
Treatment response and safety of immunotherapy for advanced non-small cell lung cancer with comorbid chronic obstructive pulmonary disease: a retrospective cohort study
Introduction
Lung cancer and chronic obstructive pulmonary disease (COPD) are two major respiratory diseases affecting the quality of human life. Lung cancer still represents the main cause cancer-related deaths, with non-small cell lung cancer (NSCLC) accounting for about 85% of all types of lung cancers. However, approximately 75% of NSCLC patients are diagnosed at an advanced stage (1). COPD is already the third leading cause of death worldwide, and its prevalence is expected to rise in the next 40 years. COPD and NSCLC are mutually important causes of death of each other. Recent research has suggested that the incidence of COPD is higher in NSCLC patients (2), and the incidence of comorbidities in both diseases is not low. Some researchers found that 69% had COPD or emphysema (39% with COPD, 59% with emphysema) in their lung cancer patients studied (2). Others also found that 50.5% patients with advanced NSCLC had COPD in their study (3). For this reason, clinicians are gradually paying more attention to this cohort, with relevant content added to the 2021 Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines. However, currently little is known about the treatment of advanced NSCLC with comorbid COPD, and there is no consensus on how to optimize treatment for these patients.
In recent years, immune checkpoint inhibitors (ICIs) have become a new standard of care for NSCLC, but evidence in NSCLC patients with comorbid COPD remain lacking. There is currently no uniform conclusion on the correlation between GOLD grading of COPD and the efficacy of immunotherapy. A study by Zhou et al. (4) found that PFS had a tendency to prolong in patients with comorbid moderate to severe COPD compared with no COPD patients, while Shin SH (5) found that PFS is better in patients with mild COPD. Therefore, this study analyzed the efficacy and tolerability of ICIs in advanced NSCLC patients with various degrees of comorbid COPD in order to optimize the use of immunotherapy in this special population. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-667/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research Ethics 2022 No. K-22). Individual consent for this retrospective analysis was waived.
Patients
We conducted a retrospective analysis of advanced NSCLC patients with and without comorbid COPD who were admitted to the First Affiliated Hospital of Guangzhou Medical University for immunotherapy between January 1, 2019, and April 30, 2021.
The inclusion criteria were as follows: (I) patients with NSCLC who had received ICIs; (II) stage IIIB/C to IV lung cancer patients evaluated unsuitable for surgery according to the 8th edition of the Union for International Cancer Control (UICC) staging criteria for NSCLC (6); (III) patients in which at least one measurable lesion was found according to the Response Evaluation Criteria in Solid Tumors (RECIST1.1); (IV) comorbid COPD according to the 2021 GOLD standard of diagnosis (https://goldcopd.org/2021-gold-reports/), i.e., the forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) after inhalation of bronchodilators <70%. The following severity rating was applied: mild (GOLD 1, FEV1% predicted ≥80%), moderate (GOLD 2, 50%≤ FEV1% predicted ≤79%), severe (GOLD 3, 30%≤ FEV1% predicted ≤49%), and extremely severe (GOLD 4, FEV1% predicted pred <30%); and (V) patients with imaging manifestations that supported the diagnosis of COPD.
The exclusion criteria were as follows: (I) patients with known intolerance or allergy to ICIs or any excipient ingredient in ICIs; (II) those with severe primary diseases in organs or systems vitally; (III) patients with disabilities either mentally or physically; (IV) patients in the active stage of infectious diseases, such as acquired immunodeficiency syndrome (AIDS), hepatitis, tuberculosis, and connective tissue diseases; (V) patients with lung infections or any other severe infections; (VI) patients participating in other clinical trials; and (VII) patients with incomplete clinical data.
The final study population included 99 patients according to the above criteria, with 19 no COPD patients (no COPD group, n1=19) and 80 advanced NSCLC coupled with various degrees of COPD patients. The latter group of patients were further divided into three groups according to the 2021 GOLD standard of diagnosis: mild COPD group (n2=24), moderate COPD group (n3=31), and severe COPD group (n4=25). All patients completed lung function tests during the study period, and were continuously observed and followed up until the occurrence of immune-related adverse events (irAEs), tumor progression, death, or loss of follow-up. Selected patients were diagnosed and evaluated by specialists in the Department of Respiratory Oncology, in strict accordance with the above guidelines.
