
Development and validation of the artificial intelligence (AI)-based diagnostic model for bronchial lumen identification
Introduction
The diagnosis of endobronchial lesions remains a major challenge in clinical practice. Lung cancer is the most common malignant lesion that causes endobronchial lesions, and it is also the leading cause of cancer-related deaths worldwide (1,2). The overall 5-year relative survival rate of lung cancer patients is only 19% (3), while that of stage IA patients exceeds 90% (4). Unfortunately, most lung cancer patients are diagnosed at an advanced stage resulting in no significant chance of a cure with palliative treatment as the only option. For this reason, the question of how to improve the early diagnosis rate of lung cancer patients has become a hot topic in recent lung cancer research.
Bronchoscopy is a key step in the diagnosis and treatment of respiratory diseases (5). Since it was first introduced in clinical medicine in China in the 1970s, bronchoscopy has become an important tool for respiratory diseases diagnosis, treatment, and emergency rescue. However, there are still a number of unmet needs related to endoscopies in China, such as lack of specialist doctors, an uneven distribution of advanced bronchoscopic units, and poor quality control (6,7). With increasing demands for bronchoscopy and their relevance in the decision-making processes, the workload and psychological pressure placed on doctors also continue to increase. As bronchoscopy is highly dependent on doctor’s clinical experience and operating skills, high-load endoscopy units can see the quality or their interventions reduced due to different factors, such as incomplete explorations of the airway, missed lesions or wrong interpretation of the findings observed.
Respiratory physicians early in their career may not be skilled in the technical aspects of bronchoscopy, or familiar with anatomic differences in airways, and physiological variations in patients which can cause challenges. The anatomy of the airway is like a labyrinth, and in performing a bronchoscopy, it is easy to get lost after entering each lumen, which can result in some segmental lumens being missed or observed repeatedly. On the other hand, respiratory physicians later in their career, can become very proficient in the bronchoscopy operation, pay too much attention to a specific lesion and ignore the observation of the rest of the lumens. To improve this variation among different specialists, there is a large number of guidelines based on expert consensus (8-10) on the optimization of bronchoscopy; however, the quality of the intervention remains very variable among doctors. Thus, for daily endoscopies, practical methods need to be developed to enable and facilitate the implementation of these guidelines.
Artificial intelligence (AI) has attracted attention since it was first proposed in 1956, especially in the last ten years (11,12). With the advent of the big-data era, the dramatic increase in computers’ computing power, and breakthroughs in algorithm research, AI has reached a period of rapid development. The combination of AI and medicine has had a profound effect on the medical system (13), especially in medical imaging (14-17), pathological examination (18-20), and endoscopy [e.g., colposcopy (21), gastroscopy (22), and colonoscopy (23)]. The combination of AI and imaging has greatly improved the accuracy and efficiency of clinicians in diagnosing various diseases. However, only limited progress has been made in the application of AI to bronchoscopy. Matava et al. (24) used 775 laryngoscopy and bronchoscopy videos as a data set, trained 3 types of CNNs to classify vocal cords and the tracheal airway anatomy in real-time during video laryngoscopy or bronchoscopy. Yoo et al. (25) used video bronchoscopy images to train an AI model through deep learning to identify anatomical locations among the carina and both the main bronchi, and the performance of the AI model was comparable to that of the most-experienced human expert.
At present, we are not aware of AI technology for bronchoscopy quality control. This research aimed to develop and validate an AI auxiliary system for bronchial lumen identification to help standardize and improve the quality of bronchoscopies. We present the following article in accordance with the TRIPOD reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-761/rc).
Methods
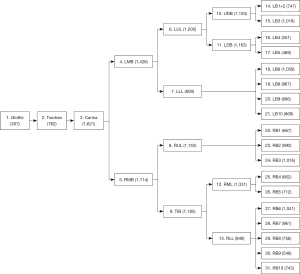
This cross-sectional study consecutively collected the operation videos of bronchoscopies from the Respiratory Endoscopy Center of Shanghai Chest Hospital from June 2020 to September 2020. For each video, 31 locations, including the glottis, trachea, carina, left main bronchus (LMB), right main bronchus (RMB), left upper lobe (LUL) bronchus, left lower lobe (LLL) bronchus, right upper lobe (RUL) bronchus, truncus intermedius bronchus (TIB), upper division bronchus (UDB), lower division bronchus (lingular bronchus) (LDB), right middle lobe (RML) bronchus, right lower lobe (RLL) bronchus, left bronchus (LB) 1+2, LB3, LB4, LB5, LB6, LB8, LB9, LB10, right bronchus (RB) 1, RB2, RB3, RB4, RB5, RB6, RB7, RB8, RB9, and RB10, were observed in order.
