
Immune thrombocytopenia in a small cell lung cancer patient treated with atezolizumab: a case report
Introduction
Immunotherapy has revolutionized the field of oncology via its blocking of the immunosuppressive effect on the interaction between cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) and its ligand, programmed death-ligand 1 (PD-L1) (1). Immune checkpoint inhibitors (ICIs) work by activating autoimmunity and relieving the suppressed state of the immune system, which is always accompanied by excessive autoimmune responses and inflammation in various systems. The most common immune-related adverse events (irAEs) occur in the endocrine system, skin, pulmonary system, and gastrointestinal tract (2).
Haematological irAEs (haem-irAEs) are relatively rare and are initially underestimated, but can be potentially life-threatening events that are occasionally irreversible and refractory (3). Recently, several studies have shown that, among haem-irAEs, thrombocytopenia and hemolytic anemia (HA) were more prevalent and occasionally occurred concomitantly (3,4). Some cases of ICI-related thrombocytopenia have been reported among patients with non-small cell lung cancer (NSCLC) and melanoma. Thrombocytopenia accounts for about 26–29% of haem-irAEs; the incidence of thrombocytopenia caused by ICIs is lowest among those treated with PD-1/L1 monotherapy (4,5). In an Asian cohort involving 141 patients receiving anti-PD-1 or anti-PD-L1 therapy, it was found that only one patient developed thrombocytopenia (6). There was only one case reported that ICI-induced thrombocytopenia in small cell lung cancer (SCLC) at present (7). Notably, unlike other irAEs, immune-related thrombocytopenia was associated with worse overall survival in patients treated with ICI. Currently, the incidence, pathogenesis, diagnosis, and management of haem-irAEs or ICI induced thrombocytopenia are not sufficiently understood.
Herein, we present for the first time a case of refractory immune-related severe thrombocytopenia and mild anemia that occurred concomitantly in a SCLC patient treated with chemotherapy combined with atezolizumab. Despite large doses of methylprednisolone, immunoglobulin and rituximab, intermittent platelet transfusion, thrombopoietin, and hemoglobinogen being administered to the patient, this glucocorticoid-refractory immune thrombocytopenia case was unfortunately fatal. To better understand and manage ICI-induced thrombocytopenia, the subjects of diagnosis of ICI-induced thrombocytopenia in the treatment pattern of chemotherapy combined ICI, strategies for the treatment of ICI-induced thrombocytopenia versus other causes of thrombocytopenia, therapeutic options for patients with glucocorticoid-resistant ICI-induced thrombocytopenia and current research progress on the incidence, potential biomarkers, mechanisms, and prognosis were been fully discussed. We present the following article in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-745/rc).
Case presentation
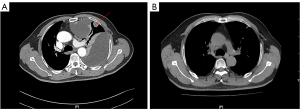
A 64-year-old man with a heavy smoking history for over 30 years presenting with left thoracalgia and shortness of breath visited The First Affiliated Hospital of Guangzhou Medical University in May 2020. The Chest computed tomography (CT) (Figure 1A) revealed a mass in the upper left lung, involving left pleural, left hilar and mediastinal lymph nodes metastases, and a large pleural effusion on the left. A biopsy of the left pleural metastases revealed SCLC and a systemic work-up diagnosed him as stage cT4N3M1a. The patient began receiving a combination of carboplatin plus etoposide and atezolizumab every 3 weeks on the 2nd of June 2020. Chest CT (Figure 1B) conducted on the 12th of October 2020 indicated partial response.
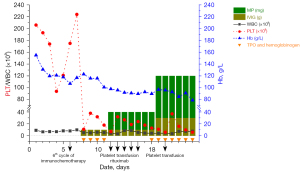
Routine blood test conducted before each cycle therapy, following administration of the sixth cycle of the combination therapy, routine blood re-examination on the third day (19th of December 2020) showed a white blood cell count of 5.2×109/L, hemoglobin of 12.3 g/dL, and a thrombocyte count of 11×109/L (Figure 2). The patient denied any overt bleeding episodes, skin bruising or ecchymoses, epistaxis, hematuria, fever, rash, diarrhea, or dyspnea. A manual review of the peripheral smear excluded pseudothrombocytopenia. The patient denied a history of thrombocytopenia, and his platelet counts had previously been within normal limits prior to and during the cancer treatment. He received recombinant human interleukin-11 thrombopoietin, hemoglobinogen (subcutaneous injection), and intravenous immunoglobulins daily during the comprehensive inspection time. During this period, we closely monitored the patient’s blood routine through finger peripheral blood or peripheral venous blood. However, his platelet counts did not improve, and his hemoglobin levels had reduced (Figure 2).
