
The landscape of cancer cachexia in advanced non-small cell lung cancer: a narrative review
Introduction
Background
Cancer cachexia mainly presents in the form of weight loss, anorexia, and fatigue, worsening the life expectancy and quality of life (QOL) of cancer patients (1). The prognosis of patients with advanced non-small cell lung cancer (NSCLC) is improving with advances in treatment, including novel molecular targeted therapy targeting KRAS G12C mutations (2). Therefore, the need for appropriate intervention for cancer cachexia is increasing. Inflammatory cytokines are produced by the immune system in response to factors produced by tumor cells. They play an important role in the pathogenesis of cancer cachexia, resulting in decreased appetite, abnormal energy metabolism, and skeletal muscle degeneration. These different phenomena further contribute to weight loss, a reportedly poor prognostic factor in patients with advanced NSCLC (3). Pharmacological therapies, such as corticosteroids and progesterone agents, have been used to alleviate symptoms associated with cancer cachexia in some countries, and there have been reports of some efficacy, whereas adverse events and other issues abound (4-6). In contrast, although the development of specific novel agents for cachexia has been attempted, it has not been successful for a variety of reasons, such as effects and toxicity (7).
In 2021, Japan became the first country in the world to approve anamorelin, a ghrelin-like agonist, for the treatment of cancer cachexia. Anamorelin continues to be used in daily clinical practice because of its therapeutic effects on cachexia. However, there is no established protocol for non-pharmacological treatment options, and clinical trials are currently underway in the world. In addition, the awareness of cancer cachexia remains low, and further educational activities for healthcare providers and patient family members are warranted.
Objectives
In this review, we provide an overview of patients with advanced lung cancer complicated with cachexia. We highlight the basic science concepts pertaining to cachexia, the advances in treatment, and the future of lung cancer treatment in the context of cancer cachexia. We present the following article in accordance with the Narrative Review reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-561/rc).
Methods
We searched published articles using the PubMed, published in the English language up to June 2022. We specifically searched using the key words “cancer cachexia”, “non-small cell lung cancer”, and in this context for “non-small cell lung cancer”, “pulmonary cachexia”, “Sarcopenia”, “COPD”, “ghrelin”, and “clinical application”. Our focus was to include mainly literature published from 2010 onwards. We also performed secondary review of bibliographies of several key meta-analyses (Table 1).
Table 1
| Items | Specification |
|---|---|
| Date of search | July 21st, 2022 |
| Databases and other sources searched | PubMed and ClinicalTrial.gov |
| Search terms used | “cancer cachexia”, “non-small cell lung cancer”, and in this context for “non-small cell lung cancer”, “pulmonary cachexia”, “sarcopenia”, “COPD”, “ghrelin” and “clinical application” |
| Timeframe | Mainly literature published from 2010 onwards |
| Inclusion and exclusion criteria | • Inclusion criteria: |
| (I) Only English-language article | |
| (II) Original publications, including the clinical trial, literature review, and review paper were included | |
| • Exclusion criteria: | |
| (I) Non-English-language article | |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Study selection and full-text articles were assessed by first authors (Tanaka Satomi and Tadaaki Yamada) and the consensus was obtained by other authors |
What is cachexia?
History of cachexia
The mention of cachexia dates back to the time of Hippocrates in ancient Greece as a condition in which a patient becomes emaciated and weak as a result of persistent and progressive chronic diseases, such as cancer, respiratory diseases, cardiac diseases, and collagen diseases. The English word cachexia is derived from the Greek words “kakos” meaning bad and “hexis” meaning condition. There was no effective treatment for cachexia, and it was considered a condition that developed in the terminal stage of the diseases. However, the pathophysiology, diagnostic criteria, and treatment methods have remained poorly understood, and until recently, cachexia was regarded as a “sign of death”.
Disease patterns have changed as medical advances have established effective treatments for many diseases. Malignant neoplasms have replaced infectious diseases as the leading cause of death (8), and according to the International Agency for Research on Cancer, the World Health Organization’s specialized agency for research on cancer, the worldwide incidence and mortality of cancer in 2018 were approximately 18.1 million cases and 9.6 million deaths, respectively. Of all cancers, lung cancer ranked first in both incidence and mortality (9).
Drug therapy for lung cancer has advanced remarkably over the years with the introduction of platinum-based drugs in the 1980s, followed by the approval of the molecularly targeted drugs from 2013 for EGFR, ALK, ROS1, KRAS, MET, and RET, and the immune checkpoint inhibitor (ICI) nivolumab in 2015 by the U.S. Food and Drug Administration (FDA). The prognosis for drug-sensitive patients has dramatically improved: the 5-year survival rates for advanced NSCLC preceding the advent of ICI ranged from 1 to 8%. The 5-year survival rates for patients treated with nivolumab, however, were reported to be 16% for squamous cell cancer and 15% for non-squamous NSCLC. Patients with high expression of PD-L1 within the tumor were reported to have a 5-year survival rate of 43% (10). In addition, the 5-year survival rate for patients with treatment-naïve advanced NSCLC treated with pembrolizumab monotherapy was reported as 23.2% (11).
With the progress of drug therapy and the improvement of overall survival, overcoming cancer cachexia, the poor prognostic factor with a major impact on QOL, has become the next main challenge. Research aimed at the development of cancer cachexia treatment methods has progressed, and in 2021, the world’s first ghrelin-like drug with anti-cancer cachexia action, “anamorelin”, was launched in Japan (Figure 1).
