of mesenchymal markers N-Cadherin and Vimentin, increased mRNA levels of the embryonic stem cell related genes Nanog and Oct 4 and increased resistance to irradiation compared to other sub-populations studied, therefore suggesting the CD44+ CD90+ population as a good candidate for the lung CSCs.
Both CD44+ CD90+ and CD44+ CD90− cells derived from SCCs formed spheroids, whereas the CD44- cells were lacking this potential. These results indicate that CD44+ CD90+ sub-population may represent CSCs in SCLC and LCCs, whereas in squamous cell cancer, CSC potentials were found within the CD44+ sub-population.
Basak et al. (45) studied the presence of CSC in malignant pleural effusions and reported CD44 as a possible stem cell marker but the characterization of stem cell-like properties was based on in vitro molecular analysis and cellular expansion only while Leung et al. (43) reported both in vitro and in vivo tumorigenecity of CD44+ cells.
CD133
CD133 (Prom1), a cell surface glycoprotein, has been used to identify CSC in lung cancer. Eramo et al. (9) studied cell lines from both SCLC and NSCLC (squamous cell/adeno/large cell neuroendocribe carcinomas) in conditions that enabled the selective growth of undifferentiated CD133+ cells. The CD133+ cells developed into a homogenous population and then proliferated as tumor spheres which in turn formed differentiated progeny that displayed phenotypic features of lung cancer cells. This has shown that lung cancer spheres are made of undifferentiated cells that under certain circumstances can differentiate into cells that resemble the majority of cells in the original tumor.
CD133-positive cells were also resistant to chemotherapy and expressed high levels of ATP-binding cassette G2, suggesting an SP phenotype. The same group of cells was linked to shorter progression-free survival of NSCLC patients treated with platinum-based regimens, therefore explaining the poor therapeutic efficacy of chemotherapy on lung cancer patients .
However, the role of CD133 expression as a prognostic marker in NSCLC has been debated (46-51). CD133 has been found to be significantly correlated with pathological stages II–IV in NSCLC and was an independent poor factor for prognosis (49) however, Salnikov et al. (51) reported CD133 may be used as a predictor for efficacy of cytotoxic therapy, but it does not represent a prognostic marker for positive in NSCLC.
Other lung CSC markers
The aldehyde dehydrogenase (ALDH) family is cytosolic isoenzyme responsible for oxidizing intracellular aldehydes, thus contributing to the oxidation of retinol to retinoic acid in early stem cell differentiation (52,53). Jiang et al. (54) were the first to isolate ALDH1-positive cells from human lung cancer cell lines and showed ALDH1-positive cancer cells exhibited the important CSC properties: in vitro self-renewal, differentiation, and multidrug resistance capacities, expression of stem cell marker, in vivo tumor initiation, and occurrence of a heterogeneous population of cancer cells. Furthermore, they analyzed ALDH1 expression in 303 lung tissues from three different populations of patients with lung cancer, and reported that increased ALDH1 protein levels were positively associated with stage and grade of the tumors and inversely related to the patients’ survival (45).
CD166 [activated leukocyte cell adhesion molecule (ALCAM)], is involved in angiogenesis, differentiation, homing, and maintenance of hematopoietic stem cells. CD166 has been recently proved to be expressed by a subpopulation of CSCs that in vivo had the capacity for self-renewal and could also generate primary and secondary xenograft tumors that phenotypically mimicked the parental patient tumor (54). CD166 positive cells formed self renewing spheres in vitro. It is of note that CD166 positive derived spheres could initiate in vivo tumorigenesis. However, knockdown of CD166 expression in the CSCs did not significantly affect tumorigenicity demonstrating CD166 is an inert surface marker capable of enriching for lung CSCs.
The urokinase plasminogen activator (uPA) and urokinase plasminogen activator receptor (uPAR/CD87) are major regulators of extracellular matrix degradation and are involved in cell migration and invasion under physiological and pathological conditions (55). uPAR are directly involved in the inhibition of apoptosis and may define a functionally important population of cancer cells in SCLC, which are resistant to traditional chemotherapies and also possess enhanced clonogenic activity in vitro. Gutova et al. (56) showed that uPAR-positive cells were more resistant to treatment with the cytotoxic agent 5-FU, while uPAR-negative cells were killed much more efficiently.