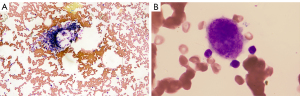
The laboratory results revealed there were no blast cells, schistocytes, or platelet clumping in the peripheral blood, and the blood serum immunological tests indicated that the patient was positive for platelet-associated GPIIb–IIIa and GPIbIx and anti-nuclear antibodies and negative for anti-neutrophil cytoplasmic antibody. The test of C-reaction protein, Cytomegalovirus DNA quantification, Epstein-Barr virus DNA quantification were negative. Bone marrow biopsy revealed that hypoplastic bone marrow and bare megakaryocytes and the production of platelet was reduced, but there was no evidence of fibrosis of the bone marrow, tumor cells, or other dysplastic cells (Figure 3).
Based on the above findings, the diagnoses of disseminated intravascular coagulation (DIC), hemolytic uremic syndrome (HUS), thrombotic thrombocytopenic purpura (TTP), pseudothrombocytopenia, infection, myelopathy, myelodysplastic syndrome, myelofibrosis, aplastic anemia, and leukaemia were excluded. Apart from the deterioration of hematology, the patient’s general condition was fine. Hence, a diagnosis of ICI-induced severe thrombocytopenia and mild anemia was considered.
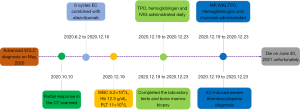
He was treated with methylprednisolone (MP, 40 mg/day) and intravenous immunoglobulin (IVIG, 10 g/day) intravenously on days 1 to 7, meanwhile, thrombopoietin, hemoglobinogen (subcutaneous injection), and intermittent platelet transfusion were administrated. Unfortunately, his platelet counts and hemoglobin did not improve at all, despite the administration of rituximab. We increased the dose of MP and IVIG to 120 mg/day and 30 g/day, respectively, and intermittent platelet transfusion, thrombopoietin, and hemoglobinogen (subcutaneous injection) were given daily. There were no signs of improvement of the patient’s platelet count and hemoglobin, so we advised the patient to receive plasma exchange or rilzabrutinib, mycophenolate mofetil and IL-6 inhibitor. The patient and his family refused further medical treatment and he was discharged automatically. During a follow-up telephone visit, the patient passed away on June 20, 2021 unfortunately. The timeline figure to describe the diagnosis, treatment, progression, and prognosis of this patient (Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient’s son for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
iMDT discussion
Discussion among physicians from The First Affiliated Hospital of Guangzhou Medical University
We conducted intradisciplinary and multidisciplinary consultation of this case. The current patient with a heavy smoking history for over 30 years, who diagnosed SCLC and a systemic work-up diagnosed him as stage cT4N3M1a, received six cycles of platinum plus etoposide combined atezolizumab. He developed refractory immune-related severe thrombocytopenia and mild anemia concomitantly. The patient denied a history of thrombocytopenia, and laboratory findings together to rule out other etiologies. Unfortunately, large doses of methylprednisolone, immunoglobulin and rituximab, intermittent platelet transfusion, thrombopoietin, and hemoglobinogen being administered to the patient, and his conditions did not improve.
Department of Oncology
Isolated case reports and case series have presented the hematologic toxicities induced by ICIs, but the incidence, diagnosis, and biological mechanisms in such patients remain unclear (7-17). Previous reports have summarized immune thrombocytopenia induced by immune checkpoint inhibitors in lung cancer (17), and ICI induced thrombocytopenia in only one case of SCLC (7). Haem-irAEs have not only been poorly understood and described but also lack consensus, whereas other common irAEs have well been characterized and managed. Haem-irAEs are relatively rare but may result in severe or even fatal outcomes due to the lack of effective treatment medicines.