Data collection
The demographic characteristics of the patients included age, gender, body mass index (BMI), smoking status, physical state (PS) score, imaging characteristics, tumor node metastasis (TNM) stage, pathological tumor type, metastasis, etc. In addition, irAEs and biochemical indexes including (but not limited to) routine blood test (e.g., creatinine, etc.), related cytokines [interleukin (IL)-6, IL-8, IL-10, etc.], and Krebs Von den Lungen (KL)-6, were also evaluated after each line of treatment. Comparability of baseline factors above and clinical characteristics were evaluated.
Endpoints and clinical efficacy
In this study, the primary endpoint was progression-free survival (PFS) and the secondary endpoints were the incidence of irAEs, the objective response rate (ORR), and the disease control rate (DCR). The PFS was defined as the time from the first administration of ICIs to disease progression, death, or last follow-up. Median PFS (mPFS) was defined as the time taken until 50% of patients experienced disease progression. The incidence of irAEs was calculated as the number of cases with irAEs/total cases ×100%.
Clinical efficacy was evaluated according to the RECIST 1.1 criteria. Complete response (CR) was defined as the disappearance of all measurable lesions, and partial response (PR) was defined as at least a 30% decrease in the sum of diameters of target lesions, taking the baseline sum diameters as a reference. Stable disease (SD) refers to a condition of insufficient shrinkage to qualify for PR nor a sufficient increase to qualify for progressive disease (PD), taking the smallest sum diameters in the study as a reference. PD was defined as at least a 20% increase in the sum of diameters of the target lesions, taking the smallest sum in the study as a reference. The appearance of one or more new lesions was also considered progression. The ORR was calculated as (CR + PR)/total number of cases ×100%, and the DCR was calculated as (CR + PR + SD)/total number of cases ×100%.
Statistical analyses
International Business Machines Corporation (IBM, America) Statistical Product Service Solutions (SPSS) 25.0 software was used for statistical analysis. The Chi-square test or Fisher’s exact test was used to compare categorical variables, and the t-test or analysis of variance was used to compare the differences between continuous variables. Survival analysis was estimated using the Kaplan Meier survival curve and log-rank test. The hazard ratio (HR) and 95% confidence interval (95% CI) of the survival time multivariate analysis were calculated using a Cox proportional hazards model. Bilateral P values less than 0.05 were considered statistically significant.
Results
Clinical characteristics of NSCLC patients with comorbid COPD
A total of 99 patients were included in this study, including 19 patients without COPD and 80 NSCLC patients with various degrees of comorbid COPD. We divided the NSCLC patients with comorbid COPD into three groups according to the GOLD guidelines: a mild group (GOLD 1), a moderate group (GOLD 2), and a severe group (GOLD 3) (shown in Figure 1).
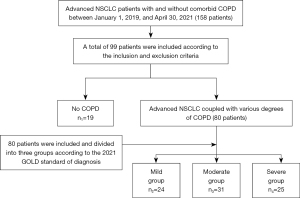
Comparability of baseline factors above and clinical characteristics were evaluated. There were statistically significant differences between the four groups of patients in terms of gender, smoking status, smoking index, Eastern Cooperative Oncology Group physical state (ECOG PS), and lung function indicators (P<0.05). However, there were no differences in terms of age, histological type, body mass index (BMI), TNM stage, and metastasis in the baseline data (P>0.05). 44 patients conducted genetic testing, none of them had EGFR mutations (shown in Table 1).
