
Association of thyroid transcription factor-1 (TTF-1) expression with efficacy of PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy in advanced non-squamous non-small cell lung cancer
Introduction
Immune checkpoint inhibitors (ICIs) that target the programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) axis have shown unprecedented clinical activity for the treatment of individuals with advanced non-small cell lung cancer (NSCLC) and have become a standard therapy for such patients (1-5). The combination of PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy is a current standard first-line treatment for patients with advanced non-squamous NSCLC without genetic alterations of EGFR or ALK. However, not all patients experience a favorable response to this combination therapy. Biomarkers that are able to predict the efficacy of such treatment are therefore needed for optimal patient selection.
Thyroid transcription factor 1 (TTF-1), also known as Nkx2-1, is a 38-kDa transcription factor that is essential for morphogenesis and differentiation of the thyroid, lung, and ventral forebrain (6). Adenocarcinoma of the lung is categorized as terminal respiratory unit (TRU) type and non-TRU type, and TTF-1 is the most sensitive and specific marker for TRU-type adenocarcinoma (7). In addition to showing TTF-1 positivity, TRU-type carcinomas are well differentiated and are derived from type II pneumocytes and Club cells, whereas TTF-1-negative non-TRU-type carcinomas originate from centrally located dysplastic mucous columnar cells (8). TTF-1 has been shown to control tumor differentiation and to limit metastatic potential in lung adenocarcinoma, with its up-regulation having been found to correlate with favorable survival and its down-regulation to be linked to loss of differentiation, enhanced tumor seeding ability, and increased metastatic proclivity (9,10).
TTF-1 expression in advanced NSCLC tumors has also been associated with the efficacy of pemetrexed plus platinum chemotherapy (11-15). However, the relation between TTF-1 expression and the efficacy of combination treatment with PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy, a standard first-line treatment regimen for advanced non-squamous NSCLC, has been unknown. We therefore investigated the potential association between TTF-1 expression and the efficacy of such combination therapy in patients with advanced non-squamous NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-393/rc).
Methods
Patients
In this multicenter retrospective study, we analyzed 122 patients (75 TTF-1-positive and 47 TTF-1-negative individuals) with advanced (stage III or IV according to the 8th edition of the TNM classification) or recurrent non-squamous NSCLC who had started combination treatment with PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy in the first-line setting between January 2019 and November 2020. This study was conducted at four hospitals in Japan: Kyushu University Hospital, Kurume University Hospital, Japan Community Health Care Organization-Kyushu Hospital, and Kitakyushu Municipal Medical Center. Patients who had previously been treated with EGFR or ALK tyrosine kinase inhibitors were excluded. Progression of disease had to be confirmed on the basis of assessment by investigators according to Response Evaluation Criteria in Solid Tumors version 1.1. Clinical characteristics and pathological data for each patient were extracted by retrospective chart inspection. Progression-free survival (PFS) and overall survival (OS) of patients on first-line treatment were also reviewed. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Kyushu University Hospital (No. 2021-78; approval date, 31 May 2021), Kurume University Hospital (No. 21037; 16 July 2021), Japan Community Health Care Organization-Kyushu Hospital (No. 746; 8 June 2021), and Kitakyushu Municipal Medical Center (No. 202106002; 8 June 2021). Individual consent for this retrospective analysis was waived.
Tumor TTF-1 analysis
All specimens with the exception of those from bone metastases were fixed in 10% neutral buffered formalin. TTF-1 was detected in biopsy specimens by immunostaining at each institute. Antigen was retrieved by using immunosaver (Nissin EM, Tokyo, Japan) and tissue-nonspecific binding sites were blocked using normal goat serum. The sections were incubated with a mouse monoclonal antibody (clone 8G7G3/1 at a dilution of 1:50–1:200 from Agilent Technologies [Santa Clara, CA, USA; Agilent Cat# M3575, RRID: AB_2877699] or ready-to-use from Biocare Medical [Concord, CA, USA; Biocare Medical Cat# CM 087 A, RRID: AB_10583041]), and then treated with Histofine MAX-PO (M) (Nichirei Bioscience Inc., Tokyo, Japan). Staining was detected using diaminobenzidine chromogen, and all sections were counterstained with hematoxylin. TTF-1 immunostaining results were based on nuclear staining of neoplastic cells and were reported as positive or negative by an experienced pathologist (Figure S1).