It has been shown that chemotherapy is a major cause of life-threatening thrombocytopenia. Other conditions, such as sepsis, liver failure, and disseminated intravascular coagulation of the malignancy also can cause thrombocytopenia. In the pattern of chemotherapy combined with ICI treatment strategy, chemotherapy-induced myelosuppression in the current patient was not completely ruled out. Since his white blood cell count did not decrease, and platelet count and hemoglobin did not improve despite the administration of thrombopoietin and hemoglobinogen, the patient’s hematological toxic side effects were considered to be caused by anti-PD-L1 therapy.
Discussion of immunology specialist
The pathophysiology of immune thrombocytopenia (ITP) is complex and remains poorly understood. Autoantibodies and abnormalities in T cells have been described as potential mechanisms of platelet destruction. Drug-induced thrombocytopenia is an acquired autoimmune disease. In the presence of the drug, platelets are destroyed by phagocytic cells through the production of autoantibodies (18,19). The main features of ITP are excessive amounts of destroyed platelets and decreased platelet production, which results in bleeding, arterial thromboembolism, or venous thrombosis. A previous study revealed that the production of platelet-associated immunoglobulin G (PA-IgG) is stimulated by PD-1 or PD-L1 inhibitors, which can block the immunoinhibitory effect of PD-1 on T cells, while the basic underlying pathophysiology of ITP is Fc gamma receptor (FcgR)-mediated phagocytosis via the targeting PA-IgG (20). Moreover, immune thrombocytopenia was found to be associated with worse overall survival in a recent retrospective study (21). The excessive stimulation of PD-1/PD-L1 signaling may develop into ITP and promote platelet destruction. The platelet counts of the current patient decreased rapidly following administration of the sixth cycle of ICI plus chemotherapy, and the antibodies against platelet-associated GPIIb-IIIa and GPIbIx were positive.
Department of Hematology
Without a standard universal treatment strategy, immunosuppressives such as corticosteroids and rituximab are administrated for ICI-induced ITP. Glucocorticoid treatment is the standard first-line option for patients with ITP, but only 60–80% of patients with ITP have an initial response, and 30–50% have a sustained response. IVIG is another option, but the effects last for only 1–2 weeks. In life-threatening situations, additional treatments should be applied, including thrombopoietin-receptor agonists, rituximab, fostamatinib, etc. Rilzabrutinib, an oral Bruton tyrosine kinase (BTK) inhibitor, has been approved for the treatment of ITP, which may provide an alternative option for glucocorticoid-refractory cases. Moreover, sutimlimab, a monoclonal antibody that targets C1s, is a novel complement-directed therapy for research in patients with ITP. Sirolimus (SRL), a mammalian target of the rapamycin (mTOR) inhibitor, has been demonstrated to inhibit lymphocyte activity, indicating the potential for SRL application in the treatment of ITP (22). In the current glucocorticoid-refractory case, despite the administration of large doses of corticosteroids, immunoglobulin, and rituximab, the patient’s platelet counts and hemoglobinogen did not improve at all. We advised him to receive the plasma exchange or rilzabrutinib, other immunosuppressive drugs, or IL-6 inhibitor, but he refused further treatment.
Several issues regarding the diagnosis and treatment of this patient are further discussed as follows
Question 1: how can we quickly identify whether thrombocytopenia is caused by chemotherapy drugs or immune checkpoint inhibitors during the treatment pattern of chemotherapy and immunotherapy?
Expert opinion 1: Nobuhiko Seki
It is very difficult to determine exactly and quickly which drug is responsible for thrombocytopenia. However, in the case of chemotherapy-induced thrombocytopenia, the time to nadir, platelet counts at nadir, and recovery time from nadir are likely to be consistent with each course of the treatment. Therefore, when severe thrombocytopenia appears suddenly for the first time, which was not seen in the previous courses of treatment as in the current case, it is natural to assume that this phenomenon is attributable to immune checkpoint inhibitors rather than chemotherapy.
However, the median time to onset of immune thrombocytopenia is known to be 40 days (range, 3–405 days), and those with anti-CTLA-4 based therapy and with anti-PD-1/PD-L1 inhibitors are also known to be 23 and 47.5 days, respectively (3). Therefore, it is important to note that we are likely to come across immune thrombocytopenia with inadequate recognition of platelet nadir pattern due to chemotherapy.