The inclusion criteria were completed video of bronchoscopy operation and clear lumen images. The exclusion criteria included unclear lumen images, and lumen lesions affecting the identification of bronchial lumen. The primary endpoint was the accuracy of AI in identifying bronchial lumens, defined as all lumens AI correctly identified divided by the total lumens AI identified. The secondary endpoints were the difference in the accuracy of AI in identifying different locations, and the comparison between AI and doctors in identifying lumens.
Data set and pre-processing
A total of 342 bronchoscopy procedure videos were collected and segmented into image frames at a frequency of 3 frames per second. For the experimental images, only a squared area containing the bronchoscopic view was cropped, and the rest of the frame was removed. All the images were resized to 1,000 by 1,000 pixels. The study design is presented in Figure 1.
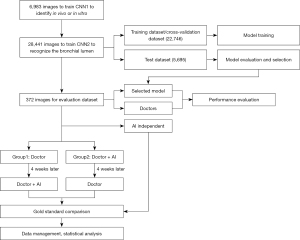
First, in-vitro, in-vivo, and unqualified images were selected randomly and marked by 3 respiratory intervention clinicians to train the convolutional neural network (CNN) to identify whether the bronchoscope was in vivo or in vitro. This model is hereafter referred to as “CNN1.”
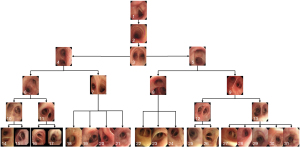
Second, the qualified bronchoscopy images were used to train the classification network to recognize the bronchial lumen. This model is hereafter referred to as “CNN2.” Using a ratio of 6:2:2, the data were randomly divided into the training dataset, cross-validation dataset, and test dataset. The respiratory tract was divided into 31 locations, including the glottis, trachea, carina, left and right main bronchi, lobar bronchi, and segmental bronchi (see Figure 2), which were independently marked by 3 professional respiratory intervention doctors. To reduce bias, the images were only included when at least 2 doctors were in agreement. The specific marking results of the 31 locations of the bronchus are shown in Figure 3.
Model training and testing
For in-vivo/in-vitro recognition, visual geometry group 16 (VGG-16) and ResNet-50 were used to train the model. For the recognition of the 31 locations of the bronchus, InceptionV3, MobileNet, ResNet-50, VGG-16, VGG-19, and Xception were used to train the model. Among the dataset, the training set was used to train the model, the cross-validation dataset was used to determine the network structure and adjust the hyperparameters of the model, and the test dataset was used to explore the generalizability of the model. Data augmentation, including reflection, rotation, shift and early stop, were used to prevent overfitting.
Clinical research design
Evaluation data set
In total, 12 complete bronchoscopy operation videos, showing 31 locations, were further included in evaluation data set. Again, each video was segmented into image frames. A typical image was selected for each location of each video, and thus a data set of 372 images was formed for clinical evaluation.
Clinical evaluation
The clinicians, who were required to identify the bronchial locations, comprised 4 senior doctors (hereafter referred to as doctors A, B, C, and D, with greater than 5 years of experience in interventional pulmonology) and 4 junior doctors (hereafter referred to as doctors a, b, c, and d, with less than 1 year of experience in interventional pulmonology), who were randomly divided into Group 1 and Group 2 respectively. Thus, Group 1 and Group 2 each comprised 2 senior doctors and 2 junior doctors, respectively. All of clinicians and the AI system were blind to the 372 images that comprised the evaluation dataset. For the evaluation dataset, the doctors in group 1 first identified independently the bronchoscopic images. AI was used 4 weeks later as a diagnosis aid in a similar process. Doctors in group 2 first used AI to assist in the identification of the images, and then independently, repeated the process, without AI help 4 weeks later. The difference in accuracy among the senior and junior doctors and the AI system was compared and analyzed.