Statistical analysis
The relation between expression of TTF-1 and patient characteristics was examined with Fisher’s exact test. Survival curves were estimated with the Kaplan-Meier method, and Cox proportional hazards regression analysis was applied to estimate the hazard ratio (HR) and its 95% confidence interval (CI). Survival outcomes were also compared between patient groups with the log-rank test. To minimize bias arising from patient background, we applied inverse probability of treatment weighting (IPTW) analysis (16). Balance before and after IPTW analysis was assessed with the standardized mean difference (SMD) between groups. An absolute SMD of <0.1 suggests adequate variable balance. The relation between patient characteristics and survival outcome was also evaluated with a multivariate Cox proportional hazards model, with the results being expressed as HR and its 95% CI. All reported p values are two-sided, and those of <0.05 were considered statistically significant. All statistical analysis was performed with Statistical Analysis System 9.4 software (SAS Institute, Cary, NC, USA; RRID:SCR_008567).
Results
Patient characteristics
A total of 122 non-squamous NSCLC patients treated with PD-1/PD-L1 inhibitors in combination with pemetrexed and platinum chemotherapy was included in the study. Seventy-five (61.5%) of these patients were positive for TTF-1 expression, and the baseline characteristics of the study patients according to TTF-1 expression status are shown in Table 1. The frequency of adenocarcinoma was higher among the TTF-1-positive patients compared with those negative for TTF-1 (98.7% vs. 76.6%, P<0.001). Histology other than adenocarcinoma is provided in Table S1. Other clinical characteristics were well balanced between the two groups.
Table 1
| Characteristic | All patients (n=122) | TTF-1 positive (n=75) | TTF-1 negative (n=47) | P value |
|---|---|---|---|---|
| Age (years) | 0.78a | |||
| Median | 67.0 | 67.0 | 70.0 | |
| Range | 36–82 | 37–82 | 36–80 | |
| Sex | 1.00 | |||
| Female | 34 (27.9) | 21 (28.0) | 13 (27.7) | |
| Male | 88 (72.1) | 54 (72.0) | 34 (72.3) | |
| ECOG PS | 0.19b | |||
| 0 | 42 (34.4) | 25 (33.3) | 17 (36.2) | |
| 1 | 76 (62.3) | 46 (61.3) | 30 (63.8) | |
| 2 | 4 (3.3) | 4 (5.3) | 0 | |
| Smoking history | 0.48 | |||
| Never-smoker | 22 (18.0) | 12 (16.0) | 10 (21.3) | |
| Smoker | 100 (82.0) | 63 (84.0) | 37 (78.7) | |
| Histology | <0.001 | |||
| Adenocarcinoma | 110 (90.2) | 74 (98.7) | 36 (76.6) | |
| Other | 12 (9.8) | 1 (1.3) | 11 (23.4) | |
| PD-L1 TPS | 0.67c | |||
| <1% | 43 (35.2) | 23 (30.7) | 20 (42.6) | |
| 1–49% | 34 (27.9) | 23 (30.7) | 11 (23.4) | |
| ≥50% | 32 (26.2) | 21 (28.0) | 11 (23.4) | |
| Stage | 1.00d | |||
| III | 6 (4.9) | 4 (5.3) | 2 (4.3) | |
| IV | 100 (82.0) | 61 (81.3) | 39 (83.0) | |
| Recurrent | 16 (13.1) | 10 (13.3) | 6 (12.8) | |
| Metastatic site at primary diagnosis | ||||
| Pleura | 45 (36.9) | 30 (40.0) | 15 (31.9) | 0.44 |
| Bone | 40 (32.8) | 25 (33.3) | 15 (31.9) | 1.00 |
| Brain | 28 (23.0) | 20 (26.7) | 8 (17.0) | 0.27 |
| Adrenal gland | 20 (16.4) | 11 (14.7) | 9 (19.1) | 0.62 |
| Liver | 9 (7.4) | 6 (8.0) | 3 (6.4) | 1.00 |
With the exception of age, all data are number (percent). All P values were calculated with Fisher’s exact test. a, <75 vs. ≥75; b, 0 or 1 vs. 2; c, <50% vs. ≥50%; d, III or IV vs. recurrent. ECOG PS, Eastern Cooperative Oncology Group performance status; TTF-1, thyroid transcription factor 1; PD-L1, programmed cell death ligand 1.