On the other hand, PA-IgG has been shown to be useful as a simple diagnostic method for immune thrombocytopenia. Of course, PA-IgG can be induced not only by immune checkpoint inhibitors but also by chemotherapy. However, in the case of a positive result, it is more natural to first consider the possibility that the immune thrombocytopenia is attributable to immune checkpoint inhibitors rather than chemotherapy. Therefore, when a patient presents with a sudden onset of thrombocytopenia as in the current case, immediate PA-IgG testing may be useful because anyone can check it in the daily clinical practice to estimate whether immune thrombocytopenia is present and which drug is most likely responsible for it.
Expert opinion 2: Satoru Miura
As the case report described, it is extremely important to know whether thrombocytopenia has occurred during the previous treatment. Additionally, the blood cell count data will be useful. Thrombocytopenia without other cytopenia sounds is highly likely to be an irAE. Onset timing will be also important. Thrombocytopenia that develops during the maintenance period of ICI, which is almost unaffected by chemotherapy, should be examined considering irAE or disseminated intravascular coagulation (DIC) associated with worsening disease.
Expert opinion 3: Takehito Shukuya
This is a difficult question, but I would suspect that thrombocytopenia is due to immune checkpoint inhibitors if the timing of onset of thrombocytopenia is early or late compared with cytotoxic chemotherapy alone, thrombocytopenia is severe enough for cytotoxic chemotherapy alone, or if thrombocytopenia is prolonged. I also believe that such signs should be noted in those associated with hemophagocytic syndrome, since it is accompanied by other blood cell decreases, fever, hepatosplenomegaly, lymphadenopathy, and skin rash. When suspected, it is important to promptly collaborate with a hematologist to proceed with a thorough examination.
Question 2: what are the treatment strategies for immune checkpoint inhibitor-induced thrombocytopenia versus other causes of thrombocytopenia?
Expert opinion 1: Nobuhiko Seki
The causes of thrombocytopenia are classified into immune and nonimmune thrombocytopenia. Even in cases of nonimmune thrombocytopenia such as infections, they are usually caused by both ‘direct action, including the reduced production and enhanced destruction of thrombocyte’ and ‘indirect action, including the etiology-induced immune antibody’. Therefore, immunosuppressants like steroids as well as the etiology-specific treatments are often needed.
On the other hand, in cases of immune thrombocytopenia, immunosuppressants like steroids will carry the mainstream of treatments. However, immune checkpoint inhibitor-induced thrombocytopenia includes not only immune thrombocytopenia but also thrombotic thrombocytopenic purpura although most of the previous reports seem to equate immune checkpoint inhibitor-induced thrombocytopenia with immune thrombocytopenia (23). In particular, because the present case showed treatment-refractory anemia, thrombotic thrombocytopenic purpura should be included in the differential diagnosis. In such a case, we have to note that PA-IgG is not useful in differentiating immune thrombocytopenia from thrombotic thrombocytopenic purpura because PA-IgG is often elevated in thrombotic thrombocytopenic purpura as well. Furthermore, it is important to note that immunosuppressants like steroids as well as plasma exchange are the first-line treatments.
Expert opinion 2: Satoru Miura
The key therapeutic agent for immune checkpoint inhibitor-induced thrombocytopenia is immunosuppressive therapy represented by prednisolone. To treat other thrombocytopenia, it is important to make an appropriate differential diagnosis using bone marrow aspiration and blood tests. DIC is often the cause of thrombocytopenia in cancer treatment, and the treatment methods are different from irAEs; therefore, it is particularly important as a differential diagnosis.
Expert opinion 3: Takehito Shukuya
Prednisolone, cyclosporine, and cyclophosphamide are indicated for thrombocytopenia associated with hemophagocytic syndrome, while prednisolone and eltrombopag olamine are indicated for thrombocytopenia due to idiopathic thrombocytopenia, and gamma globulin and splenectomy may also be considered. In addition, platelet transfusions should be administered as symptomatic therapy. Treatment should be carried out in collaboration with a hematologist.
Question 3: what are the treatment plans for corticosteroid-resistant immune checkpoint inhibitor-induced thrombocytopenia in the patient?