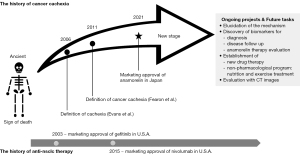
Definition of cachexia
The definitions and diagnostic criteria of “cachexia” and “cancer cachexia” were established in 2006 and 2011, respectively. The definition and diagnostic criteria for cachexia were proposed by Evans et al. at the 2006 Cachexia Consensus Conference held in the USA. Cachexia was defined at this conference as “a syndrome of complex metabolic abnormalities associated with an underlying disease, characterized by a decrease in skeletal muscle mass with or without a decrease in fat mass”. Clinical manifestations include weight loss in adults, growth retardation in children, anorexia, inflammation, insulin resistance, and muscle proteolysis. Cachexia is distinct from starvation, age-related loss of muscle mass, depression, malabsorption disorders, and hyperthyroidism. The diagnostic criteria are listed as follows: duration that is consistent with that of chronic disease, presence of weight loss of 5% or more within 12 months, or body mass index (BMI) less than 20 kg/m2 when weight change is not known, and the presence of three or more of the following items: (I) muscle weakness, (II) fatigue, (III) anorexia, (IV) low lean body mass (LBM), and (V) abnormal biochemical data (12). The definition and diagnostic criteria for cancer cachexia were published five years later in a consensus report in the European Palliative Care Research Collaborative (EPCRC). The consensus report defined cancer cachexia as “a multifactorial syndrome characterized by a persistent loss of skeletal muscle mass (with or without fat loss) that cannot be completely reversed by conventional nutritional therapy and that progresses to functional impairment.” The diagnosis of cancer cachexia is as follows: (I) weight loss of 5% or more within the past 6 months, (II) weight loss of 2% or more when BMI was less than 20 kg/m2 within the past 6 months, (III) weight loss of >2% in cases of concomitant sarcopenia within the past 6 months (1). Cancer cachexia has three stages: precachexia, cachexia, and refractory cachexia. Intervention starting from the precachexia stage is recommended. Given the multifactorial nature of the syndrome, a combination of pharmacotherapy and non-pharmacologic interventions, such as nutrition and exercise, is recommended. However, standard prescriptions for nutritional and exercise interventions are yet to be established.
Mechanism of cachexia
The pathogenesis of cachexia is complex and not fully understood. At present, the development of cancer cachexia is thought to be due to the release of various factors in response to activation of the humoral immune system, and not a direct effect of tumorigenesis. These factors include cytokines, blood cells, and humoral factors secreted by tumor cells. Tumor cell-derived cytokines, such as interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF), and interferon-γ (IFNγ), are known to be involved in the insulin-like growth factor 1 (IGF1)-Akt-forkhead box O (FoxO), transforming growth factor-β (TGF-β)-myostatin, NF-κB, and glucocorticoid pathways. Immune cells such as macrophages, neutrophils, bone marrow-derived suppressor cells, and T-cells that function in response to tumorigenesis are also implicated in cancer cachexia (13,14).
The balance of the appetite center, which is regulated by growth differentiation factor-15 (GDF-15), leptin secreted by adipocytes, and ghrelin secreted by gastric wall cells, is disrupted by inflammatory cytokines during cancer progression. Dysregulation of these processes promotes the state of appetite suppression. GDF-15, also referred to as macrophage inhibitory cytokine-1, is a divergent member of the TGF-β superfamily which regulates cell growth, differentiation, and death. GDF-15 is widely expressed in normal cells and its concentration in circulating blood is increased in inflammation, wound formation, cardiac and renal diseases, and malignancies (15). Elevated blood concentration of GDF-15 in cancer patients since the early stage of cachexia has been reported as a poor prognostic factor in patients with anorexia and weight loss due to cancer cachexia (16,17). In addition, its overexpression in skeletal muscles can promote muscle atrophy (18). In animal studies of cachexia, mice treated with anti-GDF-15 antibody experienced increased body weight, muscle mass, and fat mass (16).
GDF-11, another member of the TGF-β family of molecules, is a homologous protein of myostatin, a known inhibitor of skeletal muscle growth. Interestingly, GDF-11 and myostatin have more than 50% of their amino acid sequences in common (19). The concentration of GDF-11 in the circulating blood is associated with the degree of skeletal and cardiac muscle atrophy (20). Increased endogenous secretion of glucocorticoids, a class of corticosteroids, occurs in stressful conditions, and glucocorticoids have long been used in cancer therapy. In skeletal muscles, glucocorticoids cause increased protein catabolism and decreased synthesis (21).
Parathyroid hormone-related protein (PTHrP) in adipose tissue has been reported to be associated with increased metabolism and decreased LBM (22). Anti-PTHrP antibodies have been reported to suppress cancer cachexia (23). These diverse factors cause cachexia pathology by acting on multiple organs, such as the skeletal muscle, adipocytes, brain, liver, bone, pancreas, myocardium, and gastrointestinal tract (Figure 2A) (24).
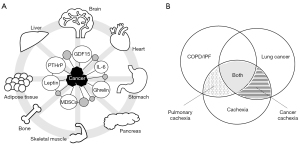
Lung cancer and cancer cachexia
Respiratory disease and cachexia, and pulmonary rehabilitation and nutritional support
Cancer cachexia occurs in 50–80% of patients with advanced cancer (25). Gastrointestinal, head and neck, and lung cancers are the most common causes of cancer cachexia (26). Gastrointestinal and head and neck cancers are directly involved in feeding, digestion, and absorption, whereas lung cancer has a less direct impact on nutritional intake. Cancer cachexia in advanced lung cancer may occur as a complication of commonly comorbid chronic lung diseases, such as chronic obstructive pulmonary disease (COPD) and idiopathic interstitial pneumonia. Cachexia is a complication of various chronic diseases, as well as cancer, and is frequently seen in COPD, congestive heart failure, cancer, and chronic kidney disease (27). The frequency of COPD and emphysema in lung cancer patients has been reported as 38.4% and 58.2%, respectively (28). The prevalence of cachexia in patients with COPD is relatively high, ranging from 5% to 15%. In addition, cachexia is known as an independent risk factor for mortality in COPD patients (27,29). A randomized controlled trial comparing administration of ghrelin versus placebo control in patients with severe COPD with a BMI of 21 kg/m2 showed that ghrelin treatment was associated with a significant reduction in dyspnea on exertion during respiratory rehabilitation (30).