Treatment regimens
Cancer drugs administered as first-line therapy for the study patients are shown in Table 2. The frequency of carboplatin-based regimens did not differ significantly between TTF-1-positive and TTF-1-negative groups (76.0% vs. 76.6%, respectively). With regard to PD-1/PD-L1 inhibitors, both pembrolizumab (66.7% vs. 66.0%) and atezolizumab (33.3% vs. 34.0%) were administered at a similar frequency in patients positive or negative for TTF-1 expression. Use of angiogenesis inhibitors was also well balanced between the two groups, with the frequency of bevacizumab treatment being similar in the TTF-1-positive and TTF-1-negative groups (17.3% vs. 17.0%, respectively). Treatment regimens for the first-line therapy are shown in Table S2.
Table 2
| Drug | TTF-1 positive (n=75) | TTF-1 negative (n=47) | P value |
|---|---|---|---|
| Platinum agent | 1.00 | ||
| Carboplatin | 57 (76.0) | 36 (76.6) | |
| Cisplatin | 18 (24.0) | 11 (23.4) | |
| Immune checkpoint inhibitor | 1.00 | ||
| Pembrolizumab | 50 (66.7) | 31 (66.0) | |
| Atezolizumab | 25 (33.3) | 16 (34.0) | |
| Angiogenesis inhibitor | 1.00 | ||
| Bevacizumab | 13 (17.3) | 8 (17.0) | |
| None | 62 (82.7) | 39 (83.0) |
Data are number (percent). All P values were calculated with Fisher’s exact test. TTF-1, thyroid transcription factor 1.
Association between TTF-1 positivity and efficacy of the combination of PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy
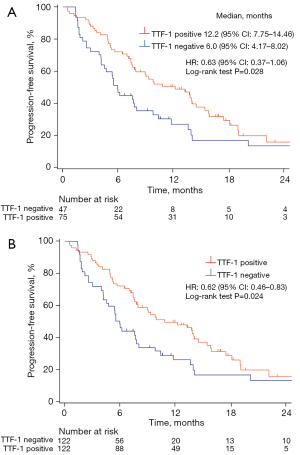
At the time of analysis, 89 (73.0%) patients had experienced a progression event (median follow-up of 14.6 months, with a range of 0.53–29.5 months). Kaplan-Meier curves for PFS of the study patients are shown in Figure 1A. TTF-1-positive patients had a longer PFS compared with TTF-1-negative patients (median, 12.2 vs. 6.0 months; HR =0.63; 95% CI: of 0.37–1.06; log-rank test P=0.028). For IPTW analysis, predicted probabilities from the propensity score model were used to calculate weight. Baseline covariates included in the propensity score calculation were age, sex, smoking history, PD-L1 tumor proportion score (TPS), and treatment with a bevacizumab-containing regimen. The distribution of patient characteristics according to TTF-1 expression status is shown for both the unweighted and weighted samples in Table S3. The differences in patient characteristics between the TTF-1 expression groups tended to be attenuated in the weighted samples compared with the unweighted samples. The IPTW-adjusted Kaplan-Meier curves for PFS are shown in Figure 1B, with PFS being significantly longer in TTF-1-positive than in TTF-1-negative patients (HR =0.62; 95% CI: 0.46–0.83; log-rank test P=0.024).