Expert opinion 1: Nobuhiko Seki
As discussed by the Department of Hematology, the treatments in the current corticosteroid-refractory case include thrombopoietin-receptor agonists, rituximab, fostamatinib, rilzabrutinib (an oral Bruton tyrosine kinase inhibitor), sutimlimab (a monoclonal antibody that targets C1s), and sirolimus (a mammalian target of the rapamycin inhibitor) in addition to plasma exchange. Furthermore, the combination therapies with corticosteroid and mycophenolate mofetil based on a phase III trial, and with corticosteroid, mycophenolate mofetil, and tocilizumab (a monoclonal antibody that targets interleukin-6 receptor) based on a case report have been attractive attention as the promising treatment options (17,24).
By the way, regarding the corticosteroid-resistance, previous investigations indicated that idiopathic thrombocytopenic purpura patients with the HLA-DRB1*0410 or HLA-DRB1*0405 allele were originally resistant to corticosteroid treatment although this needs to be validated in immune checkpoint inhibitor-induced thrombocytopenia (25,26). Furthermore, single nucleotide polymorphisms of PDCD1 (e.g., −606 G/A and +63379 C/T) have been reported to be associated with low susceptibility to corticosteroid in patients with immune thrombocytopenia (27).
Expert opinion 2: Satoru Miura
Based on the ASCO guideline, 1–2 mg/kg/day dose of prednisolone has been recommended as the initial treatment for immune-thrombocytopenia. If worsening or not improving, a dose of 1 g/kg IVIG, rituximab, thrombopoietin receptor agonists, or immunosuppressive agents will be the further treatment options (28). The treatment courses of the case were reasonable. However, I believe that a higher initial dose of prednisolone may have been better.
Expert opinion 3: Takehito Shukuya
Normally, eltrombopag olamine and gammaglobulin should be considered. In Helicobacter pylori-positive cases, its eradication should be considered. Splenectomy should also be considered, but it is unclear how effective they are and whether they were possible in the presence of severe thrombocytopenia with the complication of lung cancer.
Question 4: what is the current research progress on the incidence, potential biomarkers, mechanisms, and prognosis of immune checkpoint inhibitor-induced thrombocytopenia?
Expert opinion 1: Nobuhiko Seki
Although immune checkpoint inhibitor-induced thrombocytopenia does not necessarily refer to immune thrombocytopenia alone, I will focus on it because it is the most frequent. The epidemiology can be summarized approximately as follows.
The incidence of immune thrombocytopenia may vary depending on the factors, including cancer type, nature of study (prospective or retrospective), and treatment type (immune checkpoint inhibitor alone, combined immune checkpoint inhibitors, and immune checkpoint inhibitor in combination with chemotherapy). Therefore, the absolute incidence of immune checkpoint inhibitor-induced thrombocytopenia is difficult to estimate. However, it would be estimated to be roughly around 1% from the following reports.
The largest recent study that described immune thrombocytopenia was by Wang et al. who reviewed 125 trials involving 20,128 patients treated with immune checkpoint inhibitor and found that 1.16% developed thrombocytopenia (29). Kramer et al., who reviewed 7,626 patients treated with immune checkpoint inhibitor, found that 0.2% developed immune thrombocytopenia (30). Le Burel et al., who retrospectively reviewed 908 patients treated with anti-PD-1/PD-L1, also found that 0.2% developed immune thrombocytopenia (31). Shiuan et al. reviewed 2,360 patients with melanoma treated with immune checkpoint inhibitor and identified eleven cases (0.47%) that developed immune thrombocytopenia (16). Haddad et al., who retrospectively reviewed 1,038 patients treated with immune checkpoint inhibitor monotherapy or in combination therapy, found that 1.73% developed grade 3 or higher immune thrombocytopenia (21).
Expert opinion 2: Satoru Miura
Presumably, no biomarkers have been discovered to predict thrombocytopenia as an irAE. The incidence of thrombocytopenia due to ICI was extremely rare, indicating 0.4–1.2% in the retrospective study (30). The median duration of ICI-induced thrombocytopenia was approximately 1 month. The treatment effect should be carefully examined because it requires some time to recover, reflecting on the platelet turnover time. Based on the retrospective study, most cases improved with treatment with steroids, IVIG, and rituxan. The presented case with resistance to all treatments is believed to be extremely rare.
Expert opinion 3: Takehito Shukuya
The frequency of atezolizumab-induced hemophagocytic syndrome is estimated to be less than 0.1%, and the rate of thrombocytopenia is 5–10%. Regarding the mechanism, in addition to hemophagocytic syndrome, there may be an autoimmune mechanism similar to idiopathic thrombocytopenic purpura.