Idiopathic pulmonary fibrosis (IPF), a group of refractory interstitial pneumonia that confer poor prognosis, occurs in approximately 10–20% of patients with lung cancer (31,32). A worse prognosis has been reported in patients with IPF and signs suggesting muscle atrophy and sarcopenia, such as a low cross-sectional area of the erector spinae muscle (33).
The progression of chronic lung diseases causes chronic systemic inflammation, leading to weight loss, sarcopenia, malnutrition, and ultimately, a state of pulmonary cachexia. It has been speculated that one of the reasons for the high incidence of cachexia in lung cancer is related to the complications of chronic lung diseases, in addition to the rate of disease progression of advanced cancer (Figure 2B).
Pulmonary rehabilitation and nutritional support constitute one of the standard interventions for patients with chronic lung disease. High intensity exercise training was shown to be effective in improving lower limb muscle strength and exercise performance in COPD patients with low muscle mass and moderate airflow obstruction. Specific nutritional supplementation showed additional effects on nutritional status, inspiratory muscle strength, and physical activity (34). Despite proven physiological, symptom relief, psychosocial, and health economic benefits of pulmonary rehabilitation and nutritional support for patients with chronic respiratory diseases, these interventions are under-utilized worldwide. This is due to a lack of funding, resources, and reimbursement, as well as a lack of awareness and knowledge among healthcare professionals, payers, and patients.
Impact of weight loss in patients with advanced NSCLC
It has been reported that weight loss associated with cancer progression is a poor prognostic factor for patients with NSCLC. In the TORG0912 study, we observed weight change in 406 untreated advanced NSCLC patients with Eastern Cooperative Oncology Group-Performance status (ECOG-PS) 0–2 (median age 67 years, male to female ratio 2:1, mean BMI 21.79 kg/m2) who were prospectively followed for 12 months. The group with greater weight loss showed increased muscle weakness and a marked deterioration in life expectancy, QOL, and PS (3). In addition, another Japanese cachexia team prospectively observed untreated advanced NSCLC patients over 70 years of age during initial chemotherapy and reported significant decreases in BMI, skeletal muscle index, and shuttle walk distance at 12 weeks following the start of observation (35).
Efficacy of ICIs in advanced NSCLC patients with cancer cachexia
Complications of cachexia and sarcopenia are also known to affect treatment response in patients with advanced NSCLC. By measuring the cross-sectional area of the psoas major muscle on computed tomography (CT) images, we retrospectively evaluated the presence of sarcopenia in 38 patients with advanced NSCLC who received the ICIs, nivolumab or pembrolizumab. Progression free survival (PFS) in patients without sarcopenia was significantly greater than that in patients with sarcopenia (36). Similarly, recent studies have reported that advanced NSCLC patients with cancer cachexia-sarcopenia showed poor prognosis after treatment with immune checkpoint inhibitors (37-39).
Next, in a retrospective study of 235 patients with advanced NSCLC treated with a combined therapy of ICIs and cytotoxic anticancer agents, we reported that patients with PD-L1 expression <50% and cachexia experienced significantly lower PFS and overall survival (OS) than patients without cachexia (40). These results indicate that the presence of sarcopenia and cancer cachexia in advanced NSCLC may affect the efficacy of immunotherapy-containing regimens. In addition, inflammatory/prognostic scores, such as modified Glasgow prognostic score, prognostic nutritional index, nutritional index, and neutrophil/lymphocyte ratio, are associated with cancer cachexia (41,42) and prognosis of advanced NSCLC patients (42-45).
Such muscle weakness may cause a decline in activities of daily living (ADL) in older lung cancer patients, which may significantly hinder treatment adherence, maintenance, and effectiveness.
Early intervention, such as exercise and nutrition, to maintain muscle strength may improve life expectancy and prognosis. A previous clinical trial demonstrated that early palliative care led to significant improvements in both QOL and mood among patients with metastatic NSCLC (46). However, at present, standard prescriptions and non-pharmacological approaches for patients have not been established.
Development of novel therapeutic strategy with anamorelin for cancer cachexia
Discovery of ghrelin
Ghrelin was discovered by Kojima et al. in 1999. Ghrelin is a peptide hormone present in the stomach that increases appetite and acts as an endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a) (47). Ghrelin binds to GHS-R1a to exert its bioactivity. GHS-R1a is expressed not only in the central nervous system but also in various peripheral tissues and exhibits additional bioactivities, such as promotion of lipogenesis, involvement in glucose metabolism, regulation of gastrointestinal motility, suppression of inflammatory cytokine production, and protection of the cardiovascular system (48). Ghrelin is also known to promote protein synthesis in skeletal muscles through the following pathways: (I) ghrelin acts on the hypothalamus and enhances the secretion of growth hormone (GH); (II) secreted GH acts on the liver and promotes the secretion of IGF-1; (III) IGF-1 acts on skeletal muscles to promote protein synthesis (49).
