
Effect of histology on stereotactic body radiotherapy for non-small cell lung cancer oligometastatic pulmonary lesions
Highlight box
Key findings
• In this retrospective review of 147 oligometastatic and recurrent SBRT-treated, non-small cell lung cancer (NSCLC) pulmonary lesions from 83 patients, squamous cell carcinoma (SqCC) histology was associated with decreased overall survival (OS) and local recurrence (LR) compared to adenocarcinoma.
What is known and what is new?
• Current literature investigating the relationship between tumor histology and clinical outcomes for SBRT-treated pulmonary oligometastases has yielded mixed conclusions, and has mostly evaluated colorectal versus non-colorectal histology.
• Our data contributes to this emerging area of research and focuses specifically on the histologies of lung SqCC and adenocarcinoma.
What is the implication, and what should change now?
• This study suggests that, for SBRT-treated oligometastatic lung lesions, primary lung SqCC histology may be associated with worse clinical outcomes compared to primary lung adenocarcinoma.
• Further studies with larger sample sizes are necessary to better adjust for confounding variables and determine if these findings are reproducible.
Introduction
Metastatic spread of primary cancer continues to drive most cancer-related deaths (1,2). In the effort to improve oncologic outcomes for these patients, we have continued to refine the concept of oligometastatic disease: in carefully selected patients with a low-to-moderate burden of metastases, treating individual metastatic lesions locally can prolong survival (3-7). Radiotherapy, specifically stereotactic body radiotherapy (SBRT), has been shown to be an effective treatment modality of individual metastases in select patients across multiple disease sites (2-4,6-9). Randomized trials have published promising results with metastasis-directed radiotherapy, revealing the potential for safety, durable palliation of symptoms, and effective local control (LC) (10-13).
Since the lungs are one of the most common sites of metastatic spread, treatment of pulmonary oligometastatic lesions with SBRT has recently become a subject of considerable research scrutiny (14,15). In the search to risk-stratify pulmonary oligometastatic disease, several groups have studied tumor histology of individual pulmonary metastases as a possible predictor of clinical outcomes. Some studies, including one meta-analysis by Cao et al., revealed that colorectal adenocarcinoma histology was associated with significantly worse LC rates after SBRT compared to other histologies such as primary lung adenocarcinoma, head and neck squamous cell carcinoma (SqCC), and hepatocellular carcinoma (16,17). However, other retrospective studies and even clinical trials have found that histology is not associated with any difference in clinical outcome for SBRT-treated primary or metastatic lung tumors (3,18,19). We sought to clarify the mixed conclusions of the literature by retrospectively examining the effect of tumor histology on LC in a sample of SBRT-treated pulmonary lesions treated at our institution. As most groups examining histology in SBRT-treated metastases focused on colorectal versus non-colorectal metastases, our study was unique in that we examined only patients with non-small cell lung cancer (NSCLC). Specifically, we examined the clinical outcomes of SBRT-treated lesions of biopsy-proven lung adenocarcinoma and lung SqCC histology in patients with advanced stage disease. We believe that any information yielded by this investigation could prove useful in guiding patient selection for metastasis-directed therapy. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-538/rc).
Methods
Patient cohort
We performed a retrospective chart review of all patients’ pulmonary lesions of lung adenocarcinoma or SqCC histology treated with SBRT between January 2015 and December 2019 at our institution. SBRT was defined as any radiotherapy treatment delivered between 3–5 fractions using at least 5 Gy per fraction. We included patients who had oligometastatic disease, defined as fewer than five total metastases at distant sites including, but not limited to, contralateral lung, contralateral pulmonary lymph nodes, bones, and brain. We also included patients with previously treated, recurrent primary lung carcinoma in the lungs or regional lymph nodes who did not have distant metastases. Only patients who had received biopsies confirming lung primary adenocarcinoma or SqCC were included. Patients with locally advanced, early-stage lung cancer who received SBRT to single pulmonary nodules were excluded. Other inclusion criteria were as follows: maximum tumor diameter less than 7 cm, at least one clinical follow-up with imaging occurring at least 2 months after SBRT completion, and treatments delivered with the goal of achieving LC. Any patients who received radiation (RT) to lung metastases for symptom palliation alone were excluded.
