
The association between clinical parameters and resectability in stage III non-small cell lung cancer, and a combination of N2 lymph node burden and lung immune prognostic index score as a potential biomarker
Highlight box
Key findings
• Clinical parameters associated with resectability in stage III NSCLC patients were less smoking habit with better pulmonary function, and earlier cancer stage.
• Regarding LIPI score, LIPI 0 group showed better OS than LIPI 1 and LIPI 2 groups.
• In clinical N2 subgroup, multi-parameter scoring system combining lymph node station, lymph node volume, and LIPI score showed significant association with OS.
What is known and what is new?
• In stage III NSCLC, the patients who receive surgery as the first-line treatment exhibit better PFS and OS when compared to those who receive other treatments. LIPI score has been demonstrated to be correlated with survival outcomes for immune checkpoint inhibitor therapy in advanced NSCLC.
• In this study, LIPI score showed association with OS in stage III NSCLC patients, which was also observed in the unresectable group. In clinical N2 subgroup, multi-parameter scoring system using lymph node status combined with LIPI score may have predictive value for OS.
What is the implication, and what should change now?
• It is important to decide the candidates for surgery in stage III NSCLC patients considering the clinical parameters associated with resectability.
• We may use LIPI as potential biomarker in stage III NSCLC, combining it with lymph node status in clinical N2 subgroup.
Introduction
Lung cancer is one of the leading causes of death with non-small cell lung cancer (NSCLC) making up a high proportion of lung cancers at approximately 85%. Roughly 30% of patients in NSCLC are diagnosed as stage III (1).
Stage I–IIIA NSCLCs are considered relatively early stages, and if possible, complete resection is the treatment of choice (2). stage III NSCLC is a heterogeneous group in terms of tumor burden and distribution, and often requires a multidisciplinary team approach (3,4). The treatment of stage III NSCLC is performed using a combination of both localized and systemic therapies involving surgery, chemotherapy, and radiation therapy. Because of the curative potential of surgery, it is important to determine whether the tumor is resectable or not. The Asian Thoracic Oncology Research Group suggested in their clinical algorithm of stage III NSCLC to categorize patients into resectable, potentially resectable, or unresectable groups (5).
For unresectable stage III NSCLC, the PACIFIC trial demonstrated the survival benefits of consolidation durvalumab after concurrent chemoradiation therapy (CCRT), altering the landscape of current treatment (6). However, more real-time data are required, especially among Asian populations, due to the relatively small proportion of Asian patients included in the PACIFIC trial (27%) (5,6).
In this multicenter cohort study on stage III NSCLC patients, we investigated the clinical parameters related to the ability to undergo complete resection and compared clinical outcomes according to resectability and initial treatment modalities performed on the patients. Furthermore, we evaluated possible clinical factors predictive of prognosis in stage III NSCLC. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-642/rc).
Methods
Study population
This study was a multicenter, retrospective study from seven university hospitals: Yeouido St. Mary’s Hospital, Seoul St. Mary’s Hospital, Bucheon St. Mary’s Hospital, Eunpyeong St. Mary’s Hospital, Incheon St. Mary’s Hospital, Uijeongbu St. Mary’s Hospital, and St. Vincent’s Hospital. Among the 1,304 patients diagnosed with lung cancer between June 2008 to December 2020, patients with clinical stage III (IIIA, IIIB, and IIIC) or pathological stage III (IIIA and IIIB) NSCLC were included. Regarding pathologic type, patients diagnosed with adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and NSCLC not otherwise specified (NOS) were included for analyses. The patients diagnosed with small cell lung cancer, large cell carcinoma, and others (neuroendocrine carcinoma, sarcomatoid carcinoma, pleomorphic carcinoma, mucoepidermoid carcinoma, bronchioloalveolar carcinoma, and adenoid cystic carcinoma) were excluded. For the tumor staging, the 8th edition of TNM staging system was applied (Figure 1).
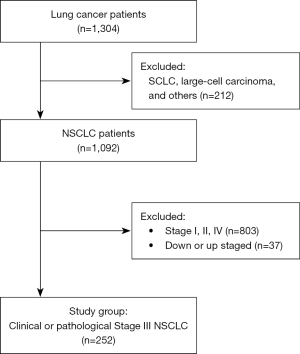
Definition of the groups
We divided the patients into two groups according to whether they received complete resections. The resectable group was defined as patients who received surgery in combination with or without perioperative therapy. The unresectable group did not undergo complete resections and were treated with other modalities.
According to the first-line treatment modalities, the patients were divided into six groups: patients who were treated with complete resections, chemotherapy, CCRT plus durvalumab, CCRT without durvalumab, radiotherapy (RT) alone, and supportive care only.
Study design
Demographic and clinical data such as age, sex, Eastern Cooperative Oncology Group (ECOG) performance score, smoking habit, comorbidities, pulmonary function testing, and treatment modalities, pathological data such as cancer stage, pathologic type, and mutation study, and laboratory data such as complete blood count, C-reactive protein, and lactate dehydrogenase (LDH) levels were collected from medical records.
The baseline characteristics were analyzed and were compared between the resectable and unresectable groups. Survival outcomes such as progression-free survival (PFS) and overall survival (OS) were also compared between the groups stratified according to resectability, first-line treatment modalities, and lung immune prognostic index (LIPI) score.
The LIPI score is the combination of derived neutrophil-to-lymphocyte ratio (dNLR) and LDH. dNLR is defined as absolute neutrophil count (ANC)/[white blood cell count (WBC) − ANC]. dNLR values greater than 3 and LDH values greater than the upper normal limit are counted as one factor. The LIPI score was used to categorize the study patients into three groups according to the number of the factors (good, 0 factor; intermediate, 1 factor; poor, 2 factors) (7). The cut-off value of LDH was defined according to the standards of each hospital.
PFS was defined as the duration from the date of the first treatment to progression or recurrence. OS was defined as the duration from the date of the first treatment to death or the last contact date.
The subgroup analysis was done to describe N2 disease more specifically. The baseline characteristics and survival outcomes of clinical N2 subgroup were analyzed using new scoring system. The parameters included in the scoring system were lymph node (LN) station (single or multi), LN volume (non-bulky or bulky), and LIPI score (0, 1, or 2). Single LN station was scored as 0, and multi-station was scored as 1. Non-bulky LN was scored as 0, while bulky LN was scored as 1. The final score was counted as the sum of LN station, LN volume, and LIPI scores, which ranges from 0 to 4 (Figure S1).