Table 1
| Variables | No COPD (n1=19) | Mild group (n2=24) | Moderate group (n3=31) | Severe group (n4=25) | P value |
|---|---|---|---|---|---|
| Age (years) | 64.11±6.24 | 62.46±7.43 | 66.29±9.51 | 66.04±7.37 | 0.276 |
| Sex | 0.049 | ||||
| Male | 15 | 23 | 29 | 25 | |
| Female | 4 | 1 | 2 | 0 | |
| BMI (kg/m2) | 22.22±2.50 | 22.25±2.67 | 23.02±2.74 | 21.88±3.77 | 0.642 |
| Smoking status | 0.022 | ||||
| Yes | 8 | 19 | 25 | 17 | |
| No | 11 | 5 | 6 | 8 | |
| Smoking index | 321±409 | 725±612 | 776±604 | 646±581 | 0.046 |
| ECOG PS | 0.038 | ||||
| 0–1 | 18 | 22 | 30 | 24 | |
| ≥2 | 1 | 2 | 1 | 1 | |
| Histology | 0.852 | ||||
| Adenocarcinoma | 10 | 11 | 14 | 12 | |
| Squamous cell carcinoma | 5 | 11 | 13 | 10 | |
| Other | 4 | 2 | 4 | 3 | |
| TNM stage | 0.649 | ||||
| IIIB/C | 5 | 8 | 10 | 11 | |
| IV | 14 | 16 | 21 | 14 | |
| Metastasis | 0.660 | ||||
| Yes | 14 | 15 | 20 | 14 | |
| No | 5 | 9 | 11 | 10 | |
| Pulmonary function | |||||
| FVC (L) | 3.45±0.50 | 3.58±0.54 | 2.88±0.47 | 2.78±0.92 | 0.000 |
| FVC% predicted | 101.8±15.16 | 100.5±15.00 | 83.62±10.37 | 79.52±22.06 | 0.000 |
| FEV1 (L) | 2.69±0.48 | 2.41±0.49 | 1.75±0.36 | 1.62±0.94 | 0.000 |
| FEV1% predicted | 99.87±15.57 | 85.01±15.40 | 64.97±8.07 | 58.86±30.63 | 0.000 |
| FEV1/FVC | 96.47±11.65 | 82.12±9.27 | 77.25±11.71 | 69.48±21.14 | 0.000 |
The data are expressed as n or mean ± SD. NSCLC, non-small cell lung cancer; COPD, chronic obstructive pulmonary disease; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group; PS, physical state; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; SD, standard deviation.
irAEs and their relationship with cytokines
Among the 99 included patients, a total of 30 experienced irAEs. The clinical manifestations included fatigue (n1 15.8%, n2 8.33%, n3 22.6%, n4 36.0%), pruritus (n1 5.26%, n2 4.17%, n3 12.9%, n4 16.0%), immune-related enteritis (n1 5.26%, n2 4.17%, n3 0, n4 8.00%), immune-related pneumonia (n1 5.26%, n2 0, n3 3.23%, n4 28.0%), and immune-related hepatitis (n1 0, n2 0, n3 0, n4 8.00%). The incidence of different irAEs varied, with that of fatigue being the highest, and the incidence of irAEs among the four groups was statistically different (P=0.003). In addition, IL-6, IL-8, IL-10, and KL-6 levels appeared statistically different among the four groups (P<0.05). In the subgroup analysis, the differences in IL-6, IL-8, and IL-10 between the mild to moderate COPD group and the severe COPD group were statistically significant (P<0.05), as detailed in Table 2.
Table 2
| irAEs/cytokines | No COPD (n1=19) | Mild group (n2=24) | Moderate group (n3=31) | Severe group (n4=25) | P value |
|---|---|---|---|---|---|
| irAEs | 21.1%i | 8.3% | 32.3%ii | 56.0% | 0.003 |
| Fatigue | 15.8% | 8.33% | 22.6% | 36.0% | |
| Pruritus | 5.26% | 4.17% | 12.9% | 16.0% | |
| Immune-related enteritis | 5.26% | 4.17% | 0 | 8.00% | |
| Immune-related pneumonia | 5.26% | 0 | 3.23% | 28.0% | |
| Immune-related hepatitis | 0 | 0 | 0 | 8.00% | |
| Cytokines | |||||
| IL-6 (pg/mL) | 7.95±9.71 | 11.65±14.81 | 14.38±12.72iii | 37.74±40.45 | 0.000 |
| IL-8 (pg/mL) | 14.89±11.28 | 27.37±32.53 | 19.30±17.72iv | 61.03±69.19 | 0.030 |
| IL-10 (pg/mL) | 2.52±2.69 | 3.37±2.00 | 3.07±2.29v | 4.67±2.42 | 0.024 |
| KL-6 (U/mL) | 1,209±1,266 | 472±263 | 537±304 | 644±521 | 0.029 |
The data are expressed as % or mean ± SD. i, no COPD group vs. severe COPD group (P=0.024); ii, mild to moderate COPD group vs. severe COPD group (P=0.003); iii, mild to moderate COPD group vs. severe COPD group (P=0.000); iv, mild to moderate COPD group vs. severe COPD group (P=0.026); v, mild to moderate COPD group vs. severe COPD group (P=0.010). irAEs, immune-related adverse events; COPD, chronic obstructive pulmonary disease; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; KL-6, Krebs Von den Lungen-6; SD, standard deviation.