Statistical analysis
The statistical analyses were performed using SPSS software (version 25.0; IBM Corp., Armonk, NY, USA). We presented standard descriptive statistics, with categorical variables presented as percentages. The Pearson’s chi-squared test was used to compare the categorical variables. All P values were two-sided. P values <0.05 were considered statistically significant.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Chest Hospital (No. IS2125). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Results
In-vivo/in-vitro recognition
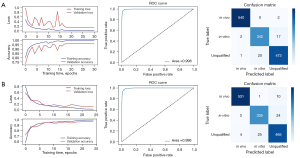
Of all the images, 1,805 in-vitro, 2,708 in-vivo, and 2,470 unqualified bronchoscopic images were selected; representative images are shown in Figure 4. VGG-16 and ResNet-50 were used to train the recognition in-vivo/in-vitro model. The results showed that the recognition accuracy rates of VGG-16 and ResNet-50 did not differ greatly, and had values of 98.02% and 98.39%, respectively. The receiver operating characteristic (ROC) curves of the 2 accuracy rates were drawn, and the areas under the ROC curve (AUCs) of both accuracy rates reached 0.99, indicating that the models’ performance was good. Performance metric changes, the ROC curves, and confusion matrixes of the ResNet50 and VGG-16 for in-vivo/in-vitro/unqualified image recognition are shown in Figure 5.
Identification of 31 locations of the bronchial lumen
A total of 28,441 qualified bronchoscopy images were selected. Multiple CNNs were used to identify 31 locations of the bronchus. The performance metric changes of each model during the training process are shown in Figure 6. The ROC curve of each model is shown in Figure 7. The AUC of each model was above 0.96, indicating that these models had good generalizability. The identification results are shown in Table 1. The results showed that in the cross-validation set, the optimal accuracy of the six models was between 91.83% and 96.62%. But in the test data, the optimal accuracy rate of the VGG-16 reached 91.88%, which also had the best recognition accuracy rate among the tested models. For this reason, VGG-16 was selected as the AI system for the next test.
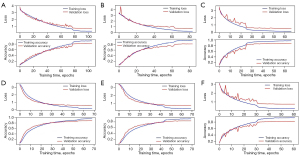
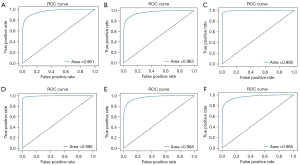
Table 1
| Model | Cross-validation set accuracy | Test set accuracy |
|---|---|---|
| VGG-16 | 95.62% | 91.88% |
| ResNet50 | 96.62% | 91.76% |
| Xception | 96.26% | 73.28% |
| VGG-19 | 91.83% | 71.77% |
| MobileNet | 95.37% | 70.97% |
| InceptionV3 | 93.42% | 70.34% |
VGG, visual geometry group.
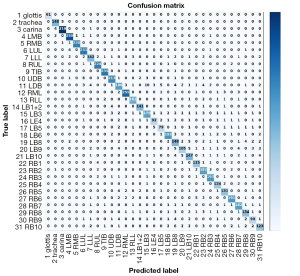
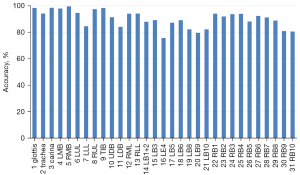
We also constructed a confusion matrix based on VGG-16 (see Figure 8), which showed the classification performance of the model for each category. The confusion matrixes for InceptionV3, MobileNet, ResNet-50, VGG-19, and Xception are shown in Figure S1. According to the confusion matrixes, there was significant difference in the ability of the model to recognize specific categories. Thus, we further measured the recognition accuracy of VGG-16 for each location. As Figure 9 shows, the recognition accuracy rates of different locations ranged from 75.67% to 99.54%. Among them, the recognition accuracy rates for the glottis, carina, and LMB, RMB, RUL bronchus, and TIB were relatively high, with values >95%. However, the recognition accuracy rates for the LLL bronchus, LDB, LB4, and most basal segment bronchi were <85%, which was significantly lower than that of the other locations. The similarity between these locations may be the reason for the lower recognition accuracy.