Statistical analysis
All statistical analyses were performed using IBM SPSS Version 24. Categorical variables were compared using Chi-square or Fisher’s exact tests, and continuous variables were analyzed using student T-tests or Mann-Whitney tests depending on the normality. The survival curves were shown using Kaplan-Meier curves, while the log-rank test was used when comparing survival outcomes between the groups. Logistic regression analysis was performed to investigate the association between clinical factors and unresectability. Cox regression analysis was used to identify the factors associated with PFS and OS. Statistical significance was set at P<0.05.
For multivariate analyses, we did not enter two or more parameters which could have correlations with each other and create potential bias. Staging parameters such as IIIA–IIIC and TNM stage were not entered in the multivariate analyses together, and separate multivariate analyses models were made.
Ethical statement
This study was approved by the Institutional Review Board of Catholic Medical Center, Korea (No. XC22RIDI0056). Informed consent was waived due to the retrospective study design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Results
Baseline characteristics
Among the 252 patients with stage III NSCLC, the median age was 69 (IQR, 62–75) years with 191 male (75.8%) patients (Table 1). Regarding the pathologic type, 112 (44.4%) patients were diagnosed with adenocarcinomas, 125 (49.6%) with squamous cell carcinomas, 3 (1.2%) with adenosquamous carcinomas, and 12 (4.8%) with NSCLCs NOS. For first-line treatment modalities, 89 (35.3%) patients received complete resections, 57 (22.6%) were treated with chemotherapy, 25 (9.9%) with CCRT plus durvalumab, 46 (18.3%) with CCRT alone, 8 (3.2%) with RT alone, and 27 (10.7%) patients received supportive care only. In terms of LIPI scores, 146 (57.9%) patients were classified as LIPI 0, 78 (31.0%) as LIPI 1, and 17 (6.7%) as LIPI 2.
Table 1
| Variables | Results |
|---|---|
| Age, median [IQR] | 69 [62–75] |
| Sex, n (%) | |
| Male | 191 (75.8) |
| Female | 61 (24.2) |
| Smoking habit, n (%) | |
| Smoker or ex-smoker | 203 (80.6) |
| Never smoker | 49 (19.4) |
| Height, cm (mean ± SD) | 162.82±7.83 |
| Weight, kg, median [IQR] | 60.50 [54.89–67.08] |
| ECOG, n (%) | |
| 0–1 | 234 (92.9) |
| 2–4 | 18 (7.1) |
| Stage at diagnosis (clinical), n (%) | |
| IA/IB | 15 (6.0)/7 (2.8) |
| IIA/IIB | 3 (1.2)/17 (6.7) |
| IIIA/IIIB/IIIC | 102 (40.5)/75 (29.8)/33 (13.1) |
| T stage (clinical), n (%) | |
| T1/T2/T3/T4 | 42 (16.7)/52 (20.6)/53 (21.0)/105 (41.7) |
| LN stage (clinical), n (%) | |
| N0/N1/N2/N3 | 59 (23.4)/45 (17.9)/93 (36.9)/55 (21.8) |
| Pathologic stage, n (%) | |
| IIIA/IIIB | 72 (28.6)/11 (4.4) |
| Not done | 3 (1.2) |
| T stage (pathologic), n (%) | |
| T1/T2/T3/T4 | 20 (7.9)/26 (10.3)/17 (6.7)/20 (7.9) |
| N stage (pathologic), n (%) | |
| N0/N1/N2/N3 | 17 (6.7)/12 (4.8)/55 (21.8)/1 (0.4) |
| Stage (clinical or pathologic) | |
| IIIA/IIIB/IIIC | 143 (56.7)/76 (30.2)/33 (13.1) |
| Pathology type, n (%) | |
| Adenocarcinoma | 112 (44.4) |
| Squamous | 125 (49.6) |
| Adenosquamous | 3 (1.2) |
| NSCLC NOS | 12 (4.8) |
| Driver mutations | |
| EGFR | 33/229 |
| ALK | 10/220 |
| ROS1 | 1/158 |
| PD-L1 expressions (22C3, SP263, SP142)† | 141/224 |
| Laboratory data, median [IQR] | |
| WBC | 7,395.0 [5,850.0–9,527.5] |
| CRP, mg/dL | 1.30 [0.27–4.54] |
| LDH | 303 [201.50–411.75] |
| NLR | 2.52 [1.83–3.96] |
| dNLR | 1.62 [1.31–2.43] |
| LIPI index, n (%) | |
| 0/1/2 | 146 (57.9)/78 (31.0)/17 (6.7) |
| First-line treatment, n (%) | |
| Surgery | 89 (35.3) |
| Chemotherapy | 57 (22.6) |
| CCRT + durvalumab | 25 (9.9) |
| CCRT | 46 (18.3) |
| RT alone | 8 (3.2) |
| Only diagnosis | 27 (10.7) |
†, positive PD-L1 expression was defined as at least one positive finding; 22C3 ≥50%, SP263 ≥10%, or SP142 TC ≥5% or IC ≥5%. NSCLC, non-small cell lung cancer; IQR, interquartile range; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; NSCLC NOS, non-small cell lung cancer not otherwise specified; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-Ros oncogene 1; PD-L1, programmed death-ligand 1; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; LIPI, lung immune prognostic index; CCRT, concurrent chemoradiation therapy; RT, radiotherapy; TC, tumor cells; IC, tumor-infiltrating immune cells.
The baseline characteristics were also compared between the groups categorized by resectability (n=225, Table 2). The resectable group consisted of the smaller proportion of male patients (64.0% vs. 80.9%, P=0.005) with less ever-smokers (65.2% vs. 89.0%, P<0.001) and less chronic obstructive pulmonary disease (COPD) patients (16.9% vs. 33.1%, P=0.007) compared to the unresectable group. The baseline pulmonary function parameters such as forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEV1/FVC ratio, and diffusing capacity of the lungs for carbon monoxide (DLCO) were higher in the resectable group than in the unresectable group. The resectable group tended to be diagnosed with earlier clinical stage III (more likely as stage IIIA than IIIC) and earlier TNM stages. Inflammatory markers such as WBC count and c-reactive protein were significantly lower in the resectable group (6,545 vs. 7,700 cell/µL, P=0.015 and 0.39 vs. 1.52 mg/dL, P=0.001, respectively). Neutrophil-to-lymphocyte ratio (NLR) and dNLR were also lower in the resectable group compared to the unresectable group (2.26 vs. 2.58, P=0.023 and 1.50 vs. 1.65, P=0.036, respectively). LIPI scores did not exhibit any statistical difference between the two groups.