Analysis of ICIs activity
The ORRs of each group were 31.6% (n1), 37.5% (n2), 35.5% (n3), 8.0% (n4), and the DCRs of each group were 84.2% (n1), 87.5% (n2), 74.2% (n3), 56.0% (n4) separately. In the subgroup analysis, there were no marked differences in the ORRs and DCRs between the no COPD group (n1) and the mild to moderate COPD group (n2/3) (P>0.05). Conversely, the differences in the ORRs and DCRs between the no COPD group (n1) and the severe COPD group (n4) were significant statistically (ORR, 31.6% vs. 8.0%; DCR, 84.2% vs. 56.0%, P<0.05). Moreover, statistical significance also existed between the mild to moderate COPD group (n2/3) and the severe COPD group (n4) (P<0.05), as detailed in Table 3.
Table 3
| Efficacy | No COPD group | Mild group | Moderate group | Severe group |
|---|---|---|---|---|
| CR | 0 | 1 | 0 | 0 |
| PR | 6 | 8 | 11 | 2 |
| SD | 10 | 12 | 12 | 12 |
| PD | 3 | 3 | 8 | 11 |
| ORR | 31.6%i | 37.5% | 35.5%ii | 8.0% |
| DCR | 84.2%iii | 87.5% | 74.2%iv | 56.0% |
i, no COPD group vs. severe COPD group (P=0.009); ii, mild to moderate COPD group vs. severe COPD group (P=0.004), no COPD group vs. mild to moderate COPD group (P=0.586); iii, no COPD group vs. severe COPD group (P=0.016); iv, mild to moderate COPD group vs. severe COPD group (P=0.037), no COPD group vs. mild to moderate COPD group (P=0.936). COPD, chronic obstructive pulmonary disease; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate; DCR, disease control rate.
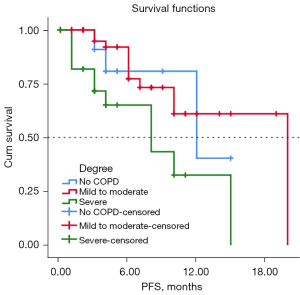
Among the 99 included patients, 17 were lost to follow-up, with a median follow-up time of 42.0 months (17.6–66.4 months). The mPFS of the four groups were 12.0 months (n1), 19.0 months (n2), not reached (n3) and 8.0 months (n4) respectively. In the subgroup analysis, the mPFS differences between the no COPD (n1), mild to moderate COPD (n2/3), and severe COPD (n4) groups were statistically significant (log-rank P=0.011), as shown in Figure 2.
Factors related to ICIs’ activity/tolerability
Univariate analysis showed that the GOLD degree (P=0.034) and the levels of cytokines IL-6 (P=0.003), IL-8 (P=0.005), and IL-10 (P=0.014) were significantly correlated with mPFS. As shown in Table 4, multivariate analysis confirmed that IL-6 levels remained the only factor significantly correlated with mPFS (P=0.007).
Table 4
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Smoking index | 1.000 (0.999–1.001) | 0.685 | |||
| ECOG PS | 0.950 (0.400–2.258) | 0.908 | |||
| Histology | |||||
| Adenocarcinoma | 1 | 0.843 | |||
| Squamous cell carcinoma | 1.292 (0.285–5.860) | 0.740 | |||
| Other | 1.514 (0.333–6.886) | 0.591 | |||
| TNM stage | 1.042 (0.409–2.651) | 0.931 | |||
| Anti-COPD drugs | 1.187 (0.352–4.004) | 0.783 | |||
| GOLD degree | 1.667 (1.038–2.677) | 0.034 | |||
| Cytokines | |||||
| IL-6 | 1.001 (1.000–1.002) | 0.003 | 1.001 (1.000–1.002) | 0.007 | |
| IL-8 | 1.014 (1.004–1.024) | 0.005 | |||
| IL-10 | 1.049 (1.010–1.090) | 0.014 | |||
| KL-6 | 1.000 (0.999–1.001) | 0.833 | |||
PFS, progression-free survival; ECOG, Eastern Cooperative Oncology Group; PS, physical state; TNM, tumor node metastasis; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; KL-6, Krebs Von den Lungen-6; HR, hazard ratio; CI, confidence interval.
The univariate analysis also indicated that the GOLD degree (P=0.003) and the levels of cytokines IL-6 (P=0.007), IL-8 (P=0.022), and IL-10 (P=0.006) were significantly related to the occurrence of irAEs, while anti-COPD drugs did not affect the occurrence of irAEs (P>0.05). Multivariate analysis found that the GOLD degree remained the only factor significantly associated to irAEs (P=0.037), as detailed in Table 5.