Treatment planning, localization, and delivery
Patients were immobilized prior to simulation computed tomography (CT) scan using a vacuum-sealed body mold. Dose was prescribed to the internal tumor volume (ITV), which was calculated from the tumor range of motion visualized from multiple CT images taken at different points during the breathing cycle. SBRT was delivered using a 6 MV photon beam on a linear accelerator with a 2.5–4 mm width multi-leaf collimator. On-board cone-beam CT (CBCT) was used prior to each treatment session to confirm tumor alignment. A 6D robotic couch was employed to align the patient and localize the target to the planning CT. Treatments were delivered with once-weekly fractionation.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Riverside Regional Medical Center Institutional Review Board and individual consent for this retrospective analysis was waived.
Patient follow-up and endpoints
The primary endpoints of the study were local progression-free survival (LPFS) and cumulative incidence of local failure in each histology arm. The secondary endpoints were overall survival (OS) in each histology arm, as well as overall LC and toxicity incidence. Patients were followed by RT oncology or hematology-oncology. As the cohort arms included patients who were initially diagnosed at varied clinical stages, our primary endpoints focused specifically on LC, as this parameter would theoretically not be influenced by disease extent at the time of treatment. Clinical assessment and most recent imaging were documented at serial follow-up. Imaging follow-up with CT or positron emission tomography (PET) performed at approximately three-month intervals were used to determine LC. LC of a discrete lesion was defined as follows: no increase in lesion size, no enhancement on CT, and no hypermetabolic activity within the SBRT treatment volume seen on CT or PET serial imaging. Time to LC was defined as time from the end of SBRT treatment to the date of follow-up imaging that first showed local failure. For patients who never showed radiographic evidence of failure, LC was calculated from end of SBRT treatment to date of last follow-up. Any radiotherapy-induced toxicities were identified by reviewing clinic follow-up notes and were graded according to the Common Terminology Criteria for Adverse Events (CTCAE).
Statistical analysis
Two-sample t-test was used to compare means for all continuous variables. Two-sample proportions test and Chi-Square test were used for all categorial variables. Survival curves for LPFS and OS were generated using the Kaplan-Meier (KM) method starting from the day of treatment completion to either date of death, date of local failure seen on imaging, or date of most recent follow-up. Log-rank testing was used to identify any statistically significant differences in LPFS and OS. The effect of tumor histology on strictly LC was assessed using Cox proportional hazards regression analysis with death as a competing risk factor. This data was used to create cumulative incidence curves of local recurrence (LR) for each histology cohort. We also performed KM Analysis separately on subsets of lesions with synchronous and metachronous disease for LPFS and OS endpoints. Synchronous disease was defined as any lesion appearing within 6 months of any additional lesions. Metachronous disease referred to lesions presenting at least 6 months before or after additional lesion development. For LPFS KM analysis, patients who were alive and without LR at the end of the study period were censored. For OS KM analysis, only patients who were alive at the end of the study period were censored. We used Cox regression to perform univariate analysis on tumor histology and other variables. For multivariate analysis, we employed two-variable Cox regression multiple times with Histology and one other variable at a time in attempt to identify specific variable relationships. We performed KM analysis and Cox-regression analysis using lesion-based and patient-based cohorts. STATA statistical software (17.0 release STATA Corporation, College Station, TX, USA) was used for data analysis.