Table 2
| Characteristics | Resectable (n=89) | Unresectable (n=136) | P value |
|---|---|---|---|
| Age, median [IQR] | 69.00 [61.50–74.00] | 69.00 [63.00–75.00] | 0.191 |
| Sex, n (%) | 0.005 | ||
| Male | 57 (64.0) | 110 (80.9) | |
| Female | 32 (36.0) | 26 (19.1) | |
| Smoking habit, n (%) | <0.001 | ||
| Smoker or ex-smoker | 58 (65.2) | 121 (89.0) | |
| Never smoker | 31 (34.8) | 15 (11.0) | |
| ECOG, n (%) | 0.484 | ||
| 0–1 | 87 (97.8) | 130 (95.6) | |
| 2–4 | 2 (2.2) | 6 (4.4) | |
| Comorbidity, n (%) | |||
| Old Tbc | 10 (11.2) | 23 (16.9) | 0.239 |
| COPD | 15 (16.9) | 45 (33.1) | 0.007 |
| Asthma | 3 (3.4) | 4 (2.9) | 1.000 |
| ILD | 3 (3.4) | 5 (3.7) | 1.000 |
| Pneumoconiosis | 0 (0.0) | 3 (2.2) | 0.280 |
| Heart disease | 7 (7.9) | 13 (9.6) | 0.662 |
| HTN | 46 (51.7) | 64 (47.1) | 0.497 |
| DM | 29 (32.6) | 35 (25.7) | 0.266 |
| Other cancer | 15 (16.9) | 16 (11.8) | 0.279 |
| PFT (post-bronchodilator) | |||
| FVC (L, absolute) (mean ± SD) | 3.17±0.87 | 3.00±0.72 | 0.002 |
| FVC (% predicted) (mean ± SD) | 89.08±18.06 | 84.65±18.60 | 0.108 |
| FEV1 (L, absolute) (mean ± SD) | 2.29±0.65 | 2.01±0.59 | 0.002 |
| FEV1 (% predicted) (mean ± SD) | 91.93±22.16 | 81.38±23.35 | 0.002 |
| FEV1/FVC, median [IQR] | 0.74 [0.66–0.79] | 0.69 [0.60–0.76] | 0.003 |
| DLCO (L, absolute) (mean ± SD) | 15.02±4.02 | 12.74±4.16 | <0.001 |
| DLCO (% predicted) (mean ± SD) | 85.03±19.96 | 74.71±21.92 | 0.001 |
| Stage at diagnosis, n (%) | <0.001 | ||
| IIIA | 78 (87.6) | 53 (39.0) | |
| IIIB | 11 (12.4) | 58 (42.6) | |
| IIIC | 0 (0.0) | 25 (18.4) | |
| T stage (clinical), n (%) | <0.001 | ||
| T1 | 24 (27.0) | 18 (13.2) | |
| T2a | 13 (14.6) | 13 (9.6) | |
| T2b | 10 (11.2) | 12 (8.8) | |
| T3 | 25 (28.1) | 23 (16.9) | |
| T4 | 17 (19.1) | 70 (51.5) | |
| LN stage (clinical), n (%) | <0.001 | ||
| N0 | 44 (49.4) | 11 (8.1) | |
| N1 | 21 (23.6) | 18 (13.2) | |
| N2 | 23 (25.8) | 63 (46.3) | |
| N3 | 1 (1.1) | 44 (32.4) | |
| Pathology type, n (%) | <0.001 | ||
| Adenocarcinoma | 58 (65.2) | 46 (33.8) | |
| Squamous | 27 (30.3) | 82 (60.3) | |
| Adenosquamous | 3 (3.4) | 0 (0.0) | |
| NSCLC NOS | 1 (1.1) | 8 (5.9) | |
| Driver mutations | |||
| EGFR | 21/74 | 10/132 | <0.001 |
| ALK | 4/69 | 5/127 | 0.002 |
| ROS1 | 1/42 | 0/98 | <0.001 |
| PD-L1 expressions† | 37/65 | 87/135 | 0.305 |
| Laboratory data, median [IQR] | |||
| WBC | 6,545 [5,650–9,023] | 7,700 [6,200–9,830] | 0.015 |
| CRP (mg/dL) | 0.39 [0.10–3.85] | 1.52 [0.44–4.75] | 0.001 |
| LDH | 223 [184–403] | 327 [218–418] | 0.006 |
| NLR | 2.26 [1.57–3.32] | 2.58 [1.87–3.89] | 0.023 |
| dNLR | 1.50 [1.09–2.19] | 1.65 [1.36–2.44] | 0.036 |
| LIPI index (n=244), n (%) | 0.556 | ||
| 0 | 58 (67.4) | 79 (61.7) | |
| 1 | 25 (29.1) | 41 (32.0) | |
| 2 | 3 (3.5) | 8 (6.3) | |
| Median PFS (month) [95% CI] | 33.70 [NR–NR] | 8.43 [6.24–10.63] | <0.001 |
| Median OS (month) [95% CI] | NR [NR–NR] | 20.53 [14.76–26.31] | <0.001 |
†, positive PD-L1 expression was defined as at least one positive finding; 22C3 ≥50%, SP263 ≥10%, or SP142 TC ≥5% or IC ≥5%. IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; Tbc, tuberculosis; COPD, chronic obstructive pulmonary disease; ILD, interstitial lung disease; HTN, hypertension; DM, diabetes mellitus; PFT, pulmonary function test; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffuse capacity for carbon monoxide; LN, lymph node; NSCLC NOS, non-small cell lung cancer not otherwise specified; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-Ros oncogene 1; PD-L1, programmed death-ligand; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; NLR, neutrophil-to-lymphocyte ratio; dNLR, derived neutrophil-to-lymphocyte ratio; LIPI, lung immune prognostic index; PFS, progression-free survival; OS, overall survival; NR, not reached; TC, tumor cells; IC, tumor-infiltrating immune cells.