Clinical evaluation results
The accuracy rate of the AI system alone in identifying the 372 bronchial images was 54.30% (202/372). For the identification of bronchi except for segmental bronchi, the accuracy was 82.69% (129/156). The recognition accuracy of segmental bronchi (B1–B10) was only 33.80% (73/216). The accuracy rates of the doctors are shown in Table 2. In Group 1, the accuracy rates of doctors A, B, a, and b increased from 42.47%, 34.68%, 28.76%, and 29.57%, respectively, when conducting the identification alone to 57.53%, 54.57%, 54.57%, and 46.24%, respectively, when conducting the identification with the assistance of the AI system. In Group 2, the accuracy rates of doctors C, D, c, and d increased from 37.90%, 41.40%, 30.91%, and 33.60%, when conducting the identification alone to 51.61%, 47.85%, 53.49%, and 54.30%, respectively, when conducting the identification with the assistance of the AI system. Except for doctor D, the recognition accuracy rates of the other 7 doctors alone (i.e., without AI assistance) and their recognition accuracy rates with AI assistance differed significantly (P<0.001). Thus, the AI system significantly improved the doctors’ recognition of the bronchial lumen, especially among the younger doctors. In relation to the specific results for the identification of the 31 locations, the doctors accurately identified the glottis, trachea, carina, LMB, RMB, RUL bronchus, and TIB. However, in relation to the identification of the segmental bronchus, the recognition accuracy of the doctors was generally low.
Table 2
| Identification results | Method | Total | P value | |
|---|---|---|---|---|
| Doctor | Doctor + AI | |||
| Group 1 | ||||
| A | <0.001 | |||
| Identification | ||||
| Yes | 158 | 214 | 372 | |
| No | 214 | 158 | 372 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 42.47% | 57.53% | 50.00% | |
| B | <0.001 | |||
| Identification | ||||
| Yes | 129 | 203 | 332 | |
| No | 243 | 169 | 412 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 34.68% | 54.57% | 44.62% | |
| a | <0.001 | |||
| Identification | ||||
| Yes | 107 | 203 | 310 | |
| No | 265 | 169 | 434 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 28.76% | 54.57% | 41.67% | |
| b | <0.001 | |||
| Identification | ||||
| Yes | 110 | 172 | 282 | |
| No | 262 | 200 | 462 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 29.57% | 46.24% | 37.90% | |
| Group 2 | ||||
| C | <0.001 | |||
| Identification | ||||
| Yes | 141 | 192 | 333 | |
| No | 231 | 180 | 411 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 37.90% | 51.61% | 44.76% | |
| D | 0.080 | |||
| Identification | ||||
| Yes | 154 | 178 | 332 | |
| No | 218 | 194 | 412 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 41.40% | 47.85% | 44.62% | |
| c | <0.001 | |||
| Identification | ||||
| Yes | 115 | 199 | 314 | |
| No | 257 | 173 | 430 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 30.91% | 53.49% | 42.20% | |
| d | <0.001 | |||
| Identification | ||||
| Yes | 125 | 202 | 327 | |
| No | 247 | 170 | 417 | |
| Total | 372 | 372 | 744 | |
| Accuracy | 33.60% | 54.30% | 43.95% | |
AI, artificial intelligence.
Discussion
Millions of people worldwide undergo bronchoscopy every year, and high-quality endoscopy can improve the robustness of respiratory diseases diagnosis (5,26-28). However, there are significant differences in the level of expertise between endoscopists, which affect the detection rate of lesions. The question of how to correctly identify and judge the bronchial lumen has become an important part of bronchoscopy examinations and treatments. This is especially important when the lesion is located at the distal end of the bronchus and is invisible under a bronchoscope, as a positioning error may directly lead to examination or treatment failure. A series of guidelines for regulating bronchoscopy operations based on expert consensus have been proposed (8-10); however, due to a lack of supervision and practical tools, these guidelines are often not well implemented, especially in developing countries.