Results
In total, 147 pulmonary lesions from 83 patients were included: 95 treated lesions from 51 patients with lung adenocarcinoma primaries and 52 treated lesions from 32 patients with lung SqCC primaries (Table 1). Mean age at treatment was 74 years for adenocarcinoma and 71 for SqCC (P=0.046). Both histology arms had mostly female patients in roughly equal proportions. Higher percentages of the adenocarcinoma cohort had received prior surgical or systemic treatment compared to SqCC, and these differences were statistically significant only for prior immunotherapy and surgery. Approximately 51% of adenocarcinoma tumors and 62% of SqCC tumors received prior thoracic radiotherapy. In most of these cases, the lesions recurred outside of the prior radiotherapy treatment fields (75% and 66% out-of-field recurrence for adenocarcinoma and SqCC, respectively). Mean OS was 30.1 months for adenocarcinoma, which was greater than the SqCC mean OS of 20.3 to a statistically significant degree (P=0.006) (Table 1). The most frequently used dose-fractionation scheme was 50 Gy in 5 fractions for the entire sample, 50 Gy in 5 fractions for the adenocarcinoma arm, and 40 Gy in 5 fractions and 50 Gy in 4 fractions for the SqCC arm. Median BED was 80 [interquartile range (IQR): 38.4–112.5] Gy for the entire sample, 80 (IQR: 38.4–112.5) Gy for adenocarcinoma, and 72 (IQR: 38.4–100) Gy for SqCC. There was no statistically significant difference in mean BED between adenocarcinoma and SqCC (P=0.22) (Table 2). It is important to note that lower doses than standard were often employed as many patients had prior thoracic radiotherapy in nearby sites and some treatments were given solely to smaller-volume regional lymph node targets. Median follow-up time for the entire sample was 18 (IQR: 7–40) months. Median follow-up times for the adenocarcinoma and SqCC histologies were 22 (IQR: 8–42) and 12 (IQR: 5–39) months, respectively (Table 2).
Table 1
| Variables | Total | Lung adenocarcinoma | Lung SqCC | P value |
|---|---|---|---|---|
| No. of patients | 83 | 51 | 32 | – |
| Patients with comorbid COPD | 46 (55%) | 32 (63%) | 14 (44%) | 0.09 |
| Patients with >1 SBRT-treated lesion | 46 (55%) | 31 (61%) | 15 (47%) | 0.214 |
| Patients with 1 SBRT-treated lesion | 37 (45%) | 20 (39%) | 17 (53%) | |
| No. of lesions | 147 | 95 | 52 | – |
| Mean age, years | 73 | 74 | 71 | 0.046 |
| Median [IQR] | 75 [68–79] | 75 [70–79] | 74 [66–80] | |
| Female | 99 (67%) | 67 (71%) | 32 (62%) | 0.267 |
| Male | 48 (33%) | 28 (29%) | 20 (38%) | |
| Prior thoracic radiotherapy | 83 (56%) | 51 (54%) | 32 (62%) | 0.11 |
| In-field recurrences | 27 (33%) | 13 (25%) | 14 (44%) | 0.08 |
| Out of field recurrences | 56 (67%) | 38 (75%) | 18 (66%) | |
| Prior thoracic surgery | 52 (35%) | 40 (42%) | 12 (23%) | 0.02 |
| Prior chemotherapy | 78 (53%) | 55 (58%) | 23 (46%) | 0.17 |
| Prior immunotherapy | 33 (22%) | 27 (28%) | 6 (11%) | 0.02 |
| Post-SBRT chemotherapy | 31 (21%) | 15 (16%) | 16 (31%) | 0.03 |
| Post-SBRT immunotherapy | 52 (35%) | 38 (40%) | 14 (27%) | 0.11 |
| Mean No. of SBRT-treated lesions | 2.35 | 2.45 | 2.23 | 0.262 |
| Median [IQR] | 2 [1–3] | 2 [2–3] | 2 [1–3] | |
| Peripheral target | 78 (53%) | 52 (55%) | 27 (52%) | 0.74 |
| Central target | 69 (47%) | 43 (45%) | 25 (48%) | |
| Synchronous disease | 104 (70.7%) | 69 (73%) | 35 (67%) | 0.498 |
| Metachronous disease | 43 (15.6%) | 26 (27%) | 17 (33%) | |
| Mean time from primary Dx to SBRT start, months | 38.86 | 46.8 | 29.8 | 0.03 |
| Median [IQR] | 29 [14–52] | 31 [16–53] | 20 [7–41.25] | |
| Mean tumor length, cm | 2.2 | 2 | 2.5 | 0.02 |
| Median [IQR] | 1.8 [1.2–3] | 1.7 [1.2–2.5] | 2.2 [1.3–3.55] |
SqCC, squamous cell carcinoma; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; SBRT, stereotactic body radiotherapy; Dx, diagnosis.