PFS and OS
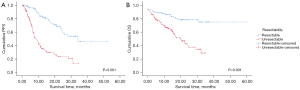
In the comparison of survival outcomes according to resectability, the resectable group demonstrated better PFS compared to the unresectable group (P<0.001, Figure 2A). The median PFS of the resectable group was 33.7 months (95% CI: NR–NR) compared to 8.43 months (95% CI: 6.24–10.63) in the unresectable group. The resectable group also exhibited better OS than the unresectable group (P<0.001, Figure 2B). The 12-month OS rate was 91.6% in the resectable group versus 70.6% in the unresectable group.
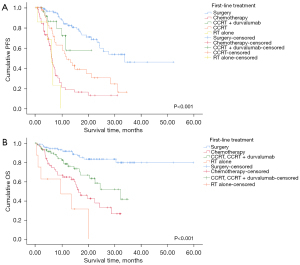
Regarding first-line treatment, there were significant differences in PFS between the groups stratified by first-line treatment modalities (P<0.001, Figure 3A). Surgery exhibited the longest median PFS (33.70 months; 95% CI: NR–NR) among the other treatment modalities, followed by CCRT alone (11.70 months; 95% CI: 7.73–15.67 months) and chemotherapy (6.10 months; 95% CI: 5.39–6.82 months). In pairwise comparisons, complete resection demonstrated significant differences when compared with most of the other treatment modalities, except for CCRT plus durvalumab. There was also a significant difference in OS among the groups categorized by first-line treatment (P<0.001, Figure 3B). The 12-month OS rate of surgery was 91.6% compared with 64.6% in chemotherapy, 78.3% in CCRT with or without durvalumab, and 46.9% in RT alone. In pairwise comparisons, surgery exhibited significant differences when compared with the other treatments.
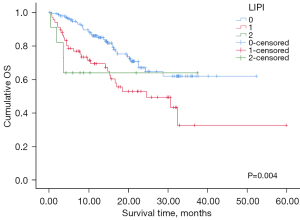
Among the 214 patients with valid LIPI scores, PFS and OS were compared between the groups stratified by LIPI score (0–2). There was no significant difference in PFS. However, OS was different among the LIPI score groups (P=0.004, Figure 4). The 12-month OS rate was 85.8% in the LIPI 0 group compared with 69.2% in the LIPI 1 group and 63.6% in the LIPI 2 group. The LIPI 0 group exhibited a significant difference when compared to the other groups in pairwise comparisons (LIPI 0 vs. LIPI 1, P=0.002; LIPI 0 vs. LIPI 2, P=0.036).
Survival outcomes according to LIPI score were also compared separately between the resectable and unresectable groups. In the resectable group, the statistics could not be calculated because the LIPI score 2 group was all censored. In the unresectable group, PFS exhibited no significant difference across the LIPI score group. However, OS was different depending on the LIPI score (P=0.003, Figure 5). The 12-month OS rate in LIPI 0 was 79.6% compared to 59.8% in LIPI 1 and 50.0% in LIPI 2. The LIPI 0 group was associated with better OS compared to LIPI 1 and 2 (P=0.049 and P<0.001, respectively).

Multivariate analysis on PFS and OS
For Cox regression analysis, two models of analyses were performed due to the different sets of cancer stages (IIIA–C vs. T, N), thus all the variables entered into each model did not have significant correlation with one another. In model 1, age (HR, 0.966; 95% CI: 0.942–0.991, P=0.009), first line treatment modalities (P<0.001), and positive targetable driver mutations (HR, 0.312; 95% CI: 0.149–0.652, P=0.002) were significantly associated with PFS (Table 3, Model 1). For first line treatment modalities, chemotherapy (HR, 7.156; 95% CI: 3.871–13.228, P<0.001), CCRT alone (HR, 2.180; 95% CI: 1.173–4.051, P=0.014), and RT alone (HR, 10.283; 95% CI: 3.685–28.700, P<0.001) exhibited a significant difference when compared to surgery. In model 2, the clinical factors that demonstrated a significant association with PFS were the same as in model 1.
Table 3
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| PFS—Model 1 | |||||
| Age | 0.999 (0.976–1.022) | 0.917 | 0.966 (0.942–0.991) | 0.009 | |
| Sex, male | 1.963 (1.231–3.131) | 0.005 | 1.530 (0.759–3.081) | 0.234 | |
| Smoking, ever | 2.283 (1.336–3.902) | 0.003 | 0.836 (0.329–2.125) | 0.707 | |
| ECOG, 2–4 vs. 0–1 | 3.229 (1.001–10.423) | 0.050 | |||
| FVC (L) | 1.198 (0.919–1.562) | 0.181 | |||
| FEV1 (L) | 0.972 (0.688–1.373) | 0.871 | |||