BMI1 polycomb ring finger oncogene (BMI-1) is also involved in stem cell cycle regulation and plays an integral role in self-renewal and maintenance of normal lung epithelial, BASCs and CSCs (57,58). In epithelial cells BMI1 represses the tumor suppressor phosphatase and tensin homolog (PTEN) (59) and BMI-1 is also overexpressed in a stem-cell like subpopulation of NSCLC cells that are tumorigenic and resistant to cisplatin therapy (60,61). It is of note that BMI1- negative patients receiving adjuvant therapy had a better disease-free survival for stage I and II NSCLC than BMI-1 positive patients (62).
Podocalyxin-like protein 1 (PODXL-1) is a glycosylated cell surface sialomucin that shares genomic and structural similarities to CD34 and endoglycan. Functionally, although it can act as an adhesive receptor on rare high endothelial venules, under most circumstances it has been found that it acts as an anti-adhesin through its negatively-charged mucin domain. This has been shown to play an important role in cell migration, adhesion and lumen formation (62). PODXL-1 and BMI-1 have been expressed in SCLC due to aberrant epigenetic changes (63) suggesting a histogenetic link between developing proximal bronchi and SCLCs supporting the role of PODXL-1 as a potential CSC marker in SCLC.
Conclusions
The CSC hypothesis states that tumor initiation, metastasis, and recurrence may be driven by a subgroup of tumor cells that have the capacity to self-renew and differentiate and are resistant to current chemotherapy regimens.
There is evidence about the existence of CSCs in lung cancer and much progress has been made in identifying the characteristics and phenotypes of lung CSC despite the fact there are multiple CSC pools that are highly heterogenous and in a dymamic state. Distinguishing lung CSC markers still remains challenging mainly due to the intratumoral heterogeneity and high degree of plasticity that can cause instability of the CSC phenotype and reversion of cell surface markers. Lung CSC markers already underpin novel therapeutic strategies for patients with lung cancer however deeper insight is needed into the roles of self-renewal and maintenance pathways in lung CSCs to accurately identify the best molecular targets for therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Hardavella G, Sethi T. Lung Cancer. Eur Respir Monogr 2015;68:1-11. Available online: , accessed on 29.01.2016.http://www.ers-education.org/publications/ers-monograph/archive/lung-cancer-2015.aspx
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta, American Cancer Society, 2014. Available online: , accessed on 29.01.2016.http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf
- International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Incidence, Mortality and Prevalence Worldwide in 2012. Available online: , accessed on June 20, 2015.http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer 2003;3:895-902. [Crossref] [PubMed]
- Eramo A, Haas TL, De Maria R. Lung cancer stem cells: tools and targets to fight lung cancer. Oncogene 2010;29:4625-35. [Crossref] [PubMed]
- Huntly BJ, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nat Rev Cancer 2005;5:311-21. [Crossref] [PubMed]
- Templeton AK, Miyamoto S, Babu A, et al. Cancer stem cells: progress and challenges in lung cancer. Stem Cell Investig 2014;1:9.