We also analyzed PFS specifically for patients with an adenocarcinoma histology (Figure S2). This analysis also revealed that TTF-1-positive patients had a longer PFS compared with TTF-1-negative patients (median, 10.8 vs. 5.7 months; HR =0.68; 95% CI: 0.37–1.26; log-rank test P=0.019).
Finally, multivariate analysis of clinicopathologic factors for PFS is shown in Table 3. With adjustment for TTF-1 expression status, age, sex, smoking history, PD-L1 TPS, and treatment with a bevacizumab-containing regimen, TTF-1 negativity remained a significant unfavorable prognostic indicator for PFS (HR =1.62; 95% CI: 1.02–2.57; P=0.04).
Table 3
| Factor | HR | 95% CI | P value |
|---|---|---|---|
| TTF-1 | |||
| Positive | Reference | ||
| Negative | 1.62 | 1.02–2.57 | 0.04 |
| Age | |||
| <75 years | Reference | ||
| ≥75 years | 1.89 | 0.90–3.96 | 0.09 |
| PD-L1 TPS | |||
| ≥50% | Reference | ||
| <50% | 1.38 | 0.82–2.32 | 0.22 |
| Smoking | |||
| Never-smoker | Reference | ||
| Smoker | 1.49 | 0.78–2.84 | 0.23 |
| Angiogenesis inhibitor added | |||
| Bevacizumab | Reference | ||
| None | 1.41 | 0.77–2.61 | 0.27 |
| Sex | |||
| Female | Reference | ||
| Male | 1.11 | 0.63–1.96 | 0.71 |
All P values were calculated with a proportional hazards regression model. PFS, progression-free survival; HR, hazard ration; CI, confidence interval; TTF-1, thyroid transcription factor 1; PD-L1, programmed cell death ligand 1; TPS, tumor proportion score.
Discussion
As far as we are aware, our study is the first to show that TTF-1-positive patients with non-squamous NSCLC have a better PFS than do TTF-1-negative patients during treatment with PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy. Whereas pemetrexed and platinum chemotherapy was previously indicated to be less effective in TTF-1-negative patients (11-15), it has remained unknown whether the addition of PD-1/PD-L1 inhibitors to this regimen might overcome this inferiority in outcome for such patients. Our results now show that TTF-1 negativity was still associated with a shorter PFS even after the addition of PD-1/PD-L1 inhibitors to pemetrexed and platinum chemotherapy.
A higher proportion of TTF1-negative cases than of TTF-1-positive cases was histologically classified as other than adenocarcinoma. Given that this difference might have influenced our results, we performed an additional analysis of PFS for only patients with adenocarcinoma. Even this analysis limited to adenocarcinoma, however, revealed that TTF-1 negativity was associated with a shorter PFS.
We conducted IPTW analysis to minimize possible confounding factors and thereby to obtain unbiased estimates of treatment effects. Such an approach has been found to be effective for balancing covariates across patient groups, and has often shown superior performance compared with propensity score matching, in particular with small sample sizes (17), because it allows all cases to be considered in the final analysis. In the present study, IPTW analysis revealed a significant association between TTF-1 expression and PFS in the study patients. Moreover, multivariate analysis with Cox proportional hazards regression showed that TTF-1 expression was an independent factor influencing PFS. These statistical approaches thus confirm that TTF-1 expression in non-squamous NSCLC was associated with the efficacy of combination treatment with PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy.
Our results are consistent with recent basic research findings that TTF-1 influences the immune status of tumors, with a study of lung adenocarcinoma showing that TTF-1 is able to activate PD-L1 expression in vitro (18). On the other hand, loss of TTF-1 was thus shown to increase the production of transforming growth factor–β in lung cancer, which attenuates the tumor response to PD-L1 blockade by promoting T cell exclusion (19). Moreover, TTF-1 negativity in lung cancer was found to increase the recruitment of tumor-associated neutrophils (20), which contribute to cancer progression by establishing immune exclusion and are related to a poor outcome in patients treated with PD-1/PD-L1 inhibitors (21,22). These data suggest that TTF-1 negativity reduces the effectiveness of PD-1/PD-L1 inhibitors by influencing the tumor microenvironment through cytokine production and neutrophil recruitment.