| DLCO (L) | 0.967 (0.918–1.018) | 0.202 | |||
| LIPI 1–2, vs.0 | 1.286 (0.854–1.936) | 0.229 | |||
| Cancer type | |||||
| Adenocarcinoma | 1 (ref) | 0.155 | |||
| Squamous | 1.358 (0.914–2.017) | 0.130 | |||
| Adenosquamous | NA | 0.960 | |||
| NSCLC NOS | 2.438 (1.036–5.734) | 0.041 | |||
| Stage | |||||
| IIIA | 1 (ref) | <0.001 | 1 (ref) | 0.149 | |
| IIIB | 1.700 (1.117–2.587) | 0.013 | 0.738 (0.451–1.207) | 0.226 | |
| IIIC | 2.879 (1.585–5.227) | <0.001 | 1.367 (0.700–2.671) | 0.361 | |
| First-line treatment | |||||
| Surgery | 1 (ref) | <0.001 | 1 (ref) | <0.001 | |
| Chemotherapy | 6.684 (4.026–11.097) | <0.001 | 7.156 (3.871–13.228) | <0.001 | |
| CCRT + durvalumab | 1.716 (0.696–4.233) | 0.241 | 1.235 (0.478–3.196) | 0.663 | |
| CCRT | 2.975 (1.723–5.139) | <0.001 | 2.180 (1.173–4.051) | 0.014 | |
| RT alone | 10.891 (4.051–29.281) | <0.001 | 10.283 (3.685–28.700) | <0.001 | |
| Positive driver mutations† | 0.348 (0.200–0.606) | <0.001 | 0.312 (0.149–0.652) | 0.002 | |
| PFS—Model 2 | |||||
| Age | 0.999 (0.976–1.022) | 0.917 | 0.964 (0.940–0.989) | 0.006 | |
| Sex, male | 1.963 (1.231–3.131) | 0.005 | 1.586 (0.793–3.171) | 0.192 | |
| Smoking, ever | 2.283 (1.336–3.902) | 0.003 | 0.753 (0.298–1.899) | 0.547 | |
| ECOG, 2–4 vs. 0–1 | 3.229 (1.001–10.423) | 0.050 | |||
| FVC (L) | 1.198 (0.919–1.562) | 0.181 | |||
| FEV1 (L) | 0.972 (0.688–1.373) | 0.871 | |||
| DLCO (L) | 0.967 (0.918–1.018) | 0.202 | |||
| LIPI 1–2, vs. 0 | 1.286 (0.854–1.936) | 0.229 | |||
| Cancer type | |||||
| Adenocarcinoma | 1 (ref) | 0.155 | |||
| Squamous | 1.358 (0.914–2.017) | 0.130 | |||
| Adenosquamous | NA | 0.960 | |||
| NSCLC NOS | 2.438 (1.036–5.734) | 0.041 | |||
| T Stage | |||||
| T1 | 1 (ref) | 0.272 | |||
| T2 | 1.101 (0.581–2.085) | 0.767 | |||
| T3 | 1.532 (0.797–2.944) | 0.201 | |||
| T4 | 1.593 (0.898–2.825) | 0.111 | |||
| N stage | |||||
| N0 | 1 (ref) | 0.020 | 1 (ref) | 0.405 | |
| N1 | 1.475 (0.690–3.154) | 0.316 | 2.051 (0.897–4.691) | 0.089 | |
| N2 | 1.147 (0.612–2.151) | 0.669 | 1.481 (0.726–3.020) | 0.280 | |
| N3 | 2.313 (1.164–4.594) | 0.017 | 1.544 (0.699–3.411) | 0.283 | |
| First-line treatment | |||||
| Surgery | 1 (ref) | <0.001 | 1 (ref) | <0.001 | |
| Chemotherapy | 6.684 (4.026–11.097) | <0.001 | 6.086 (3.379–10.962) | <0.001 | |
| CCRT + durvalumab | 1.716 (0.696–4.233) | 0.241 | 1.068 (0.418–2.728) | 0.891 | |
| CCRT | 2.975 (1.723–5.139) | <0.001 | 1.835 (1.005–3.349) | 0.048 | |
| RT alone | 10.891 (4.051–29.281) | <0.001 | 12.816 (4.283–38.344) | <0.001 | |
| Positive driver mutations† | 0.348 (0.200–0.606) | <0.001 | 0.288 (0.137–0.605) | 0.001 | |
†, at least one positive result among three mutation studies; EGFR, ALK, or ROS1. PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffuse capacity for carbon monoxide; LIPI, lung immune prognostic index; NSCLC NOS, non-small cell lung cancer not otherwise specified; CCRT, concurrent chemoradiation therapy; RT, radiotherapy; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; ROS1, c-Ros oncogene 1.
Regarding the factors associated with OS, in model 1, ECOG 2–4 (compared with 0–1; HR, 3.386; 95% CI: 1.114–10.289, P=0.031), LIPI 1–2 (compared with LIPI 0; HR, 2.564; 95% CI: 1.454–4.519, P=0.001), and first line treatment modalities (P=0.002) were independent prognostic factors (Table 4, Model 1). In terms of first line treatment modalities, chemotherapy (HR, 2.740 95% CI: 1.102–6.812, P=0.030) and RT alone (HR, 8.170; 95% CI: 2.766–24.134, P<0.001) exhibited a significant difference when compared with surgery. In model 2, ECOG 2–4 (compared with 0–1; HR, 3.733; 95% CI: 1.278–10.902, P=0.016), LIPI 1–2 (compared with LIPI 0; HR, 2.520; 95% CI: 1.441–4.408, P=0.001), and first line treatment modalities (P=0.001) demonstrated a significant association. Chemotherapy (HR, 3.180; 95% CI: 1.376–7.350, P=0.007), CCRT with or without durvalumab (HR, 2.234; 95% CI: 1.048–4.761, P=0.037), and RT alone (HR, 8.020; 95% CI: 2.743–23.448, P<0.001) showed a significant difference when compared with surgery.