It was previously reported that some scholars applied AI to bronchoscopy. Tan et al. (29) proposed a new migration learning method on DenseNet for the recognition of lung diseases, including cancer and tuberculosis, under bronchoscopy. Using this method, the authors identified 87% of cancers, 54% of tuberculosis cases, and 91% of normal cases with good detection accuracy. Feng et al. (30) used fluorescent bronchoscopy pictures of 12 cases of adenocarcinoma and 11 cases of squamous cell carcinoma as a data set, and linear regression machine learning methods for classification, and ultimately achieved an accuracy of 83% for lung cancer classification. Hotta et al. (31) also trained CNN model to predict benign or malignant lesions based on endobronchial ultrasonography findings. These studies mainly focused on AI assisted diagnosis of bronchial lesions. However, the recognition of bronchial lumen was only limited to the recognition of vocal cords and tracheal (24), carina and both the main bronchi (25).
Our AI system was able to accurately identify the main and lobar bronchi. AI systems could help inexperienced doctors to quickly identify the lumen and prevent missed diagnoses and misdiagnoses. For senior doctors, bronchoscopies can be standardized by AI systems, thereby improving the quality of the interventions performed.
In terms of endoscopy, Wu et al. combined a deep CNN with deep reinforcement learning to train a real-time quality improvement system (WINSENSE) for esophageal gastroduodenoscopy. Monitoring blind spots during the examination has become a powerful auxiliary tool to reduce the different skills of endoscopists and improve the quality of daily endoscopies (32); however, in relation to bronchoscopy, most studies have focused on the identification of the pathological specimens of transbronchial biopsies (33,34). To our knowledge, this is the first study to apply CNN training to identify bronchial anatomy.
Due to the hierarchical relationship between the bronchial segments, the bronchial lumens are usually checked in order during bronchoscopy; thus, there is no need to actually consider the specific shape of each lumen. In addition, the segmental bronchi, especially the lingual segment and the basal segment of the lower lobes, are very similar; making it extremely difficult to identify the bronchial lumen based on images. In this study, neither junior nor senior doctors had very high accuracy rates for recognizing bronchial lumen images. This indicates that image-based bronchial lumen recognition is challenging. In relation to the recognition accuracy of the doctors for each location, the 2 groups of doctors could better identify the glottis, trachea, carina, LMB, RMB, RUL bronchus, and TIB than the remaining bronchial lumens of the 31 different locations. For these locations, the senior doctors had a slightly higher recognition accuracy rate than the junior doctors, which indicates that these locations have more obvious characteristics that facilitate their recognition. However, for the segmental bronchi B1-B10, the recognition accuracy rates of both the senior and junior doctors declined steeply, and the accuracy rates of all the doctors were between 2.31% and 20.37%. These results demonstrate that these locations are difficult to recognize based on images alone. Conversely, the AI system had a better ability to recognize the lumen, especially the segmental bronchus. The recognition accuracy rate of the AI system was significantly higher than that of all the doctors. Thus, the AI system was able to identify lumen that the doctors could not properly identify.
An understanding of the specific shape of each lumen could increase the ability of junior doctors to identify and more quickly familiarize themselves with the bronchial structure, thereby improving their ability to recognize the lumen and reducing the time spent identifying them during bronchoscopy. With the assistance of AI, the recognition accuracy of both junior and senior doctors increased significantly. The improvement in the recognition accuracy of the junior doctors was more obvious than of the senior ones. This showed that the AI system improved doctors’ ability to recognize the bronchial lumen, and enabled the junior doctors to achieve a level of accuracy similar to that of the senior doctors.
The introduction of an AI system in bronchoscopy has a number of advantages. First, it can help junior doctors to quickly familiarize themselves with bronchoscopy techniques and thus avoid missing or repeating the viewing of certain lumens during bronchoscopy. Second, it can improve the operation quality by enabling doctors to gain a comprehensive understanding of the inspection process. Third, it can improve the detection rate of lesions, and enable the automatic identification of possible lesions.
Although the results of our study warrant further investigations, it has some limitations. First, our results were obtained using Olympus endoscopes (Olympus is a mainstream model); however, we hope that the AI system can be applied to endoscopes from other vendors. Theoretically, this could be achieved by using the transfer learning method without additional algorithm tuning (35). Second, this study only focused on the recognition of bronchial lumen based on images, and complete video bronchoscopy training was not carried out. Third, this was a single-center study, and the number of clinicians involved was small. Thus, we intend to train and test the AI system using more videos of bronchoscopy operations from multiple centers in the future.