We also assessed OS in the present study, with 44 (36.1%) patients having died at the time of analysis. Kaplan-Meier curves and IPTW-adjusted Kaplan-Meier curves of OS for the study patients are shown in Figure S3. Whereas there was no significant difference in OS between the TTF-1-positive and TTF-1-negative groups before IPTW adjustment (median of not reached vs. 23.3 months, respectively; HR =0.64; 95% CI: 0.33–1.26; log-rank test P=0.054), the IPTW-adjusted curves showed that TTF-1-positive patients had a significantly longer OS than did TTF-1-negative patients (HR =0.55; 95% CI: 0.36–0.84; log-rank test P=0.047). However, the number of censored patients was high in the OS analysis as a result of the relatively short observation period, suggesting that the observed relation of TTF-1 positivity to OS should be viewed with caution.
A recent study also suggested that TTF-1 expression is related to PFS and OS in non-squamous NSCLC patients treated with ICIs. However, this study differs from ours in that it included patients who received different ICIs in different lines of treatment and in that it pooled patients treated with immunotherapy alone together with those treated with a combination of ICIs plus chemotherapy (23).
Our study has several limitations. First, it was retrospective in nature, although this limitation is mitigated by the multicenter design of the study and its adoption of an analytic approach to minimize the risk of confounding factors. Second, although we found that TTF-1 is a promising candidate for further study with regard to understanding the effects of PD-1/PD-L1 inhibitors plus chemotherapy, we are not able to suggest a better regimen than PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy for TTF-1–negative patients. Given that our study did not compare regimens, our results might reflect only the prognostic effect of TTF-1. However, it remains possible that PD-1/PD-L1 inhibitors combined with platinum plus paclitaxel or nab-paclitaxel chemotherapy might be a better option for TTF-1-negative patients, on the basis of the suggestion that pemetrexed plus platinum chemotherapy is inferior to platinum regimens not containing pemetrexed in such patients (13). We are planning a prospective study of PD-1/PD-L1 inhibitors in combination with carboplatin plus nab-paclitaxel for patients with TTF-1-negative non-squamous NSCLC (jRCTs071220008). Third, the number of patients included in the study was relatively small and the observation period was relatively short, both of which limit evaluation of the impact of TTF-1 expression on OS in particular.
Conclusions
In summary, we have evaluated TTF-1 expression in advanced non-squamous NSCLC and found that TTF-1 positivity is associated with a better PFS for patients receiving PD-1/PD-L1 inhibitors plus pemetrexed and platinum chemotherapy in the first-line setting. This result suggests that TTF-1 expression can serve to predict PFS in patients with advanced non-squamous NSCLC who receive such first-line treatment.