Table 4
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| OS—Model 1 | |||||
| Age | 1.047 (1.017–1.078) | 0.002 | 1.014 (0.978–1.050) | 0.454 | |
| Sex, male | 4.701 (2.146–10.302) | <0.001 | 5.667 (0.836–38.389) | 0.076 | |
| Smoking, ever | 6.201 (2.255–17.054) | <0.001 | 0.781 (0.094–6.466) | 0.819 | |
| ECOG, 2–4 vs. 0–1 | 7.448 (3.120–17.780) | <0.001 | 3.386 (1.114–10.289) | 0.031 | |
| FVC (L) | 1.010 (0.746–1.366) | 0.950 | |||
| FEV1 (L) | 0.848 (0.571–1.258) | 0.412 | |||
| DLCO (L) | 0.880 (0.826–0.939) | <0.001 | 0.944 (0.871–1.023) | 0.161 | |
| LIPI, 1–2 vs.0 | 2.181 (1.345–3.537) | 0.002 | 2.564 (1.454–4.519) | 0.001 | |
| Cancer type | |||||
| Adenocarcinoma | 1 (ref) | <0.001 | 1 (ref) | 0.283 | |
| Squamous | 3.367 (1.957–5.794) | <0.001 | 1.536 (0.777–3.034) | 0.217 | |
| Adenosquamous | 3.049 (0.406–22.906) | 0.279 | 6.189 (0.722–53.089) | 0.096 | |
| NSCLC NOS | 2.080 (0.480–9.015) | 0.328 | 0.870 (0.096–7.854) | 0.901 | |
| Stage | |||||
| IIIA | 1 (ref) | 0.018 | 1 (ref) | 0.636 | |
| IIIB | 2.022 (1.226–3.335) | 0.006 | 1.359 (0.712–2.596) | 0.353 | |
| IIIC | 1.780 (0.820–3.861) | 0.145 | 1.295 (0.501–3.350) | 0.594 | |
| First-line treatment | |||||
| Surgery | 1 (ref) | <0.001 | 1(ref) | 0.002 | |
| Chemotherapy | 5.437 (2.812–10.513) | <0.001 | 2.740 (1.102–6.812) | 0.030 | |
| CCRT (with or without durvalumab) | 2.945 (1.467–5.912) | 0.002 | 2.009 (0.904–4.469) | 0.087 | |
| RT alone | 12.560 (4.690–33.633) | <0.001 | 8.170 (2.766–24.134) | <0.001 | |
| OS—Model 2 | |||||
| Age | 1.047 (1.017–1.078) | 0.002 | 1.012 (0.978–1.048) | 0.499 | |
| Sex, male | 4.701 (2.146–10.302) | <0.001 | 5.532 (0.827–36.991) | 0.078 | |
| Smoking, ever | 6.201 (2.255–17.054) | <0.001 | 0.810 (0.100–6.566) | 0.843 | |
| ECOG, 2–4 vs. 0–1 | 7.448 (3.120–17.780) | <0.001 | 3.733 (1.278–10.902) | 0.016 | |
| FVC (L) | 1.010 (0.746–1.366) | 0.950 | |||
| FEV1 (L) | 0.848 (0.571–1.258) | 0.412 | |||
| DLCO (L) | 0.880 (0.826–0.939) | <0.001 | 0.944 (0.872–1.022) | 0.157 | |
| LIPI, 1–2 vs. 0 | 2.181 (1.345–3.537) | 0.002 | 2.520 (1.441–4.408) | 0.001 | |
| Cancer type | |||||
| Adenocarcinoma | 1 (ref) | <0.001 | 1 (ref) | 0.303 | |
| Squamous | 3.367 (1.957–5.794) | <0.001 | 1.483 (0.758–2.900) | 0.249 | |
| Adenosquamous | 3.049 (0.406–22.906) | 0.279 | 5.646 (0.668–47.715) | 0.112 | |
| NSCLC NOS | 2.080 (0.480–9.015) | 0.328 | 0.731 (0.082–6.555) | 0.780 | |
| T Stage | |||||
| T1 | 1 (ref) | 0.573 | |||
| T2 | 0.872 (0.408–1.864) | 0.724 | |||
| T3 | 0.870 (0.391–1.938) | 0.734 | |||
| T4 | 1.262 (0.647–2.459) | 0.495 | |||
| N stage | |||||
| N0 | 1 (ref) | 0.085 | |||
| N1 | 2.106 (0.778–5.704) | 0.143 | |||
| N2 | 1.596 (0.670–3.804) | 0.291 | |||
| N3 | 2.839 (1.131–7.125) | 0.026 | |||
| First-line treatment | |||||
| Surgery | 1 (ref) | <0.001 | 1 (ref) | 0.001 | |
| Chemotherapy | 5.437 (2.812–10.513) | <0.001 | 3.180 (1.376–7.350) | 0.007 | |
| CCRT (with or without durvalumab) | 2.945 (1.467–5.912) | 0.002 | 2.234 (1.048–4.761) | 0.037 | |
| RT alone | 12.560 (4.690–33.633) | <0.001 | 8.020 (2.743–23.448) | <0.001 | |
OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffuse capacity for carbon monoxide; LIPI, lung immune prognostic index; NSCLC NOS, non-small cell lung cancer not otherwise specified; CCRT, concurrent chemoradiation therapy; RT, radiotherapy.
Association with unresectability
The potential factors associated with unresectability were evaluated using two models for cancer stages which are similar to those used in the Cox regression analysis (Table 5). After adjusting for potential confounding factors, ever-smoker (OR, 12.401; 95% CI: 1.205–127.579, P=0.034), lower FEV1 (OR, 0.408; 95% CI: 0.179–0.931, P=0.033), and more advanced cancer stages, especially stage IIIB rather than IIIA (OR, 5.750; 95% CI: 2.426–13.631, P<0.001) in model 1 remained significantly associated with unresectability. Likewise, in model 2, ever-smoker (OR 11.550; 95% CI: 1.036–128.789, P=0.047), T4 rather than the T1 stage (OR, 16.729; 95% CI: 3.476–80.503, P=0.001), and N1–3 rather than the N0 stage (P<0.001) remained significantly associated.