Conclusions
In conclusion, this AI model is an endoscopy quality improvement system based on CNNs. The AI system was better able to recognize lumen than the junior and senior doctors alone were. Furthermore, our findings suggest that the supplemental application of the AI system could reduce the differences in the endoscopic skills of doctors with different levels of experience, and it could become a powerful auxiliary tool to improve the quality of daily endoscopic examinations. In the future, the AI system is expected to be used to monitor the blind spot rate during bronchoscopy and determine the inspection time.
Acknowledgments
The authors would like to thank Dr. Wu Weijin for his help in training the CNN models. The authors also appreciate the academic support from AME Artificial Intelligence Collaborative Group.
Funding: This work was supported by funding from the SJTU Trans-med Awards Research Grant (No. 20210101), and the Multi-Disciplinary Collaborative Clinical Research Innovation Project of Shanghai Chest Hospital (No. YJXT20190205).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-761/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-761/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-761/coif). MR reports personal fees from Abex, Intuitive, and AstraZeneca, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Shanghai Chest Hospital (No. IS2125). Because of the retrospective nature of the study, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Schabath MB, Cote ML. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol Biomarkers Prev 2019;28:1563-79. [Crossref] [PubMed]
- Murakami S, Ito H, Tsubokawa N, et al. Prognostic value of the new IASLC/ATS/ERS classification of clinical stage IA lung adenocarcinoma. Lung Cancer 2015;90:199-204. [Crossref] [PubMed]
- Gasparini S, Bonifazi M. Management of endobronchial tumors. Curr Opin Pulm Med 2016;22:245-51. [Crossref] [PubMed]
- Lin J, Tao X, Xia W, et al. A multicenter survey of pediatric flexible bronchoscopy in western China. Transl Pediatr 2021;10:83-91. [Crossref] [PubMed]
- Nie XM, Cai G, Shen X, et al. Bronchoscopy in some tertiary grade A hospitals in China: two years' development. Chin Med J (Engl) 2012;125:2115-9. [PubMed]
- Jin F, Li Q, Li S, et al. Interventional Bronchoscopy for the Treatment of Malignant Central Airway Stenosis: An Expert Recommendation for China. Respiration 2019;97:484-94. [Crossref] [PubMed]
- Ernst A, Wahidi MM, Read CA, et al. Adult Bronchoscopy Training: Current State and Suggestions for the Future: CHEST Expert Panel Report. Chest 2015;148:321-32. [Crossref] [PubMed]
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax 2013;68:i1-i44. [Crossref] [PubMed]
- Howard J. Artificial intelligence: Implications for the future of work. Am J Ind Med 2019;62:917-26. [Crossref] [PubMed]
- Xiang Y, Zhao L, Liu Z, et al. Implementation of artificial intelligence in medicine: Status analysis and development suggestions. Artif Intell Med 2020;102:101780. [Crossref] [PubMed]
- Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med 2019;25:44-56. [Crossref] [PubMed]
- Lassau N, Estienne T, de Vomecourt P, et al. Five simultaneous artificial intelligence data challenges on ultrasound, CT, and MRI. Diagn Interv Imaging 2019;100:199-209. [Crossref] [PubMed]
- Masood A, Sheng B, Li P, et al. Computer-Assisted Decision Support System in Pulmonary Cancer detection and stage classification on CT images. J Biomed Inform 2018;79:117-28. [Crossref] [PubMed]
- Becker AS, Marcon M, Ghafoor S, et al. Deep Learning in Mammography: Diagnostic Accuracy of a Multipurpose Image Analysis Software in the Detection of Breast Cancer. Invest Radiol 2017;52:434-40. [Crossref] [PubMed]