Clinical development and challenges of anamorelin
In 2001, peripheral administration of ghrelin to healthy volunteers showed a significant increase in appetite and food intake (50). However, the short half-life of ghrelin of approximately 10 min and the need for intravenous administration were problematic for its clinical application. To overcome these problems, anamorelin, a ghrelin agonist, was developed as a ghrelin-like compound that can be orally administered. Anamorelin is a low-molecular-weight compound with a molecular weight of 583.16 and a half-life of approximately 9 hours in blood, making it feasible for daily oral administration. In clinical trials done in Japan (ONO-7643-04) investigating anamorelin in patients with NSCLC and gastrointestinal cancer, treatment with 100 mg/day of anamorelin once daily for 12 weeks showed a significant increase in LBM; the change from baseline was 1.38±0.18 and −0.17±0.17 kg in the anamorelin and placebo groups, respectively (P<0.0001). In addition, the LBM of anamorelin group increased one week after anamorelin treatment and it continued thereafter with no safety concern. Appetite was determined using the QOL Questionnaire for Cancer Patients Treated with Anticancer Drugs (QOL-ACD), and the scores also improved from the first week of treatment, and the effect was maintained until the 12th week. In contrast, the study did not show any improvement in non-dominant handgrip strength or 6-min walking distance (51). Following these results, the anamorelin was launched in Japan in 2021. However, it was not licensed in Europe and the US due to lack of adequate data on patient benefits and safety. Currently, two phase III SCALA studies on anamorelin are underway in the US, Europe, Russia, and Australia for treatment of malignancy-associated weight loss and anorexia in adult patients with advanced NSCLC (NCT03743051 and NCT03743064).
Regarding the introduction of anamorelin therapy, attention should be paid to two points: side effects and selection of treatment eligibility. In terms of side effects, adverse cardiovascular events, such as first-degree atrioventricular block, tachycardia, and hypertension, have been reported. From the results of nonclinical studies (49,52), anamorelin has been reported to have an inhibitory activity on ligand binding to sodium channels and L-type calcium channels and is known to inhibit sodium channel currents in human cardiomyocytes. Therefore, it should be noted that anamorelin is administered with caution or is contraindicated in patients with cardiac diseases, such as congestive heart failure, myocardial infarction, and angina pectoris, as well as severe heart conduction disorders, including complete atrioventricular block. In addition, because the drug is metabolized via CYP3A4, concomitant use with drugs that inhibit the activity of this enzyme is contraindicated. Particular attention should be paid to patients with comorbid chronic respiratory diseases, as frequently used drugs, such as clarithromycin, itraconazole, and voriconazole, are among the contraindications. In addition, caution must be taken when determining patient eligibility for clinical trials of anamorelin. In this study, patients with an ECOG-PS of 0–2 and an expected prognosis of at least 4 months were included. Therefore, the use of anamorelin in patients with poor PS should be judged with caution. The Japanese Association of Supportive Care in Cancer formulated a guideline for the proper use of anamorelin, considering the above precautions. Currently, anamorelin is available for patients with cachexia in daily practice but is not routinely used in Japan.
In contrast, the IGF-1 molecule secreted from the liver as a result of anamorelin administration is known to be a tumor growth factor. There was a concern about the enhancement of tumorigenicity during the drug development process; however, preclinical studies and clinical trials have shown no enhancement of tumorigenic effects with anamorelin administration (53).
Clinical interventional study for cancer cachexia
The aforementioned clinical trials showed that anamorelin treatment did not restore muscle strength or improve physical activity in patients with advanced cancer (51). To date, efficacy and tolerability have not been proven for the multidisciplinary treatment of patients with cancer cachexia using non-pharmacological therapies. Currently, randomized controlled clinical trials are underway in Japan and Europe to evaluate the efficacy of concurrent anamorelin and non-pharmacological treatment in the form of exercise and nutritional therapy. The Nutritional and Exercise Treatment for Advanced Cancer (NEXTAC) study, a multicenter study in Japan aiming to assess the efficacy of early nutritional and exercise interventions in older patients with advanced NSCLC/pancreatic cancer, is underway. The goal of this study is to establish interventions for muscle strength and physical function in patients with cancer cachexia. A phase I trial (NEXTAC-ONE) prospectively evaluated the feasibility of exercise therapy and its impact on QOL in 30 patients with advanced-stage untreated NSCLC and pancreatic cancer. These patients were aged 70 years or older, belonged to ECOG-PS 0–1, scored >90 points on the Barthel index, and were scheduled for induction of first-line anticancer therapy (54). A physiotherapist, dietitian, and nurse provided counseling to promote daily physical activity throughout the 8-week intervention. Investigators assessed patient attendance and compliance with counseling, as well as the effectiveness of the intervention. Intensity of exercise was set at three levels (≤2,000 steps, >2,000 steps/<8,000 steps, and ≥8,000 steps) and was tailored to each patient based on their baseline average number of steps per day during the screening period. The median age of the patients was 75 years [70–84], and 12 (40%) patients met the criteria for cachexia at inclusion. Twenty-eight (93%) patients participated in all courses, resulting in 6 (15%) patients with increased indoor activities and 15 (52%) patients with increased outdoor activities. There were significant differences in physical activity, QOL, and mental activity between the patients who increased their activity and those who did not. We conclude that the NEXTAC program of exercise therapy is feasible and well tolerated by older patients with advanced-stage cancer. In fact, many patients showed behavioral changes and improved QOL (55). A multicenter randomized phase II trial (NEXTAC-TWO) is currently underway to test the usefulness of exercise therapy by evaluating two similar patient groups (UMIN000028801) (56). A multicenter, randomized phase II trial (NEXTAC-THREE) is also underway (JPRN-jRCTs 041210053). In European countries, a phase III study Multimodal-Exercise, Nutrition and Anti-inflammatory medication (MENAC) for the treatment of advanced NSCLC and advanced pancreatic cancer in patients aged 18 years and older with Karnofsky Performance Status >70 is also ongoing (NCT02330926) (57).