Table 5
| Variables | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | ||
| Model 1 | |||||
| Age | 1.020 (0.989–1.053) | 0.213 | 0.995 (0.949–1.044) | 0.851 | |
| Sex, male | 2.375 (1.293–4.364) | 0.005 | 0.213 (0.023–2.006) | 0.176 | |
| Smoking, ever | 4.311 (2.160–8.608) | <0.001 | 12.401 (1.205–127.579) | 0.034 | |
| FVC (L) | 0.759 (0.520–1.107) | 0.152 | |||
| FEV1 (L) | 0.475 (0.289–0.780) | 0.003 | 0.408 (0.179–0.931) | 0.033 | |
| DLCO (L) | 0.871 (0.809–0.937) | <0.001 | 0.939 (0.835–1.056) | 0.294 | |
| WBC | 1.000 (1.000–1.000) | 0.027 | 1.000 (1.000–1.000) | 0.626 | |
| CRP | 1.051 (0.983–1.124) | 0.148 | |||
| LDH, > ULN | 1.407 (0.758–2.610) | 0.279 | |||
| dNLR | 1.093 (0.882–1.354) | 0.416 | |||
| Stage | |||||
| IIIA | 1 (ref) | <0.001 | 1 (ref) | <0.001 | |
| IIIB | 7.760 (3.729–16.150) | <0.001 | 5.750 (2.426–13.631) | <0.001 | |
| IIIC | NA | 0.998 | NA | 0.998 | |
| Model 2 | |||||
| Age | 1.020 (0.989–1.053) | 0.213 | 0.996 (0.946–1.049) | 0.89 | |
| Sex, male | 2.375 (1.293–4.364) | 0.005 | 0.268 (0.026–2.811) | 0.272 | |
| Smoking, ever | 4.311 (2.160–8.608) | <0.001 | 11.550 (1.036–128.789) | 0.047 | |
| FVC (L) | 0.759 (0.520–1.107) | 0.152 | |||
| FEV1 (L) | 0.475 (0.289–0.780) | 0.003 | 0.441 (0.179–1.087) | 0.075 | |
| DLCO (L) | 0.871 (0.809–0.937) | <0.001 | 0.899 (0.788–1.027) | 0.117 | |
| WBC | 1.000 (1.000–1.000) | 0.027 | 1.000 (1.000–1.000) | 0.623 | |
| CRP | 1.051 (0.983–1.124) | 0.148 | |||
| LDH, > ULN | 1.407 (0.758–2.610) | 0.279 | |||
| dNLR | 1.093 (0.882–1.354) | 0.416 | |||
| T Stage | |||||
| T1 | 1 (ref) | 0.001 | 1 (ref) | <0.001 | |
| T2 | 1.029 (0.445–2.377) | 0.947 | 1.103 (0.327–3.725) | 0.874 | |
| T3 | 1.217 (0.510–2.902) | 0.658 | 1.477 (0.348–6.259) | 0.597 | |
| T4 | 3.704 (1.661–8.260) | 0.001 | 16.729 (3.476–80.503) | 0.001 | |
| N stage | |||||
| N0 | 1 (ref) | 0.001 | 1 (ref) | <0.001 | |
| N1 | 2.266 (0.805–6.379) | 0.121 | 5.972 (1.358–26.264) | 0.018 | |
| N2 | 1.809 (0.788–4.153) | 0.162 | 16.869 (3.886–73.230) | <0.001 | |
| N3 | 72.000 (8.648–599.418) | <0.001 | 673.627 (44.789–10131.384) | <0.001 | |
OR, odds ratio; CI, confidence interval; FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; DLCO, diffuse capacity for carbon monoxide; WBC, white blood cell; CRP, C-reactive protein; LDH, lactate dehydrogenase; ULN, upper limit of normal; dNLR, derived neutrophil-to-lymphocyte ratio.
N2 subgroup analysis
The baseline characteristics were analyzed among clinical N2 subgroup patients (n=93, Table S1). The median age was 68 (IQR, 61–75) years, and 71 (76.3%) patients were male. Regarding LN status, 40 (43%) were single station, while 35 (37.6%) were multi-station. Twelve (12.9%) patients showed bulky mediastinal LN, and 63 patients (67.7%) had non-bulky N2 nodes. Among the N2 subgroup, 23 (24.7%) patients received surgery as the first-line treatment, and same number of patients were treated with chemotherapy and CCRT.
Kaplan-Meier curves for PFS and OS comparison according to the combinatorial scoring system which is the sum of LN station (single/multi-station), LN volume (bulky/non-bulky), and LIPI score parameters showed statistically significant difference (P=0.002, and P=0.003, respectively, Figure S2 and Figure S3).
In Cox regression analysis on PFS, however, the combinatorial scores (LN station + LN volume + LIPI score) did not show statistical significance in univariate analysis (P=0.057). The score was significantly associated with OS in both univariate and multivariate analysis (Table S2, P=0.018, and P=0.016, respectively). The risk of mortality increased significantly as the combinatorial score increased. When compared to the score 0 group (reference), score 2 group showed HR of 9.498 (95% CI: 2.200–41.011, P=0.003) and score 3 group showed HR of 20.083 (95% CI: 2.454–164.356, P=0.005), respectively.
Discussion
The aim of this study was to evaluate patients with stage III NSCLC with a focus on resectability. Smoking history and low FEV1 were significantly related to patients not receiving complete resections. Patients with tumors of stage IIIB, T4, and with LN metastasis were less likely to receive complete resections as the first-line treatment. The LIPI score exhibited potential predictable value in OS among the entire study population and unresectable subgroup, specifically. LIPI score 0 was significantly related to better OS when compared to LIPI 1 and 2. Most of the recent studies have used LIPI as a prognostic factor for outcomes of immune checkpoint inhibitor therapy. This study demonstrated the possibility of using LIPI as a prognostic factor for stage III NSCLC, especially for patients who cannot receive surgery.
Since complete resection has curative potential, there have been attempts to define resectability in stage III NSCLC. Several patient- and tumor-related factors contribute to the possibilities of successful complete resections. The large size of tumors is a risk factor for unresectability. In terms of T stage, tumors staged as T4 tend to invade adjacent normal structures, making it difficult to achieve complete resections. Tumors classified as N3 stage also exhibit limits on successful complete resections. Decreased pulmonary function and underlying cardiovascular diseases decrease the chance of operability (8,9). Patients with these risk factors are considered unlikely candidates for complete resections. The previous findings are consistent with the results of our study, as patients with poor lung function (low FEV1), T4 rather than T1 staging, and N1–3 rather than N0 classifications tended to be considered unresectable patients.
In recent studies, patients with stage III NSCLC who received surgical resections exhibited better PFS and OS compared to those who did not. In a study by Myall et al., 5-year OS was significantly improved in complete resections [33% (R0) vs. 19% (R1) vs. 12% (R2), P<0.0001 for R0 vs. R1] (8). In a real-world international observational study (KINDLE), median PFS (mPFS) and median OS (mOS) were 19.9 and 65.4 months, respectively in resectable NSCLC patients, while 10.6 and 25.0 months, respectively, were the values in unresectable patients (P<0.0001) (10). This study used the definition of resectability that differed from our study, as the resectable group also included patients who did not receive surgery. The mPFS of the resectable group was longer in our study, and one of the reasons is that our study defined resectable patients as those who actually underwent surgery. The patients who were considered as operable that were selected by clinicians were sorted into the resectable group.
In patients with unresectable stage III NSCLC, CCRT followed by consolidation durvalumab therapy significantly improved survival outcomes in our study. The PACIFIC trial reported that PFS and OS were significantly longer in patients with CCRT followed by durvalumab compared to the placebo (16.8 vs. 5.6 months, P<0.001 for PFS and 66.3% vs. 55.6%, P=0.005 for 24-month OS rate, respectively) (6,11,12). In the real-world setting, durvalumab maintenance therapy after CCRT also exhibited significantly improved local-regional-progression-free-survival (LRPFS; P=0.002), PFS (P=0.018), and OS (P=0.005) compared to CCRT alone (13). Our study is consistent with the results from present studies. The CCRT with durvalumab group exhibited longer PFS compared to other treatments except for surgery, and OS could not be calculated because all the patients in this group survived during the observation time.