Table 5
| Factors | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Smoking index | 1.000 (0.999–1.001) | 0.850 | |||
| ECOG PS | 1.396 (0.589–3.311) | 0.449 | |||
| Histology | |||||
| Adenocarcinoma | 1 | 0.478 | |||
| Squamous cell carcinoma | 1.889 (0.456–7.821) | 0.380 | |||
| Other | 1.149 (0.262–5.034) | 0.853 | |||
| TNM stage | 1.326 (0.527–3.335) | 0.549 | |||
| Anti-COPD drugs | 1.806 (0.545–5.983) | 0.334 | |||
| GOLD degree | 2.063 (1.286–3.309) | 0.003 | 1.774 (1.035–3.039) | 0.037 | |
| Cytokines | |||||
| IL-6 | 1.032 (1.008–1.056) | 0.007 | 1.013 (0.989–1.038) | 0.275 | |
| IL-8 | 1.036 (1.005–1.067) | 0.022 | |||
| IL-10 | 1.349 (1.091–1.668) | 0.006 | 1.219 (0.976–1.521) | 0.080 | |
| KL-6 | 1.001 (1.000–1.002) | 0.060 | |||
irAEs, immune-related adverse events; ECOG, Eastern Cooperative Oncology Group; PS, physical state; TNM, tumor node metastasis; COPD, chronic obstructive pulmonary disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; KL-6, Krebs Von den Lungen-6; HR, hazard ratio; CI, confidence interval.
Discussion
COPD and lung cancer are two major respiratory diseases affecting patients’ survival and quality of life. COPD patients are 3–6 times more likely to develop lung cancer than people with normal lung function, and about 40% of COPD patients die within 1 year after the diagnosis of lung cancer, which accounts for 33% of COPD-related deaths (7). On these bases clinicians have gradually paid greater attention to these patients’ cohorts. Our team first proposed the concept of “Severe Lung Cancer” in 2017 (8,9). The first edition of the International Consensus on Severe Lung Cancer (10) in 2021 offers a clear definition of the concept of severe lung cancer, i.e., a disease in which the patient with stage IIIB, IIIC, and IV lung cancer has a PS score between 2 and 4 in certain stages due to various acute or chronic comorbidities, the tumor itself, and/or treatment-related adverse events. However, these patients will likely benefit from existing systemic anti-tumor therapy following supportive care and anti-tumor treatment based on dynamic and precise testing. With this clear definition, this special population has been gradually regarded as patients with severe lung cancer for clinical diagnosis, treatment, and management. However, there is currently insufficient clinical experience in the diagnosis and treatment of such severe lung cancer patients, and the efficacy of immunotherapy is unclear. Therefore, we conducted this retrospective cohort study of advanced NSCLC patients with comorbid COPD who received immunotherapy.
A previous study has shown that lung cancer and COPD may have similar physiological and pathological mechanisms, such as genetic susceptibility, telomere shortening, mitochondrial dysfunction, or premature aging (11). A prospective trial following nearly 500,000 non-smokers showed that lung cancer was more common in non-smokers with COPD (12), emphasizing the importance of increased chronic inflammation and oxidative stress. Lung damage in COPD can be caused by exogenous or endogenous oxidative stress and the release of inflammatory cytokines, which in turn leads to airway destruction and obstruction. COPD drives the occurrence of lung cancer by increasing oxidative stress and chronic exposure to pro-inflammatory cytokines (13). Study (14) has shown that inflammation is the main source of persistent reactive oxygen species in COPD, and patients with lung cancer also exhibit increased levels of inflammation. Our research showed that smoking is not an essential factor in the occurrence of the disease. Smoking has no statistically significant effects on tumor progression and irAEs in NSCLC patients, while IL-6, an inflammatory cytokine, is an independent factor influencing tumor progression. This indicates that chronic inflammation of COPD may be a powerful factor driving NSCLC. Furthermore, the enrolled patients conducting genetic testing exhibited no EGFR mutations, which was consistent with previous studies that COPD was also significantly correlated with low prevalence of EGFR mutations in non-smoker NSCLC patients (15).