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med 1997;3:730-7. [Crossref] [PubMed]
- Eramo A, Lotti F, Sette G, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ 2008;15:504-14. [Crossref] [PubMed]
- Yang ZF, Ho DW, Ng MN, et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008;13:153-66. [Crossref] [PubMed]
- O'Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445:106-10. [Crossref] [PubMed]
- Li C, Lee CJ, Simeone DM. Identification of human pancreatic cancer stem cells. Methods Mol Biol 2009;568:161-73. [Crossref] [PubMed]
- Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res 2008;68:4311-20. [Crossref] [PubMed]
- Collins AT, Berry PA, Hyde C, et al. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res 2005;65:10946-51. [Crossref] [PubMed]
- Martínez-Climent JA, Andreu EJ, Prosper F. Somatic stem cells and the origin of cancer. Clin Transl Oncol 2006;8:647-63. [Crossref] [PubMed]
- Pathak S. Organ- and tissue-specific stem cells and carcinogenesis. Anticancer Res 2002;22:1353-6. [PubMed]
- Borthwick DW, Shahbazian M, Krantz QT, et al. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol 2001;24:662-70. [Crossref] [PubMed]
- Teta M, Rankin MM, Long SY, et al. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007;12:817-26. [Crossref] [PubMed]
- Dor Y, Melton DA. How important are adult stem cells for tissue maintenance? Cell Cycle 2004;3:1104-6. [Crossref] [PubMed]
- Rawlins EL, Ostrowski LE, Randell SH, et al. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A 2007;104:410-7. [Crossref] [PubMed]
- Stripp BR, Reynolds SD. Maintenance and repair of the bronchiolar epithelium. Proc Am Thorac Soc 2008;5:328-33. [Crossref] [PubMed]
- Stripp BR, Maxson K, Mera R, et al. Plasticity of airway cell proliferation and gene expression after acute naphthalene injury. Am J Physiol 1995;269:L791-9. [PubMed]
- Schoch KG, Lori A, Burns KA, et al. A subset of mouse tracheal epithelial basal cells generates large colonies in vitro. Am J Physiol Lung Cell Mol Physiol 2004;286:L631-42. [Crossref] [PubMed]
- Hajj R, Baranek T, Le Naour R, et al. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells 2007;25:139-48. [Crossref] [PubMed]
- Rock JR, Onaitis MW, Rawlins EL, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci U S A 2009;106:12771-5. [Crossref] [PubMed]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech 2010;3:545-56. [Crossref] [PubMed]
- Jeremy George P, Banerjee AK, Read CA, et al. Surveillance for the detection of early lung cancer in patients with bronchial dysplasia. Thorax 2007;62:43-50. [Crossref] [PubMed]
- Evans MJ, Johnson LV, Stephens RJ, et al. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Lab Invest 1976;35:246-57. [PubMed]
- Wang XY, Keefe KM, Jensen-Taubman SM, et al. Novel method for isolation of murine clara cell secretory protein-expressing cells with traces of stemness. PLoS One 2012;7:e43008. [Crossref] [PubMed]
- Reynolds SD, Giangreco A, Power JH, et al. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol 2000;156:269-78. [Crossref] [PubMed]
- Reynolds SD, Hong KU, Giangreco A, et al. Conditional clara cell ablation reveals a self-renewing progenitor function of pulmonary neuroendocrine cells. Am J Physiol Lung Cell Mol Physiol 2000;278:L1256-63. [PubMed]
- Stevens TP, McBride JT, Peake JL, et al. Cell proliferation contributes to PNEC hyperplasia after acute airway injury. Am J Physiol 1997;272:L486-93. [PubMed]
- Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell 2005;121:823-35. [Crossref] [PubMed]
- Jackson EL, Willis N, Mercer K, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev 2001;15:3243-8. [Crossref] [PubMed]
- Kajstura J, Rota M, Hall SR, et al. Evidence for human lung stem cells. N Engl J Med 2011;364:1795-806. [Crossref] [PubMed]
- Bearzi C, Rota M, Hosoda T, et al. Human cardiac stem cells. Proc Natl Acad Sci U S A 2007;104:14068-73. [Crossref] [PubMed]
- Leri A. Human cardiac stem cells: the heart of a truth. Circulation 2009;120:2515-8. [Crossref] [PubMed]
- Alamgeer M, Peacock CD, Matsui W, et al. Cancer stem cells in lung cancer: Evidence and controversies. Respirology 2013;18:757-64. [Crossref] [PubMed]
- Goodell MA, Brose K, Paradis G, et al. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med 1996;183:1797-806. [Crossref] [PubMed]
- Zhong Y, Zhou C, Ma W, et al. Most MCF7 and SK-OV3 cells were deprived of their stem nature by Hoechst 33342. Biochem Biophys Res Commun 2007;364:338-43. [Crossref] [PubMed]
- Solis MA, Chen YH, Wong TY, et al. Hyaluronan regulates cell behavior: a potential niche matrix for stem cells. Biochem Res Int 2012;2012:346972.