Conclusions
ICI-induced ITP is exceedingly rare reported, but a life-threatening adverse event given the lack of universally effective strategies. Close monitoring and early recognition of the relevant signs are the main interventions for ITP. In clinical practice, platelet count and PA-IgG fluctuations should be closely monitored during ICI therapy for cancer patients. Meanwhile, pathophysiology and differential diagnosis were necessary considered. Also, corticosteroids should be considered when necessary, and additional treatments, including thrombopoietin-receptor agonists, rituximab, mycophenolate mofetil, IL-6 inhibitor, fostamatinib, rilzabrutinib, and sutimlimab, should be used if the condition develops.
Acknowledgments
We appreciate the academic support from the AME Lung Cancer Collaborative Group.
We would like to offer sincere thanks to the patient and his family for giving their consent the publication of this case.
Funding: This study was supported by the Natural Science Foundation of Guangdong Province of China (No. 2020A1515011384), the Science and Technology Program of Guangzhou, China (No. 202102010371), and the State Key Laboratory of Respiratory Disease the Open Project (Nos. SKLRD-OP-202101; SKLRD-Z-202010).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-745/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-745/coif). NS obtained commercial research grants from Eli Lilly, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer Japan, Ono Pharmaceutical, and Nippon Boehringer Ingelheim, and has received speaking honoraria from Eli Lilly, AstraZeneca, MSD Oncology, Chugai Pharmaceutical, Taiho Pharmaceutical, Pfizer Japan, Ono Pharmaceutical, Nippon Boehringer Ingelheim, and Bristol-Myers Squibb Japan. SM reports personal fees from AstraZeneca K.K., Bristol-Myers Squibb Co., Ltd., Boehringer Ingelheim, MSD K.K., Elli Lilly Japan, Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Pfizer, Novartis, and Taiho Pharmaceutical Co., Ltd. TS reports grants from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis and MSD; honoraria from AstraZeneca, Chugai Pharmaceutical, Boehringer Ingelheim, Novartis, MSD, Taiho Pharma, Daiichi-Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Kayaku and Pfizer. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (No. 2021-38) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient’ son for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov 2018;8:1069-86. [Crossref] [PubMed]
- Champiat S, Lambotte O, Barreau E, et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 2016;27:559-74. [Crossref] [PubMed]
- Davis EJ, Salem JE, Young A, et al. Hematologic Complications of Immune Checkpoint Inhibitors. Oncologist 2019;24:584-8. [Crossref] [PubMed]
- Delanoy N, Michot JM, Comont T, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 2019;6:e48-e57. [Crossref] [PubMed]
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer 2019;122:72-90. [Crossref] [PubMed]
- Huang Y, Soon YY, Aminkeng F, et al. Risk factors for immune-related adverse events from anti-PD-1 or anti-PD-L1 treatment in an Asian cohort of nonsmall cell lung cancer patients. Int J Cancer 2022;150:636-44. [Crossref] [PubMed]
- Zhou H, Li N, Tang H, et al. Delayed thrombocytopenia as a rare but serious adverse event secondary to immune checkpoint inhibitor: a case report. Ann Palliat Med 2021;10:5881-6. [Crossref] [PubMed]
- Tardy MP, Gastaud L, Boscagli A, et al. Autoimmune hemolytic anemia after nivolumab treatment in Hodgkin lymphoma responsive to immunosuppressive treatment. A case report. Hematol Oncol 2017;35:875-7. [Crossref] [PubMed]
- Palla AR, Kennedy D, Mosharraf H, et al. Autoimmune Hemolytic Anemia as a Complication of Nivolumab Therapy. Case Rep Oncol 2016;9:691-7. [Crossref] [PubMed]
- Kong BY, Micklethwaite KP, Swaminathan S, et al. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 2016;26:202-4. [Crossref] [PubMed]