Current status of cancer cachexia treatment
Nutrition impact symptoms that can be intervened
Many NSCLC patients have a high symptom burden and are at risk of malnutrition prior to starting systemic anticancer therapy, suggesting that the improvement of nutritional conditions might enhance clinical outcomes (58). However, simple nutritional interventions did not improve clinical outcomes, including nutritional outcomes and QOL (59).
Practical interventions for cancer cachexia have not yet been well established. Management should appropriately address treatable factors associated with cancer. Symptoms that produce secondary hunger are termed nutrition impact symptoms and are thought to be caused by complications such as chemotherapy-related oral mucosal damage, diarrhea, nausea, and vomiting, as well as cancer-related depression (60). These complications for most cases are manageable by therapeutic intervention. The aforementioned TORG0912 trial showed that weight loss itself is a poor prognostic factor in patients with advanced cancer (3), and it is important to intervene appropriately through patient/family communication and QOL active weight follow-up.
Patient and family education
In a survey of cancer patients and their families in Japan, 8.2% of patients and 14.5% of family members reported being aware of the term cancer cachexia (61). Moreover, anorexia and weight loss were not reported or discussed with healthcare professionals when the main symptoms of cancer cachexia occurred in 42.4% and 57.9% of cases, respectively (62). Although the data were obtained before the launch of anamorelin, it is clear that there is insufficient awareness regarding cancer cachexia, highlighting the need for proper patient and family education.
Prospects for cancer cachexia
Development of novel therapeutics
New anti-cachectic agents are under development following anamorelin. Visugromab, a neutralizing antibody against GDF-15, is currently attracting attention as a novel therapeutic target against cachexia. GDF-15 has been reported to induce anorexia by acting on the brain’s feeding center and to be involved in the reduction of LBM and fat mass in cancer patients (17). Visugromab has been evaluated for tolerability in a phase 1 study and is currently being evaluated in a phase 2 study. Bermekimab, an anti-IL-1α antibody targeting inflammatory cytokines, was evaluated in a phase 1 study (NCT01021072) in patients with advanced cancer to identify the appropriate dose of the antibody drug, as well as its antitumor effects and response to cachexia. In this study, no dose-limiting toxicity of Bermekimab was observed until 3.75 mg/kg, and disease control was obtained (63). They also reported that Bermekimab was well tolerated, with gains in LBM [1.0±2.5 kg, mean 0.4 kg (SD: −0.5 to 2.6)] in patients with metastatic non-small cell lung cancer (64). A phase III study in patients with advanced colorectal cancer refractory to treatment with oxaliplatin plus irinotecan treatment showed that Bermekimab treatment resulted in changes in LBM and improvement in subjective symptoms, such as pain, fatigue, and anorexia (65). Bermekimab is currently in clinical trials for inflammatory diseases, including a phase 2 study (NCT03496974) for atopic dermatitis. In addition, rapamycin has a therapeutic potential for sarcopenia by slowing age-related muscle wasting through regulation of mTORC1 (66).
Basic science research has also revealed several factors that are potential therapeutic targets, including fibroblast growth factor inducible 14 (Fn14), muscle RING finger 1 (MuRF1), SPRY domain-containing SOCS box protein 1 (Spsb1), serum amyloid A1 (SAA1), and ZIP14.
Fn14 is a receptor for TNF-related weak inducer of apoptosis (TWEAK), one of the super family of TNF. Anti-Fn14 antibody treatment improved cachexia symptoms, increased body weight, and prolonged survival in mice with carcinoma in situ (67). Spsb1 induces destabilization of p21, a CDK inhibitor, and contributes to cancer cell survival (68). SAA1, which is increased in acute phase inflammation, induces activation of TLR2/TLR4/NF-κB signaling and causes muscle cell atrophy (69). ZIP14, a transporter of zinc ions, has been reported to promote cachexia when its levels are elevated in skeletal muscles of advanced cancer mouse models (70). These factors are being investigated as next-generation therapeutic targets for cancer cachexia and are expected to be developed clinically in the future.
Biomarker development for cancer cachexia
Currently, the indicators used in the diagnosis and evaluation of cancer cachexia include body weight and BMI, as well as the diagnostic criteria for cachexia proposed by Evans et al. (12). These criteria include serum CRP, hemoglobin, and albumin levels and a QOL questionnaire that is used to score subjective symptoms, such as appetite and fatigue.
The FAACT and its Anorexia-Cachexia subscale (A/Cs) are representative QOL questionnaires. In particular, the A/Cs has been shown to be useful in identifying patients with cancer cachexia (71). The QOL-ACD was used to survey QOL during the development of anamorelin. However, owing to inadequate reimbursement coverage in real-world practice, team-based care for cancer cachexia, such as QOL surveys and patient-family education, is yet to be adequately developed.
For biomarker studies with muscle tissue, blood, and urine samples, various analytical approaches were used, including nuclear resonance spectrometry, gas chromatography-mass spectrometry, and liquid chromatography-mass spectrometry. The levels of metabolites, such as phenylalanine, asymmetric dimethylarginine (ADMA), paraxanthine, 3-hydroxybutyrate, lysoPC 18:2, lysoPC 16:1, hexadecanoic acid (palmitic acid), and octadecanoic acid (stearic acid), were elevated in patients with cachexia and weight loss (72). Among them, ADMA has been identified as a potential therapeutic target for cachexia (73). Although such a comprehensive analysis poses the challenges of procedural complexity and elevated costs, ADMA is expected to develop into a novel biomarker for the diagnosis and disease assessment of cancer cachexia in the future.