- Penno MB, August JT, Baylin SB, et al. Expression of CD44 in human lung tumors. Cancer Res 1994;54:1381-7. [PubMed]
- Leung EL, Fiscus RR, Tung JW, et al. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One 2010;5:e14062. [Crossref] [PubMed]
- Wang P, Gao Q, Suo Z, et al. Identification and characterization of cells with cancer stem cell properties in human primary lung cancer cell lines. PLoS One 2013;8:e57020. [Crossref] [PubMed]
- Basak SK, Veena MS, Oh S, et al. The malignant pleural effusion as a model to investigate intratumoral heterogeneity in lung cancer. PLoS One 2009;4:e5884. [Crossref] [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of CD133 expression in stage I lung adenocarcinomas. Int J Clin Exp Pathol 2010;4:32-42. [PubMed]
- Mihatsch J, Toulany M, Bareiss PM, et al. Selection of radioresistant tumor cells and presence of ALDH1 activity in vitro. Radiother Oncol 2011;99:300-6. [Crossref] [PubMed]
- Li F, Zeng H, Ying K. The combination of stem cell markers CD133 and ABCG2 predicts relapse in stage I non-small cell lung carcinomas. Med Oncol 2011;28:1458-62. [Crossref] [PubMed]
- Mizugaki H, Sakakibara-Konishi J, Kikuchi J, et al. CD133 expression: a potential prognostic marker for non-small cell lung cancers. Int J Clin Oncol 2014;19:254-9. [Crossref] [PubMed]
- Okudela K, Woo T, Mitsui H, et al. Expression of the potential cancer stem cell markers, CD133, CD44, ALDH1, and β-catenin, in primary lung adenocarcinoma--their prognostic significance. Pathol Int 2012;62:792-801. [Crossref] [PubMed]
- Salnikov AV, Gladkich J, Moldenhauer G, et al. CD133 is indicative for a resistance phenotype but does not represent a prognostic marker for survival of non-small cell lung cancer patients. Int J Cancer 2010;126:950-8. [PubMed]
- Yoshida A, Hsu LC, Davé V. Retinal oxidation activity and biological role of human cytosolic aldehyde dehydrogenase. Enzyme 1992;46:239-44. [PubMed]
- Zhang WC, Shyh-Chang N, Yang H, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell 2012;148:259-72. [Crossref] [PubMed]
- Jiang F, Qiu Q, Khanna A, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res 2009;7:330-8. [Crossref] [PubMed]
- Soh BS, Zheng D, Li Yeo JS, et al. CD166(pos) subpopulation from differentiated human ES and iPS cells support repair of acute lung injury. Mol Ther 2012;20:2335-46. [Crossref] [PubMed]
- Gutova M, Najbauer J, Gevorgyan A, et al. Identification of uPAR-positive chemoresistant cells in small cell lung cancer. PLoS One 2007;2:e243. [Crossref] [PubMed]
- Zacharek SJ, Fillmore CM, Lau AN, et al. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell 2011;9:272-81. [Crossref] [PubMed]
- Lessard J, Sauvageau G. Bmi-1 determines the proliferative capacity of normal and leukaemic stem cells. Nature 2003;423:255-60. [Crossref] [PubMed]
- Song LB, Li J, Liao WT, et al. The polycomb group protein Bmi-1 represses the tumor suppressor PTEN and induces epithelial-mesenchymal transition in human nasopharyngeal epithelial cells. J Clin Invest 2009;119:3626-36. [Crossref] [PubMed]
- Bertolini G, Roz L, Perego P, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A 2009;106:16281-6. [Crossref] [PubMed]
- Vrzalikova K, Skarda J, Ehrmann J, et al. Prognostic value of Bmi-1 oncoprotein expression in NSCLC patients: a tissue microarray study. J Cancer Res Clin Oncol 2008;134:1037-42. [Crossref] [PubMed]
- Debruin EJ, Hughes MR, Sina C, et al. Podocalyxin regulates murine lung vascular permeability by altering endothelial cell adhesion. PLoS One 2014;9:e108881. [Crossref] [PubMed]
- Koch LK, Zhou H, Ellinger J, et al. Stem cell marker expression in small cell lung carcinoma and developing lung tissue. Hum Pathol 2008;39:1597-605. [Crossref] [PubMed]