Regarding the immune mechanism, a study (16) used immunohistochemistry to detect the immune-related cell subtypes and cytokines in the tumor and non-tumor specimens of 33 lung cancer patients with comorbid COPD and 20 simple lung cancer patients. This study found that IL-10 is significantly higher in patients with COPD, suggesting that lung cancer patients with comorbid COPD have a certain immune effect on the application of immunotherapy. Basic research (17) has demonstrated that chronic inflammation similar with COPD creates a good immunosuppressive microenvironment for tumor development, and COPD-related lung tumors may respond better to immune checkpoint therapy. Mark et al. (18) found that the helper T 1 (Th1) cell phenotype differentiation was enhanced in COPD patients, and this phenotype also existed in the tumor microenvironment. The abovementioned research indicates that the state of chronic inflammation characterized by persistent lung injury increases the activity of the adaptive system. This increased activity also activates a regulatory mechanism that reduces the immune response.
In our study, we also found that the cytokines IL-6, IL-8, and IL-10 varied markedly between the different COPD groups. Moreover, significant differences also existed between the mild to moderate COPD group and the severe COPD group. NSCLC patients with severe comorbid COPD have poorer pulmonary function and other basic conditions, which may lead to more severe autoimmune system damage and immune response overexpression compared to patients with mild to moderate COPD. Therefore, the poor sensitivity of such patients to ICIs leads to poor final immunotherapy efficacy.
In terms of irAEs, the American Society of Clinical Oncology (ASCO) and National Comprehensive Cancer Network (NCCN) jointly issued the Immunotherapy-related Adverse Events Management Guidelines in 2018 (19), which pointed out that the adverse events caused by immunotherapy can affect many individual organs, such as the skin, digestive tract, lungs, endocrine, etc. IrAEs involved in our study were consistent with those in the guidelines. At the same time, our study also found that the incidence of irAEs in patients with mild to moderate COPD was relatively lower than that in patients with severe COPD. Mark et al. (18) found that the proportion of cluster of differentiation 3 (CD3), CD4+, and CD8+ cells increased in the non-tumor lung tissue of COPD patients compared with that in non-COPD patients. Other studies (20,21) have shown that T cells are exposed to persistent inflammatory signals and their exhaustion was usually related to the ineffective control of persistent infections and tumors. Exhausted T cells exhibit dysfunctional tumor-specific immunity. The response is also accompanied by an increased expression of inhibitory receptors such as programmed cell death protein-1 (PD-1), T cell immunoglobulin domain and mucin domain-3 (TIM-3), cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), lymphocyte activation gene-3 (LAG-3), and B- and T-lymphocyte attenuator (BTLA), and a decrease in cytokines. Therefore, we believe that the destruction of immune checkpoint molecular functions during immunotherapy may lead to the failure of immune tolerance. However, patients with severe COPD have worse inflammation and infection, immune imbalance, and poorer immune response than patients with mild to moderate COPD, which leads to more irAEs. Therefore, immunotherapy must be carefully considered for NSCLC patients with severe COPD. On the other hand, the GOLD guidelines (22) indicate that inhaled bronchodilators and/or glucocorticoids should be used according to the disease in stable periods. Antibiotics should be used reasonably in the acute exacerbation phase according to the disease, and inhalants should be appropriately increased. Also, patients who are followed up regularly in outpatient clinics are suitable for COPD treatment (23). Patients with COPD in our study were treated with standardized medications strictly according to the guidelines, which did not increase the occurrence of adverse events, and the efficacy of immunotherapy was not affected. Therefore, we also advocate that for severe lung cancer patients with advanced NSCLC and comorbid COPD, standardized drug treatment should also be strictly carried out to achieve the simultaneous treatment of both cancer and the lung.
In terms of survival and prognosis, although this type of severe lung cancer patient is associated with COPD and has a poor basic status, the overall survival is not high. However, the results of a retrospective cohort study (18) showed that the presence of COPD significantly improved the response to ICI treatment in patients with COPD or simple lung cancer who received ICI treatment, and the PFS and overall survival in the COPD group were longer than those in non-COPD patients. This suggested that advanced NSCLC patients with comorbid COPD are not contraindicated for immunotherapy. However, of the patients who are clinically diagnosed with COPD, the lung volume is recorded in only 30% of patients.