Table 2
| Variables | Total | Lung adenocarcinoma | Lung squamous cell carcinoma | P value |
|---|---|---|---|---|
| Mean follow-up, months | 25.3 | 26.5 | 23.1 | 0.364 |
| Median [IQR] | 18 [7–40] | 22 [8–42] | 12 [5–39] | |
| Mean dose, Gy | 39 | 39.3 | 37.3 | 0.37 |
| Median [IQR] | 40 [24–50] | 40 [24–50] | 40 [24–50] | |
| Mean No. of fractions | 4.22 | 4.16 | 4.32 | 0.143 |
| Median [IQR] | 4 [4–5] | 4 [4–5] | 4 [4–5] | |
| Mean BED, Gy | 78 | 79.8 | 72.6 | 0.22 |
| Median [IQR] | 80 [38.4–112.5] | 80 [38.4–112.5] | 72 [38.4–100] | |
| Mean ITV, cm3 | 37.63 | 18.8 | 30.79 | 0.11 |
| Median [IQR] | 20.8 [ 12.1–53.3] | 18.4 [11.9–48.8] | 30.2 [13.9–62.3] | |
| Concurrent systemic therapy | 14 (9.5%) | 12 (13%) | 2 (3.8%) | 0.08 |
| 1-year LC | 87% (128/147) | 90.5% (86/95) | 80.7% (42/52) | 0.09 |
| 2-year LC | 85% (125/147) | 89% (85/95) | 77% (40/52) | 0.04 |
| Mean OS, months | 26.6 | 30.1 | 20.3 | 0.006 |
| Median [IQR] | 21 [9–42] | 24 [10–46] | 14.5 [7.5–33] | |
| Deceased | 96 (65%) | 60 (63%) | 36 (69%) | 0.55 |
| Alive | 52 (35%) | 35 (37%) | 16 (31%) | |
| Total G1–G2 toxicity events | 12 (8.2%) | 10 (10.5%) | 2 (3.8%) | 0.16 |
| Total G3 toxicity events | 0 (0%) | 0 (0%) | 0 (0%) | – |
IQR, interquartile range; BED, biologically effective dose; ITV, internal tumor volume; LC, local recurrence; OS, overall survival; G1–3, Grade 1–3.
Two-year LC for the entire cohort was 85%. Two-year LC was 89% for adenocarcinoma and 77% for SqCC (P=0.04) (Table 2). There was no statistically significant difference between groups in 1-year LC (P=0.09). KM survival analysis revealed that adenocarcinoma histology was associated with improved LPFS compared to SqCC (P=0.014) (Figure 1). Adenocarcinoma was also found to have improved OS compared to SqCC (P=0.04) by log rank test (Figure 2). Cox proportional hazards regression of LR with death as a competing risk factor was then performed. This analysis revealed an increased LR risk in the SqCC group compared to the adenocarcinoma group that approached statistical significance (P=0.061) (Table 3, Figure 3A).
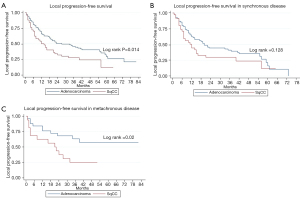
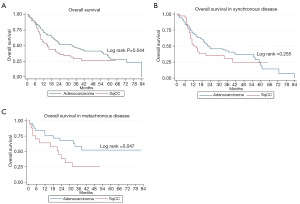
Table 3
| Cohort | SHR | SE | z | P>|z| | (95% CI) |
|---|---|---|---|---|---|
| Total sample | 2.174 | 0.899 | 1.88 | 0.061 | (0.9661–4.8937) |
| Synchronous disease | 3.029 | 1.387 | 2.42 | 0.016 | (1.234–7.436) |
| Metachronous disease | 0.827 | 1.01 | −0.15 | 0.877 | (0.075–9.132) |
SHR, subdistribution hazard ratio; SE, standard error; CI, confidence interval.