Attempts have also been made to evaluate cancer cachexia using imaging data. Although the analysis of skeletal muscle mass using CT images has been widely used to evaluate sarcopenia in the past, most of these methods require labor-intensive manual measurement. Recently, Analyzer using Computed tomography image Segmentation (ABACS), a software that analyzes CT images to quantify skeletal muscle mass and fat mass, was developed in the United States and Canada and was shown to measure with high accuracy (74). Measurement of skeletal muscle mass and fat mass has the potential to assess the pathophysiology of cachexia more accurately than weight loss rate or BMI, and its efficient reproducibility through software is expected to be applied to routine clinical practice similar to estimation of bone density in the diagnosis of osteoporosis.
The clinical biomarkers regarding response to anamorelin therapy remain unclear. Therefore, development of predictive biomarkers for anamorelin therapy remains an issue. In post-marketing surveillance of anamorelin in Japan, gastrointestinal disturbances were the most frequently reported adverse reactions, with nausea reported by half of the patients (75). The action of anamorelin differs from patient to patient, and further clinical investigations are warranted.
Conclusions
The pathogenesis and treatment of cancer cachexia is being established. Patients who are not eligible for conventional supportive care but who have progressive weight loss may have advanced cancer cachexia and require earlier, more aggressive intervention. Beneficial interventions for diseases require an understanding of cancer cachexia by both providers and patients and the development of effective drugs. The Society for Sarcopenia, Cachexia and Wasting Disorder was established in 2008, mainly in Europe and the United States, and is promoting global awareness of cancer cachexia. Cancer cachexia treatment is expected to become the future of drug therapy in advanced lung cancer in conjunction with anticancer therapy.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-561/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-561/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-561/coif). TY serves as an unpaid editorial board member of Translational Lung Cancer Research from October 2021 to September 2023. TY received grants from Pfizer, Ono Pharmaceutical, Janssen Pharmaceutical, AstraZeneca plc, and Takeda Pharmaceutical and personal fees from Eli Lilly. KT received grants from Chugai Pharmaceutical and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai Pharmaceutical, MSD, Eli Lilly, Boehringer Ingelheim, and Daiichi Sankyo. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 2011;12:489-95. [Crossref] [PubMed]
- Skoulidis F, Li BT, Dy GK, et al. Sotorasib for Lung Cancers with KRAS p.G12C Mutation. N Engl J Med 2021;384:2371-81. [Crossref] [PubMed]
- Takayama K, Atagi S, Imamura F, et al. Quality of life and survival survey of cancer cachexia in advanced non-small cell lung cancer patients-Japan nutrition and QOL survey in patients with advanced non-small cell lung cancer study. Support Care Cancer 2016;24:3473-80. [Crossref] [PubMed]
- Paulsen O, Klepstad P, Rosland JH, et al. Efficacy of methylprednisolone on pain, fatigue, and appetite loss in patients with advanced cancer using opioids: a randomized, placebo-controlled, double-blind trial. J Clin Oncol 2014;32:3221-8. [Crossref] [PubMed]
- Madeddu C, Mantovani G, Gramignano G, et al. Advances in pharmacologic strategies for cancer cachexia. Expert Opin Pharmacother 2015;16:2163-77. [Crossref] [PubMed]
- Ruiz Garcia V, López-Briz E, Carbonell Sanchis R, et al. Megestrol acetate for treatment of anorexia-cachexia syndrome. Cochrane Database Syst Rev 2013;2013:CD004310. [Crossref] [PubMed]
- Mantovani G, Macciò A, Madeddu C, et al. Randomized phase III clinical trial of five different arms of treatment in 332 patients with cancer cachexia. Oncologist 2010;15:200-11. [Crossref] [PubMed]
- The top 10 causes of death. [cited 2020 Dec 9]. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Garon EB, Hellmann MD, Rizvi NA, et al. Five-Year Overall Survival for Patients With Advanced Non‒Small-Cell Lung Cancer Treated With Pembrolizumab: Results From the Phase I KEYNOTE-001 Study. J Clin Oncol 2019;37:2518-27. [Crossref] [PubMed]
- Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr 2008;27:793-9. [Crossref] [PubMed]
- Baazim H, Antonio-Herrera L, Bergthaler A. The interplay of immunology and cachexia in infection and cancer. Nat Rev Immunol 2022;22:309-21. [Crossref] [PubMed]
- Li Z, Zhu L, Zheng H, et al. Serum IL-35 levels is a new candidate biomarker of cancer-related cachexia in stage IV non-small cell lung cancer. Thorac Cancer 2022;13:716-23. [Crossref] [PubMed]
- Mimeault M, Batra SK. Divergent molecular mechanisms underlying the pleiotropic functions of macrophage inhibitory cytokine-1 in cancer. J Cell Physiol 2010;224:626-35. [Crossref] [PubMed]
- Lerner L, Tao J, Liu Q, et al. MAP3K11/GDF15 axis is a critical driver of cancer cachexia. J Cachexia Sarcopenia Muscle 2016;7:467-82. [Crossref] [PubMed]
- Saarma M, Goldman A. Obesity: Receptors identified for a weight regulator. Nature 2017;550:195-7. [Crossref] [PubMed]
- Patel MS, Lee J, Baz M, et al. Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle 2016;7:436-48. [Crossref] [PubMed]
- Parsons SA, Millay DP, Sargent MA, et al. Age-dependent effect of myostatin blockade on disease severity in a murine model of limb-girdle muscular dystrophy. Am J Pathol 2006;168:1975-85. [Crossref] [PubMed]
- Hammers DW, Merscham-Banda M, Hsiao JY, et al. Supraphysiological levels of GDF11 induce striated muscle atrophy. EMBO Mol Med 2017;9:531-44. [Crossref] [PubMed]
- Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol 2008;197:1-10. [Crossref] [PubMed]
- Kir S, Komaba H, Garcia AP, et al. PTH/PTHrP Receptor Mediates Cachexia in Models of Kidney Failure and Cancer. Cell Metab 2016;23:315-23. [Crossref] [PubMed]