Despite its limitations, this study reminds us that advanced NSCLC patients with comorbid COPD should be strictly evaluated and graded for the severity of COPD before undergoing immunotherapy to be clear about the impact of COPD with different severities on the efficacy of immunotherapy. There are also similar studies by different scholars in the early stage. Biton et al. (24) observed a greater response to nivolumab in COPD patients and the PFS of COPD patients was longer, suggesting that COPD patients are more sensitive to PD-1 blockade. Shin et al. (5) pointed out that NSCLC patients with comorbid COPD treated with pembrolizumab had a longer overall survival and PFS, and a higher ORR compared to patients with simple lung cancer, and patients with mild COPD benefited most. This provides favorable evidence for the use of ICI therapy in NSCLC patients with comorbid COPD and suggests that lung cancer patients with mild COPD may benefit the most. However, this study was limited to patients receiving second-line NSCLC treatment and programmed cell death-ligand 1 tumor proportion score (PD-L1 TPS) ≥50%. Put simply, previous studies have not emphasized the grading of lung function and it is impossible to clarify the different efficacies of immunotherapy in NSCLC patients with various degrees of comorbid COPD.
Our study is an independent exploration of immunotherapy in NSCLC patients with various degrees of comorbid COPD. We classified the patients’ lung function indicators strictly according to the GOLD guidelines, and not only compared the differences in immunotherapy between patients with and without COPD but also divided the NSCLC patients with comorbid COPD into detailed groups that were not limited to the types of ICIs. Finally, we found that the PFS of patients with mild to moderate COPD was longer than patients with severe COPD following immunotherapy, and the incidence of irAEs was lower, confirming the necessity of lung function assessment for severe lung cancer patients (such as those with NSCLC and comorbid COPD) before receiving immunotherapy.
There are limitations in this study that should be noted. Firstly, this is a single-center retrospective cohort study, which involves certain limitations on the general applicability and versatility of the research results. Thus, a larger-scale multi-center clinical study is necessary. Secondly, the follow-up time of this study was relatively short and the study cohort was relatively small. Also, the overall survival of immunotherapy for NSCLC patients with various degrees of comorbid COPD remains unknown, and thus, longer follow-up periods are needed to obtain more stable results. Tumor mutational burden (TMB), a biomarker which was missed to test in our study, should also be noted since it is in general associated to response to ICIs.
In summary, advanced NSCLC patients with comorbid COPD are not contraindicated for immunotherapy, and more attention should be paid to the assessment and grading of their lung function before immunotherapy. Moreover, the PFS of patients with mild-to-moderate COPD is longer than that in patients with severe COPD after immunotherapy. Also, the incidence of irAEs is lower in this group, and the efficacy of immunotherapy is better. However, NSCLC patients with severe COPD should receive immunotherapy cautiously because of its high toxicity and poor efficacy. At the same time, attention should be paid to the independent influence of IL-6 on tumor progression. Immunotherapy could bring new hope for advanced NSCLC patients with comorbid COPD, which are considered to be among the populations with severe lung cancer. Immunotherapy may be the preferred treatment plan to improve the prognosis of this special cohort. However, standardized anti-COPD drug treatment, which does not increase toxicity and will not affect the occurrence of irAEs, should be also strictly administered to achieve the simultaneous treatment of both cancer and the lung (9).
Acknowledgments
The authors appreciate the academic support from AME Lung Cancer Collaborative Group.
Funding: This study was funded by the High Level University Academic Backbone Cultivation Plan of Guangzhou Medical University Established (No. 201721020), the Characteristic innovation projects in ordinary universities in Guangdong Province, the Mandatory project of Guangdong Medical Research Fund (No. 2018KTSCX181), the Mandatory subject project of medical research fund of Guangdong province (No. C2019029)/Self-funded science and technology plan project of Guangdong department of science and technology (No. 2019ZC0029), Guangzhou Teaching Achievement Cultivation Project (No. 201982310), the General Program of the State Key Laboratory of Respiratory Diseases (No. SKLRD-MS-201905), the New Coronary Pneumonia Scientific Research Project of Guangdong Provincial Department of Education in 2020 (No. 2020KZDZX1162), and the Guangzhou Municipal Bureau of Science and Technology Basic Research Program Municipal School (Institute) Joint Funding Project (Guangdong Zhong Nanshan Medical Foundation) in 2021 (No. 202201020445).