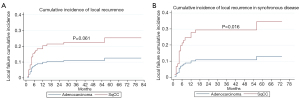
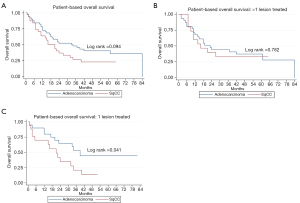
Multiple subset analyses were performed to identify any confounding factors affecting the relationship of histology to clinical outcomes. Lesion-based KM analyses of histology in separate subsets of synchronous and metachronous disease showed adenocarcinoma to have improved LPFS and OS compared to SqCC (Figures 1,2). However, this difference was statistically significant by log rank in only the metachronous subset for both LPFS and OS. SqCC was associated with a statistically significant LR incidence with death as a competing risk in the synchronous subset (SYN) (P=0.016) but not in the metachronous subset (Table 3, Figure 3B). Patient-based KM curves revealed improved OS for adenocarcinoma that was only statistically significant among patients with one SBRT-treated lung lesion, and not for those with more than one SBRT-treated lesion (Figure 4).
Lesion-based univariate regression showed increased risk of SqCC compared to adenocarcinoma in LPFS (P=0.014) and OS (P=0.046) (Table 4). Univariate Cox regression analysis revealed statistically significant associations of age, female gender, prior chemotherapy, prior surgery, concurrent systemic therapy, ITV, tumor length, peripheral tumor location, total RT dose, and time from primary diagnosis to SBRT with LC and survival (Table 4). SqCC continued to show a statistically significant increased risk of local failure when adjusted for these variables. Only prior thoracic surgery significantly diluted the effect of SqCC histology on LPFS.
Table 4
| Factors | LPFS | OS | |||
|---|---|---|---|---|---|
| HR | P | HR | P | ||
| SqCC vs. AdCA histology | 1.68 | 0.014 | 1.537 | 0.046 | |
| Age | 0.97 | 0.029 | 0.973 | 0.024 | |
| Female vs. male gender | 0.64 | 0.040 | 0.633 | 0.037 | |
| Prior RT | 1.482 | 0.095 | 1.461 | 0.109 | |
| Prior immunotherapy | 1.532 | 0.072 | 1.697 | 0.027 | |
| Prior Chemo | 1.519 | 0.044 | 1.616 | 0.022 | |
| Prior surgery | 0.345 | <0.001 | 0.423 | <0.001 | |
| Concurrent systemic therapy | 2.071 | 0.020 | 2.01 | 0.025 | |
| Post-SBRT Chemo | 1.285 | 0.295 | 1.242 | 0.368 | |
| Post-SBRT immunotherapy | 1.22 | 0.345 | 1.357 | 0.149 | |
| ITV | 1.01 | 0.003 | 1.007 | 0.006 | |
| BED | 0.99 | 0.001 | 0.99 | 0.002 | |
| Tumor length1 | 1.25 | 0.004 | 1.24 | 0.006 | |
| Number of SBRT-treated lesions | 1.103 | 0.248 | 1.032 | 0.704 | |
| Peripheral vs. central tumor location | 0.615 | 0.018 | 0.532 | 0.002 | |
| Total dose | 0.98 | 0.002 | 0.976 | 0.002 | |
| Time from primary diagnosis to SBRT | 0.989 | 0.001 | 0.99 | 0.003 | |
| Metachronous vs. synchronous disease | 0.58 | 0.025 | 0.691 | 0.125 | |
1, longest dimension of tumor. LPFS, local progression-free survival; OS, overall survival; HR, hazard ratio; SqCC, squamous cell carcinoma; AdCA, adenocarcinoma; RT, radiation; SBRT, stereotactic body radiotherapy; ITV, internal tumor volume; BED, biologically effective dose.
The effect of SqCC histology on OS was weakened when adjusted for most of these variables except for prior chemotherapy and prior immunotherapy (Tables 4,5). Notably, prior RT, post-SBRT chemo, post-SBRT immunotherapy, and metachronous disease (compared to synchronous disease) did not affect the increased risk of SqCC with death (Tables 4,5). Patient-based regression analysis revealed an increased risk of SqCC with death that was not statistically significant (P=0.1). Prior surgery, greater BED, greater total RT dose, and peripheral tumor location were associated with a decreased risk of death and ITV was associated with a statistically significant but miniscule increased risk of death (Table 6).