- Kir S, White JP, Kleiner S, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Nature 2014;513:100-4. [Crossref] [PubMed]
- Siddiqui JA, Pothuraju R, Jain M, et al. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim Biophys Acta Rev Cancer 2020;1873:188359. [Crossref] [PubMed]
- Argilés JM, Busquets S, Stemmler B, et al. Cancer cachexia: understanding the molecular basis. Nat Rev Cancer 2014;14:754-62. [Crossref] [PubMed]
- Baracos VE, Martin L, Korc M, et al. Cancer-associated cachexia. Nat Rev Dis Primers 2018;4:17105. [Crossref] [PubMed]
- von Haehling S, Anker SD. Cachexia as a major underestimated and unmet medical need: facts and numbers. J Cachexia Sarcopenia Muscle 2010;1:1-5. [Crossref] [PubMed]
- Mizuno S, Takiguchi Y, Fujikawa A, et al. Chronic obstructive pulmonary disease and interstitial lung disease in patients with lung cancer. Respirology 2009;14:377-83. [Crossref] [PubMed]
- Collins PF, Yang IA, Chang YC, et al. Nutritional support in chronic obstructive pulmonary disease (COPD): an evidence update. J Thorac Dis 2019;11:S2230-7. [Crossref] [PubMed]
- Miki K, Maekura R, Nagaya N, et al. Effects of ghrelin treatment on exertional dyspnea in COPD: an exploratory analysis. J Physiol Sci 2015;65:277-84. [Crossref] [PubMed]
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788-824. [Crossref] [PubMed]
- Ogura T, Takigawa N, Tomii K, et al. Summary of the Japanese Respiratory Society statement for the treatment of lung cancer with comorbid interstitial pneumonia. Respir Investig 2019;57:512-33. [Crossref] [PubMed]
- Suzuki Y, Yoshimura K, Enomoto Y, et al. Distinct profile and prognostic impact of body composition changes in idiopathic pulmonary fibrosis and idiopathic pleuroparenchymal fibroelastosis. Sci Rep 2018;8:14074. [Crossref] [PubMed]
- van de Bool C, Rutten EPA, van Helvoort A, et al. A randomized clinical trial investigating the efficacy of targeted nutrition as adjunct to exercise training in COPD. J Cachexia Sarcopenia Muscle 2017;8:748-58. [Crossref] [PubMed]
- Naito T, Okayama T, Aoyama T, et al. Skeletal muscle depletion during chemotherapy has a large impact on physical function in elderly Japanese patients with advanced non-small-cell lung cancer. BMC Cancer 2017;17:571. [Crossref] [PubMed]
- Nishioka N, Uchino J, Hirai S, et al. Association of Sarcopenia with and Efficacy of Anti-PD-1/PD-L1 Therapy in Non-Small-Cell Lung Cancer. J Clin Med 2019;8:450. [Crossref] [PubMed]
- Roch B, Coffy A, Jean-Baptiste S, et al. Cachexia - sarcopenia as a determinant of disease control rate and survival in non-small lung cancer patients receiving immune-checkpoint inhibitors. Lung Cancer 2020;143:19-26. [Crossref] [PubMed]
- Rounis K, Makrakis D, Tsigkas AP, et al. Cancer cachexia syndrome and clinical outcome in patients with metastatic non-small cell lung cancer treated with PD-1/PD-L1 inhibitors: results from a prospective, observational study. Transl Lung Cancer Res 2021;10:3538-49. [Crossref] [PubMed]
- Degens JHRJ, Dingemans AC, Willemsen ACH, et al. The prognostic value of weight and body composition changes in patients with non-small-cell lung cancer treated with nivolumab. J Cachexia Sarcopenia Muscle 2021;12:657-64. [Crossref] [PubMed]
- Morimoto K, Uchino J, Yokoi T, et al. Impact of cancer cachexia on the therapeutic outcome of combined chemoimmunotherapy in patients with non-small cell lung cancer: a retrospective study. Oncoimmunology 2021;10:1950411. [Crossref] [PubMed]
- Baldessari C, Guaitoli G, Valoriani F, et al. Impact of body composition, nutritional and inflammatory status on outcome of non-small cell lung cancer patients treated with immunotherapy. Clin Nutr ESPEN 2021;43:64-75. [Crossref] [PubMed]
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534-40. [Crossref] [PubMed]
- Silva GAD, Wiegert EVM, Calixto-Lima L, et al. Clinical utility of the modified Glasgow Prognostic Score to classify cachexia in patients with advanced cancer in palliative care. Clin Nutr 2020;39:1587-92. [Crossref] [PubMed]
- Tanaka S, Uchino J, Yokoi T, et al. Prognostic Nutritional Index and Lung Immune Prognostic Index as Prognostic Predictors for Combination Therapies of Immune Checkpoint Inhibitors and Cytotoxic Anticancer Chemotherapy for Patients with Advanced Non-Small Cell Lung Cancer. Diagnostics (Basel) 2022;12:423. [Crossref] [PubMed]
- Katayama Y, Yamada T, Chihara Y, et al. Significance of inflammatory indexes in atezolizumab monotherapy outcomes in previously treated non-small-cell lung cancer patients. Sci Rep 2020;10:17495. [Crossref] [PubMed]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 2010;363:733-42. [Crossref] [PubMed]
- Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999;402:656-60. [Crossref] [PubMed]
- Colldén G, Tschöp MH, Müller TD. Therapeutic Potential of Targeting the Ghrelin Pathway. Int J Mol Sci 2017;18:798. [Crossref] [PubMed]
- Pietra C, Takeda Y, Tazawa-Ogata N, et al. Anamorelin HCl (ONO-7643), a novel ghrelin receptor agonist, for the treatment of cancer anorexia-cachexia syndrome: preclinical profile. J Cachexia Sarcopenia Muscle 2014;5:329-37. [Crossref] [PubMed]
- Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001;86:5992. [Crossref] [PubMed]
- Katakami N, Uchino J, Yokoyama T, et al. Anamorelin (ONO-7643) for the treatment of patients with non-small cell lung cancer and cachexia: Results from a randomized, double-blind, placebo-controlled, multicenter study of Japanese patients (ONO-7643-04). Cancer 2018;124:606-16. [Crossref] [PubMed]
- Adlumiz, INN-anamorelin - European Medicines Agency. [Cited 2022 Jul 27]. Available online: https://www.ema.europa.eu/en/documents/assessment-report/adlumiz-epar-refusal-public-assessment-report_en.pdf
- Northrup R, Kuroda K, Duus EM, et al. Effect of ghrelin and anamorelin (ONO-7643), a selective ghrelin receptor agonist, on tumor growth in a lung cancer mouse xenograft model. Support Care Cancer 2013;21:2409-15. [Crossref] [PubMed]
- MAHONEY FI. BARTHEL DW. FUNCTIONAL EVALUATION: THE BARTHEL INDEX. Md State Med J 1965;14:61-5.