- Schwab KS, Heine A, Weimann T, et al. Development of Hemolytic Anemia in a Nivolumab-Treated Patient with Refractory Metastatic Squamous Cell Skin Cancer and Chronic Lymphatic Leukemia. Case Rep Oncol 2016;9:373-8. [Crossref] [PubMed]
- Dougherty SC, Lynch AC, Hall RD. Drug-induced immune-mediated thrombocytopenia secondary to durvalumab use. Clin Case Rep 2021;9:e04227. [Crossref] [PubMed]
- Nair R, Gheith S, Nair SG. Immunotherapy-Associated Hemolytic Anemia with Pure Red-Cell Aplasia. N Engl J Med 2016;374:1096-7. [Crossref] [PubMed]
- Gergi M, Landry KK, Ades S, et al. Nivolumab-Induced Thrombotic Thrombocytopenic Purpura in a Patient with Anal Squamous Cell Carcinoma: A Lesson on Hematologic Toxicity from Immunotherapy. Oncologist 2020;25:1009-12. [Crossref] [PubMed]
- Kanai O, Nakatani K, Fujita K, et al. No need to hesitate: immune-related neutropenia and thrombocytopenia that improved by corticosteroids. Respirol Case Rep 2021;9:e00799. [Crossref] [PubMed]
- Shiuan E, Beckermann KE, Ozgun A, et al. Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy. J Immunother Cancer 2017;5:8. [Crossref] [PubMed]
- Xie W, Hu N, Cao L. Immune Thrombocytopenia Induced by Immune Checkpoint Inhibitrs in Lung Cancer: Case Report and Literature Review. Front Immunol 2021;12:790051. [Crossref] [PubMed]
- Liu XG, Ma SH, Sun JZ, et al. High-dose dexamethasone shifts the balance of stimulatory and inhibitory Fcgamma receptors on monocytes in patients with primary immune thrombocytopenia. Blood 2011;117:2061-9. [Crossref] [PubMed]
- Morodomi Y, Kanaji S, Won E, et al. Mechanisms of anti-GPIbalpha antibody-induced thrombocytopenia in mice. Blood 2020;135:2292-301. [Crossref] [PubMed]
- Nie M, Liu Y, Li XX, et al. PD-1/PD-L Pathway Potentially Involved in ITP Immunopathogenesis. Thromb Haemost 2019;119:758-65. [Crossref] [PubMed]
- Haddad TC, Zhao S, Li M, et al. Immune checkpoint inhibitor-related thrombocytopenia: incidence, risk factors and effect on survival. Cancer Immunol Immunother 2022;71:1157-65. [Crossref] [PubMed]
- Feng Y, Xiao Y, Yan H, et al. Sirolimus as Rescue Therapy for Refractory/Relapsed Immune Thrombocytopenia: Results of a Single-Center, Prospective, Single-Arm Study. Front Med (Lausanne) 2020;7:110. [Crossref] [PubMed]
- Schneider BJ, Naidoo J, Santomasso BD, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J Clin Oncol 2021;39:4073-126. [Crossref] [PubMed]
- Bradbury CA, Pell J, Hill Q, et al. Mycophenolate Mofetil for First-Line Treatment of Immune Thrombocytopenia. N Engl J Med 2021;385:885-95. [Crossref] [PubMed]
- Hasegawa T, Ozaki Y, Inoue T, et al. Nivolumab-related severe thrombocytopenia in a patient with relapsed lung adenocarcinoma: a case report and review of the literature. J Med Case Rep 2019;13:316. [Crossref] [PubMed]
- Nomura S, Matsuzaki T, Ozaki Y, et al. Clinical significance of HLA-DRB1*0410 in Japanese patients with idiopathic thrombocytopenic purpura. Blood 1998;91:3616-22. [Crossref] [PubMed]
- Kasamatsu T, Ino R, Takahashi N, et al. PDCD1 and CTLA4 polymorphisms affect the susceptibility to, and clinical features of, chronic immune thrombocytopenia. Br J Haematol 2018;180:705-14. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1714-68. [Crossref] [PubMed]
- Wang Y, Zhou S, Yang F, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol 2019;5:1008-19. [Crossref] [PubMed]
- Kramer R, Zaremba A, Moreira A, et al. Hematological immune related adverse events after treatment with immune checkpoint inhibitors. Eur J Cancer 2021;147:170-81. [Crossref] [PubMed]
- Le Burel S, Champiat S, Mateus C, et al. Prevalence of immune-related systemic adverse events in patients treated with anti-Programmed cell Death 1/anti-Programmed cell Death-Ligand 1 agents: A single-centre pharmacovigilance database analysis. Eur J Cancer 2017;82:34-44. [Crossref] [PubMed]