Table 5
| Factors | LPFS | OS | |||
|---|---|---|---|---|---|
| HR | P | HR | P | ||
| Age | 0.98 | 0.108 | 0.977 | 0.063 | |
| SqCC vs. AdCA histology | 1.55 | 0.045 | 1.419 | 0.114 | |
| Female vs. male gender | 0.68 | 0.083 | 0.67 | 0.066 | |
| SqCC vs. AdCA histology | 1.60 | 0.028 | 1.46 | 0.081 | |
| Prior Chemo | 1.60 | 0.023 | 1.72 | 0.011 | |
| SqCC vs. AdCA histology | 1.77 | 0.007 | 1.66 | 0.02 | |
| Prior immunotherapy | 1.76 | 0.020 | 1.935 | 0.007 | |
| SqCC vs. AdCA histology | 1.852 | 0.005 | 1.732 | 0.013 | |
| Prior surgery | 0.36 | <0.001 | 0.441 | 0.001 | |
| SqCC vs. AdCA histology | 1.49 | 0.063 | 1.386 | 0.133 | |
| ITV | 1.01 | 0.003 | 1.01 | 0.007 | |
| SqCC vs. AdCA histology | 1.61 | 0.030 | 1.42 | 0.116 | |
| BED | 0.99 | 0.002 | 0.991 | 0.003 | |
| SqCC vs. AdCA histology | 1.58 | 0.030 | 1.43 | 0.099 | |
| Tumor length | 1.24 | 0.007 | 1.22 | 0.011 | |
| SqCC vs. AdCA histology | 1.61 | 0.025 | 1.44 | 0.093 | |
| Total dose | 0.98 | 0.003 | 0.977 | 0.004 | |
| SqCC vs. AdCA histology | 1.62 | 0.023 | 1.465 | 0.078 | |
| Peripheral vs central tumor location | 0.621 | 0.020 | 0.538 | 0.003 | |
| SqCC vs. AdCA histology | 1.66 | 0.016 | 1.51 | 0.056 | |
| Metachronous vs. synchronous disease | 0.56 | 0.021 | – | – | |
| SqCC vs. AdCA histology | 1.70 | 0.012 | – | – | |
LPFS, local progression-free survival; OS, overall survival; HR, hazard ratio; SqCC, squamous cell carcinoma; AdCA, adenocarcinoma; ITV, internal tumor volume; BED, biologically effective dose.
Table 6
| Factors | OS | |
|---|---|---|
| HR | P | |
| Univariate analysis | ||
| SqCC vs. AdCA histology | 1.582 | 0.1 |
| Age | 0.98 | 0.204 |
| Female vs. male gender | 0.746 | 0.308 |
| Prior immunotherapy | 1.45 | 0.250 |
| Prior Chemo | 1.528 | 0.140 |
| Prior surgery | 0.516 | 0.031 |
| Concurrent systemic therapy | 1.76 | 0.196 |
| ITV | 1.01 | 0.041 |
| BED | 0.99 | 0.009 |
| Tumor length1 | 1.36 | 0.006 |
| >1 vs. 1 SBRT-treated lesion | 1.06 | 0.831 |
| Peripheral vs. central tumor | 0.546 | 0.030 |
| Total dose | 0.975 | 0.012 |
| Time from primary Dx to SBRT | 0.992 | 0.059 |
| Metachronous vs. synchronous | 0.817 | 0.470 |
| Multivariate analysis | ||
| Prior surgery | 0.564 | 0.075 |
| SqCC vs. AdCA histology | 1.327 | 0.334 |
| ITV | 1.007 | 0.049 |
| SqCC vs. AdCA histology | 1.537 | 0.135 |
| BED | 0.991 | 0.020 |
| SqCC vs. AdCA histology | 1.371 | 0.267 |
| Tumor length | 1.32 | 0.017 |
| SqCC vs. AdCA histology | 1.365 | 0.281 |
| Total dose | 0.977 | 0.024 |
| SqCC vs. AdCA histology | 1.408 | 0.227 |
| Peripheral vs. central tumor | 0.511 | 0.016 |
| SqCC vs. AdCA histology | 1.728 | 0.051 |
1, longest dimension of tumor. OS, overall survival; HR, hazard ratio; SqCC, squamous cell carcinoma; AdCA, adenocarcinoma; ITV, internal tumor volume; BED, biologically effective dose; SBRT, stereotactic body radiotherapy; Dx, diagnosis.