- Naito T, Mitsunaga S, Miura S, et al. Feasibility of early multimodal interventions for elderly patients with advanced pancreatic and non-small-cell lung cancer. J Cachexia Sarcopenia Muscle 2019;10:73-83. [Crossref] [PubMed]
- Miura S, Naito T, Mitsunaga S, et al. A randomized phase II study of nutritional and exercise treatment for elderly patients with advanced non-small cell lung or pancreatic cancer: the NEXTAC-TWO study protocol. BMC Cancer 2019;19:528. [Crossref] [PubMed]
- Solheim TS, Laird BJA, Balstad TR, et al. Cancer cachexia: rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support Palliat Care 2018;8:258-65. [Crossref] [PubMed]
- Phillips I, Allan L, Hug A, et al. Nutritional status and symptom burden in advanced non-small cell lung cancer: results of the dietetic assessment and intervention in lung cancer (DAIL) trial. BMJ Support Palliat Care 2021;bmjspcare-2020-002838.
- Baldwin C, Spiro A, McGough C, et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J Hum Nutr Diet 2011;24:431-40. [Crossref] [PubMed]
- Khorasanchi A, Nemani S, Pandey S, et al. Managing Nutrition Impact Symptoms in Cancer Cachexia: A Case Series and Mini Review. Front Nutr 2022;9:831934. [Crossref] [PubMed]
- Morimoto T, Machii K, Matsumoto H, et al. Web questionnaire survey on appetite loss and weight loss associated with cancer cachexia Japanese evidence for patients of cancer cachexia(J-EPOCC)-the understanding of cancer cachexia. Gan To Kagaku Ryoho 2020;47:1075-80. [PubMed]
- Morimoto T, Machii K, Matsumoto H, et al. Web questionnaire survey on appetite loss and weight loss associated with cancer cachexia Japanese evidence for patients of cancer cachexia (J-EPOCC)-the problem awareness of appetite loss and weight loss. Gan To Kagaku Ryoho 2020;47:947-53. [PubMed]
- Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol 2014;15:656-66. [Crossref] [PubMed]
- Hong DS, Janku F, Naing A, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs 2015;33:621-31. [Crossref] [PubMed]
- Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2017;18:192-201. [Crossref] [PubMed]
- Ham DJ, Börsch A, Lin S, et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat Commun 2020;11:4510. [Crossref] [PubMed]
- Johnston AJ, Murphy KT, Jenkinson L, et al. Targeting of Fn14 Prevents Cancer-Induced Cachexia and Prolongs Survival. Cell 2015;162:1365-78. [Crossref] [PubMed]
- Kim HJ, Kim HJ, Kim MK, et al. SPSB1 enhances ovarian cancer cell survival by destabilizing p21. Biochem Biophys Res Commun 2019;510:364-9. [Crossref] [PubMed]
- Hahn A, Kny M, Pablo-Tortola C, et al. Serum amyloid A1 mediates myotube atrophy via Toll-like receptors. J Cachexia Sarcopenia Muscle 2020;11:103-19. [Crossref] [PubMed]
- Wang G, Biswas AK, Ma W, et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat Med 2018;24:770-81. [Crossref] [PubMed]
- LeBlanc TW, Samsa GP, Wolf SP, et al. Validation and real-world assessment of the Functional Assessment of Anorexia-Cachexia Therapy (FAACT) scale in patients with advanced non-small cell lung cancer and the cancer anorexia-cachexia syndrome (CACS). Support Care Cancer 2015;23:2341-7. [Crossref] [PubMed]
- Cui P, Li X, Huang C, et al. Metabolomics and its Applications in Cancer Cachexia. Front Mol Biosci 2022;9:789889. [Crossref] [PubMed]
- Kunz HE, Dorschner JM, Berent TE, et al. Methylarginine metabolites are associated with attenuated muscle protein synthesis in cancer-associated muscle wasting. J Biol Chem 2020;295:17441-59. [Crossref] [PubMed]
- Cespedes Feliciano EM, Popuri K, Cobzas D, et al. Evaluation of automated computed tomography segmentation to assess body composition and mortality associations in cancer patients. J Cachexia Sarcopenia Muscle 2020;11:1258-69. [Crossref] [PubMed]
- Post Marketing Surveillance of ADLUMIZ Tablets. 2022 [cited 2022 September 14th]; Available online: https://www.ono-oncology.jp/system/files/2022-02/ADM_houkoku.pdf