- Aramendía-Vidaurreta V, Cabeza R, Villanueva A, et al. Ultrasound Image Discrimination between Benign and Malignant Adnexal Masses Based on a Neural Network Approach. Ultrasound Med Biol 2016;42:742-52. [Crossref] [PubMed]
- Bera K, Schalper KA, Rimm DL, et al. Artificial intelligence in digital pathology - new tools for diagnosis and precision oncology. Nat Rev Clin Oncol 2019;16:703-15. [Crossref] [PubMed]
- Awan R, Sirinukunwattana K, Epstein D, et al. Glandular Morphometrics for Objective Grading of Colorectal Adenocarcinoma Histology Images. Sci Rep 2017;7:16852. [Crossref] [PubMed]
- Komura D, Ishikawa S. Machine Learning Methods for Histopathological Image Analysis. Comput Struct Biotechnol J 2018;16:34-42. [Crossref] [PubMed]
- Miyagi Y, Takehara K, Miyake T. Application of deep learning to the classification of uterine cervical squamous epithelial lesion from colposcopy images. Mol Clin Oncol 2019;11:583-9. [PubMed]
- Itoh T, Kawahira H, Nakashima H, et al. Deep learning analyzes Helicobacter pylori infection by upper gastrointestinal endoscopy images. Endosc Int Open 2018;6:E139-44. [Crossref] [PubMed]
- Urban G, Tripathi P, Alkayali T, et al. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology 2018;155:1069-1078.e8. [Crossref] [PubMed]
- Matava C, Pankiv E, Raisbeck S, et al. A Convolutional Neural Network for Real Time Classification, Identification, and Labelling of Vocal Cord and Tracheal Using Laryngoscopy and Bronchoscopy Video. J Med Syst 2020;44:44. [Crossref] [PubMed]
- Yoo JY, Kang SY, Park JS, et al. Deep learning for anatomical interpretation of video bronchoscopy images. Sci Rep 2021;11:23765. [Crossref] [PubMed]
- Beaudoin EL, Chee A, Stather DR. Interventional pulmonology: an update for internal medicine physicians. Minerva Med 2014;105:197-209. [PubMed]
- d'Hooghe J, Alvarez Martinez H, Pietersen PI, et al. ERS International Congress, Madrid, 2019: highlights from the Clinical Techniques, Imaging and Endoscopy Assembly. ERJ Open Res 2020;6:e00116-2020. [Crossref] [PubMed]
- Rutter MD, Senore C, Bisschops R, et al. The European Society of Gastrointestinal Endoscopy Quality Improvement Initiative: developing performance measures. Endoscopy 2016;48:81-9. [PubMed]
- Tan T, Li Z, Liu H, et al. Optimize Transfer Learning for Lung Diseases in Bronchoscopy Using a New Concept: Sequential Fine-Tuning. IEEE J Transl Eng Health Med 2018;6:1800808. [Crossref] [PubMed]
- Feng PH, Lin YT, Lo CM. A machine learning texture model for classifying lung cancer subtypes using preliminary bronchoscopic findings. Med Phys 2018;45:5509-14. [Crossref] [PubMed]
- Hotta T, Kurimoto N, Shiratsuki Y, et al. Deep learning-based diagnosis from endobronchial ultrasonography images of pulmonary lesions. Sci Rep 2022;12:13710. [Crossref] [PubMed]
- Wu L, Zhang J, Zhou W, et al. Randomised controlled trial of WISENSE, a real-time quality improving system for monitoring blind spots during esophagogastroduodenoscopy. Gut 2019;68:2161-9. [Crossref] [PubMed]
- Asfahan S, Elhence P, Dutt N, et al. Digital-Rapid On-site Examination in Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration (DEBUT): a proof of concept study for the application of artificial intelligence in the bronchoscopy suite. Eur Respir J 2021;58:2100915. [Crossref] [PubMed]
- Zaizen Y, Kanahori Y, Ishijima S, et al. Deep-Learning-Aided Detection of Mycobacteria in Pathology Specimens Increases the Sensitivity in Early Diagnosis of Pulmonary Tuberculosis Compared with Bacteriology Tests. Diagnostics (Basel) 2022;12:709. [Crossref] [PubMed]
- Chang P, Grinband J, Weinberg BD, et al. Deep-Learning Convolutional Neural Networks Accurately Classify Genetic Mutations in Gliomas. AJNR Am J Neuroradiol 2018;39:1201-7. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