Acknowledgments
We thank the participating patients and their families as well as the investigators and institutions involved in this study. We also thank members of the Center for Clinical and Translational Research at Kyushu University Hospital for their contribution to this study.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-393/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-393/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-393/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-393/coif). EI reports personal fees from Chugai Pharmaceutical, AstraZeneca, and Lilly. KT reports personal fees from Chugai Pharmaceutical, Lilly, and MSD. IO reports grants and personal fees from Chugai Pharmaceutical, AstraZeneca, MSD, Lilly, Boehringer Ingelheim, Ono Pharmaceutical, Taiho Pharmaceutical, and Bristol-Myers Squibb; grants from Astellas Pharma, Novartis, and AbbVie; and personal fees from Pfizer outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the ethics committee of Kyushu University Hospital (No. 2021-78; approval date, 31 May 2021), Kurume University Hospital (No. 21037; 16 July 2021), Japan Community Health Care Organization–Kyushu Hospital (No. 746; 8 June 2021), and Kitakyushu Municipal Medical Center (No. 202106002; 8 June 2021). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Kimura S, Hara Y, Pineau T, et al. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev 1996;10:60-9. [Crossref] [PubMed]
- Yatabe Y, Mitsudomi T. Epidermal growth factor receptor mutations in lung cancers. Pathol Int 2007;57:233-44. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Winslow MM, Dayton TL, Verhaak RG, et al. Suppression of lung adenocarcinoma progression by Nkx2-1. Nature 2011;473:101-4. [Crossref] [PubMed]
- Kwei KA, Kim YH, Girard L, et al. Genomic profiling identifies TITF1 as a lineage-specific oncogene amplified in lung cancer. Oncogene 2008;27:3635-40. [Crossref] [PubMed]
- Schilsky JB, Ni A, Ahn L, et al. Prognostic impact of TTF-1 expression in patients with stage IV lung adenocarcinomas. Lung Cancer 2017;108:205-11. [Crossref] [PubMed]
- Doherty MK, O'Connor E, Hannon D, et al. Absence of thyroid transcription factor-1 expression is associated with poor survival in patients with advanced pulmonary adenocarcinoma treated with pemetrexed-based chemotherapy. Ir J Med Sci 2019;188:69-74. [Crossref] [PubMed]
- Frost N, Zhamurashvili T, von Laffert M, et al. Pemetrexed-Based Chemotherapy Is Inferior to Pemetrexed-Free Regimens in Thyroid Transcription Factor 1 (TTF-1)-Negative, EGFR/ALK-Negative Lung Adenocarcinoma: A Propensity Score Matched Pairs Analysis. Clin Lung Cancer 2020;21:e607-21. [Crossref] [PubMed]
- Sun JM, Han J, Ahn JS, et al. Significance of thymidylate synthase and thyroid transcription factor 1 expression in patients with nonsquamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. J Thorac Oncol 2011;6:1392-9. [Crossref] [PubMed]
- Fiala O, Pesek M, Skrickova J, et al. Thyroid transcription factor 1 expression is associated with outcome of patients with non-squamous non-small cell lung cancer treated with pemetrexed-based chemotherapy. Tumour Biol 2017;39:1010428317691186. [Crossref] [PubMed]
- Xie J, Liu C. Adjusted Kaplan-Meier estimator and log-rank test with inverse probability of treatment weighting for survival data. Stat Med 2005;24:3089-110. [Crossref] [PubMed]
- Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol 2012;12:70. [Crossref] [PubMed]
- Guo M, Tomoshige K, Meister M, et al. Gene signature driving invasive mucinous adenocarcinoma of the lung. EMBO Mol Med 2017;9:462-81. [Crossref] [PubMed]
- Mariathasan S, Turley SJ, Nickles D, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8. [Crossref] [PubMed]
- Mollaoglu G, Jones A, Wait SJ, et al. The Lineage-Defining Transcription Factors SOX2 and NKX2-1 Determine Lung Cancer Cell Fate and Shape the Tumor Immune Microenvironment. Immunity 2018;49:764-779.e9. [Crossref] [PubMed]
- Faget J, Groeneveld S, Boivin G, et al. Neutrophils and Snail Orchestrate the Establishment of a Pro-tumor Microenvironment in Lung Cancer. Cell Rep 2017;21:3190-204. [Crossref] [PubMed]
- Park W, Lopes G. Perspectives: Neutrophil-to-lymphocyte Ratio as a Potential Biomarker in Immune Checkpoint Inhibitor for Non-Small-Cell Lung Cancer. Clin Lung Cancer 2019;20:143-7. [Crossref] [PubMed]
- Galland L, Le Page AL, Lecuelle J, et al. Prognostic value of Thyroid Transcription Factor-1 expression in lung adenocarcinoma in patients treated with anti PD-1/PD-L1. Oncoimmunology 2021;10:1957603. [Crossref] [PubMed]