Several biomarkers reflecting systemic inflammatory status have been suggested for various types of cancers. Baseline ANC and dNLR were significantly associated with disease progression and death in ipilimumab-treated melanoma patients (P<0.0001 for all) (14). An elevated platelet to lymphocyte ratio (PLR), a marker of inflammation, has been associated with poor prognosis in several malignancies (15). LDH is a biomarker of tumor burden and is also considered to have prognostic value in several types of cancers (16). For lung cancer, Mezquita et al. suggested that LIPI, a combination of pre-treatment dNLR and LDH, was correlated with poor outcomes for immune checkpoint inhibitor therapy in patients with advanced NSCLC (7). Since LIPI reflects inflammatory status, we attempted to apply LIPI as a biomarker for patients with stage III NSCLC in this study. High LIPI score was correlated with outcomes exhibiting poor OS in both overall patients with stage III NSCLC and the unresectable patients group. Further validation in larger study populations is necessary, but our study suggested the potential value of LIPI scores as a biomarker predicting clinical outcomes in stage III NSCLC.
Making the decision of treatment modalities in stage IIIA-N2 disease is more challenging. As N2 involvement exhibits heterogeneous disease entity, treatment often requires bi- or trimodalities, and the clinical role of complete resection requires further investigation (17). In guidelines on decision of treatment options for stage III N2 disease, LN extent (single station or multi-station, single zone or multizone) and LN volume (non-bulky or bulky) are included as criteria (18). Stage III N2 disease is heterogeneous in terms of tumor burden, anatomical distribution of tumor cells, and treatment modalities. We believe that multiple factors should be considered when predicting outcomes of this heterogenous patients group. In the subgroup analysis of patients with N2 diseases, we attempted to make new scoring system. LN station and volume parameters reflect the anatomical distribution and tumor burden of the N2 disease. LIPI score focuses on the onco-immunological background, as this biomarker has shown association with clinical outcomes in NSCLC patients undergoing immunotherapy (7). As was shown in our results, this multi-parameter scoring system may predict survival outcomes of the N2 disease. However, larger N2 populations are necessary for validation.
Our study has several limitations. First, due to the retrospective design of the study, accurate comparisons of clinical outcomes between groups categorized by initial treatment modalities may be limited. Second, assessments of OS in patients who received CCRT followed durvalumab maintenance were limited due to the relatively short observation time.
Conclusions
In this study, the resectable group, which consisted of patients who received surgery as the first-line treatment, exhibited better PFS and OS compared to the other patients. Unresectability was associated with significant smoking history, lower FEV1, and higher cancer stages. Lower LIPI scores demonstrated a higher OS rate that was also observed in the unresectable group. In clinical N2 subgroup, LN status combined with LIPI score may have predictive value for OS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-642/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-642/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-642/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-642/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of Catholic Medical Center, Korea (No. XC22RIDI0056). Informed consent was waived due to the retrospective study design. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Lim JU, Yeo CD. Update on adjuvant therapy in completely resected NSCLC patients. Thorac Cancer 2022;13:277-83. [Crossref] [PubMed]
- Majem M, Hernández-Hernández J, Hernando-Trancho F, et al. Multidisciplinary consensus statement on the clinical management of patients with stage III non-small cell lung cancer. Clin Transl Oncol 2020;22:21-36. [Crossref] [PubMed]
- Casal-Mouriño A, Ruano-Ravina A, Lorenzo-González M, et al. Epidemiology of stage III lung cancer: frequency, diagnostic characteristics, and survival. Transl Lung Cancer Res 2021;10:506-18. [Crossref] [PubMed]
- Tan WL, Chua KLM, Lin CC, et al. Asian Thoracic Oncology Research Group Expert Consensus Statement on Optimal Management of Stage III NSCLC. J Thorac Oncol 2020;15:324-43. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Mezquita L, Auclin E, Ferrara R, et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2018;4:351-7. [Crossref] [PubMed]
- Myall NJ, Das M. Advances in the Treatment of Stage III Non-Small Cell Lung Cancer. Clin Chest Med 2020;41:211-22. [Crossref] [PubMed]
- Van Schil PE, Berzenji L, Yogeswaran SK, et al. Surgical Management of Stage IIIA Non-Small Cell Lung Cancer. Front Oncol 2017;7:249. [Crossref] [PubMed]
- Jazieh AR, Onal HC, Tan DSW, et al. Real-World Treatment Patterns and Clinical Outcomes in Patients With Stage III NSCLC: Results of KINDLE, a Multicountry Observational Study. J Thorac Oncol 2021;16:1733-44. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Kim HC, Choi CM. Current Status of Immunotherapy for Lung Cancer and Future Perspectives. Tuberc Respir Dis (Seoul) 2020;83:14-9. [Crossref] [PubMed]
- Taugner J, Käsmann L, Eze C, et al. Durvalumab after Chemoradiotherapy for PD-L1 Expressing Inoperable Stage III NSCLC Leads to Significant Improvement of Local-Regional Control and Overall Survival in the Real-World Setting. Cancers (Basel) 2021;13:1613. [Crossref] [PubMed]
- Ferrucci PF, Ascierto PA, Pigozzo J, et al. Baseline neutrophils and derived neutrophil-to-lymphocyte ratio: prognostic relevance in metastatic melanoma patients receiving ipilimumab. Ann Oncol 2016;27:732-8. [Crossref] [PubMed]
- Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2014;23:1204-12. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Coinu A, et al. Prognostic role of lactate dehydrogenase in solid tumors: a systematic review and meta-analysis of 76 studies. Acta Oncol 2015;54:961-70. [Crossref] [PubMed]
- Yamaguchi M, Sugio K. Current status of induction treatment for N2-Stage III non-small cell lung cancer. Gen Thorac Cardiovasc Surg 2014;62:651-9. [Crossref] [PubMed]
- Putora PM, Leskow P, McDonald F, et al. International guidelines on stage III N2 nonsmall cell lung cancer: surgery or radiotherapy? ERJ Open Res 2020; [Crossref] [PubMed]