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-667/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-667/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-667/coif). FP declares consulting/advisory fee from Merck Sharp Dohme, Amgen, Thermo Fisher Scientifics, AstraZeneca, Janssen, Sanofi, and Beigene. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Medical Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (Medical Research Ethics 2022 No. K-22). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Yi YS, Ban WH, Sohng KY. Effect of COPD on symptoms, quality of life and prognosis in patients with advanced non-small cell lung cancer. BMC Cancer 2018;18:1053. [Crossref] [PubMed]
- Ytterstad E, Moe PC, Hjalmarsen A. COPD in primary lung cancer patients: prevalence and mortality. Int J Chron Obstruct Pulmon Dis 2016;11:625-36. [Crossref] [PubMed]
- Zhou J, Chao Y, Yao D, et al. Impact of chronic obstructive pulmonary disease on immune checkpoint inhibitor efficacy in advanced lung cancer and the potential prognostic factors. Transl Lung Cancer Res 2021;10:2148-62. [Crossref] [PubMed]
- Shin SH, Park HY, Im Y, et al. Improved treatment outcome of pembrolizumab in patients with nonsmall cell lung cancer and chronic obstructive pulmonary disease. Int J Cancer 2019;145:2433-9. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Machida H, Inoue S, Shibata Y, et al. The Incidence and Risk Analysis of Lung Cancer Development in Patients with Chronic Obstructive Pulmonary Disease: Possible Effectiveness of Annual CT-Screening. Int J Chron Obstruct Pulmon Dis 2021;16:739-49. [Crossref] [PubMed]
- Qin YY, Zhang DH, Lin XQ, et al. Clinical analysis of 36 cases of advanced non-small cell lung cancer (NSCLC) with performance status (PS) scores between 2 and 4. Zhonghua Zhong Liu Za Zhi 2017;39:855-61. [PubMed]
- Qin YY, Zhou CZ, Zhu Z, et al. Case report: dermatomyositis associated with lung cancer with heterogeneous morphology. J Thorac Dis 2017;9:E1110-7. [Crossref] [PubMed]
- Zhou C, Li S, Liu J, et al. International consensus on severe lung cancer-the first edition. Transl Lung Cancer Res 2021;10:2633-66.
- Miron O, Afrasanie VA, Paduraru MI, et al. The relationship between chronic lung diseases and lung cancer - a narrative review. J BUON 2020;25:1687-92. [PubMed]
- Turner MC, Chen Y, Krewski D, et al. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. Am J Respir Crit Care Med 2007;176:285-90. [Crossref] [PubMed]
- Durham AL, Adcock IM. The relationship between COPD and lung cancer. Lung Cancer 2015;90:121-7. [Crossref] [PubMed]
- Koshiol J, Rotunno M, Consonni D, et al. Chronic obstructive pulmonary disease and altered risk of lung cancer in a population-based case-control study. PLoS One 2009;4:e7380. [Crossref] [PubMed]
- Lim JU, Yeo CD, Rhee CK, et al. Chronic Obstructive Pulmonary Disease-Related Non-Small-Cell Lung Cancer Exhibits a Low Prevalence of EGFR and ALK Driver Mutations. PLoS One 2015;10:e0142306. [Crossref] [PubMed]
- Tang J, Ramis-Cabrer D, Curull V, et al. Immune Cell Subtypes and Cytokines in Lung Tumor Microenvironment: Influence of COPD. Cancers (Basel) 2020;12:1217. [Crossref] [PubMed]
- Narayanapillai SC, Han YH, Song JM, et al. Modulation of the PD-1/PD-L1 immune checkpoint axis during inflammation-associated lung tumorigenesis. Carcinogenesis 2020;41:1518-28. [Crossref] [PubMed]
- Mark NM, Kargl J, Busch SE, et al. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non-Small Cell Lung Cancer. Am J Respir Crit Care Med 2018;197:325-36. [Crossref] [PubMed]
- Brahmer JR, Lacchetti C, Thompson JA. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract 2018;14:247-9. [Crossref] [PubMed]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015;15:486-99. [Crossref] [PubMed]
- Thommen DS, Schreiner J, Müller P, et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol Res 2015;3:1344-55. [Crossref] [PubMed]
- Gupta N, Agrawal S, Chakrabarti S, et al. COPD 2020 Guidelines - what is new and why? Adv Respir Med 2020;88:38-40. [Crossref] [PubMed]
- Wang F, Xie XH, Lin XQ, et al. Exploration of the treatment model for patients with advanced non-small cell lung cancer complicated with chronic obstructive pulmonary disease based on real-world data. Zhonghua Jie He He Hu Xi Za Zhi 2020;43:450-4. [PubMed]
- Biton J, Ouakrim H, Dechartres A, et al. Impaired Tumor-Infiltrating T Cells in Patients with Chronic Obstructive Pulmonary Disease Impact Lung Cancer Response to PD-1 Blockade. Am J Respir Crit Care Med 2018;198:928-40. [Crossref] [PubMed]