Overall toxicity incidence was minimal at 8.2% and all toxicities were grades 1 and 2. There were 12 total post-treatment toxicity events. The most common toxicity was cough (new or worsened from baseline), of which there were 5 cases (3.4%). There were 3 cases of pneumonitis (2%) based on clinical and radiographic evidence. There were also 2 cases of lateral rib pain (1.4%) and one case of nausea (0.7%). All toxicity cases were mild and resolved within one month of treatment completion. There were no toxicity events of CTCAE stage 3 or greater.
Discussion
The results of our study add to the growing body of evidence suggesting that SBRT is a safe and effective modality to treat metastatic lung disease, even in the case of reirradiation (2-4,14-20). Our study showed an overall 2-year LC rate of 85%, which is consistent with most of the published 2-year, post-SBRT LC rates that range from 66–94% (16-22). Our toxicity rate was 8.2% and consisted of mild, transient cases of cough, pneumonitis, fatigue, nausea, and lateral rib pain. These findings reflect the SBRT literature, much of which has shown that SBRT to pulmonary metastases is associated with a low incidence of mostly mild and transient toxicities (2-4,14-24).
The primary aim of our study was to investigate the role of tumor histology in clinical outcomes of recurrent and metastatic pulmonary lesions treated with SBRT. There is evidence suggesting that for early-stage NSCLC, SqCC histology is associated with worse clinical outcomes after SBRT compared to adenocarcinoma (25,26). The biological mechanisms for this difference are unclear. Some have hypothesized that since SqCC usually develops in areas of chronic inflammation-induced dysplasia, patients with SqCC histology are more likely to have locoregional disease recurrence (26). This research of histology in SBRT-treated early-stage NSCLC led to the examination of histology’s role in SBRT-treated metastatic lesions. However, studies in this area have thus far yielded many conflicting results. Retrospective studies performed by Cao et al. and Takeda et al. both found SBRT-treated pulmonary metastases of colorectal histology to be associated with worse LC compared to those of non-colorectal histology (16,17). Zhang et al. performed a retrospective analysis which consisted mostly of primary lung, colorectal, and head and neck tumors, and found no statistically significant effect of tumor location or histology on LC (3) These results were echoed by several other retrospective studies and even clinical trials such as the SAFRON phase II trial (18-20,22,27,28). Most of these groups divided their cohorts by histology irrespective of primary site, or by primary site alone (3,18-20,28). Our study was unique in that we specifically evaluated pulmonary lung lesions that were biopsy-proven to be lung adenocarcinoma or SqCC. By limiting our analysis to NSCLC histology, our investigation focused on an emerging area of study in treating pulmonary oligometastatic disease. Our results revealed adenocarcinoma was associated with increased OS and 2-year LC compared to SqCC. Additionally, KM survival analysis showed a statistically significant improvement in LPFS of adenocarcinoma compared to SqCC. There were relatively few overall instances of local failure compared to deaths within the study period. We thus performed Cox proportional hazard regression analysis and used this information to plot cumulative incidence curves of local failure to more reliably assess LC alone. This analysis revealed an increased risk of LR in the SqCC group that trended towards statistical significance.
Since our sample was comprised of patients with a wide spectrum of disease burden at time of treatment, it was possible that this heterogeneity was responsible for the increased risk of poor clinical outcomes with SqCC. We thus performed subset analysis of synchronous versus metachronous disease. KM analyses in all subsets showed increased risk of SqCC compared to adenocarcinoma in both OS and LPFS, but this risk was only statistically significant in metachronous disease. Similarly, for patient-specific OS analysis, the poorer OS of SqCC compared to adenocarcinoma was statistically significant in patients with only one lesion treated with SBRT, but not in those with more than one SBRT-treated lesion.
The defining feature of synchronous disease is more rapid development of new lesions, leading to the co-existence of multiple tumors. Synchronous disease is thus associated with a higher burden of gross, and presumably microscopic, disease throughout the body. Therefore, a possible interpretation of these results is that pulmonary SqCC is associated with poorer treatment outcomes compared to adenocarcinoma, but that this difference becomes negligible beyond a certain threshold of total-body disease burden. This interpretation is further validated by our findings that the histology-clinical outcome relationship appears stronger for lesion-specific outcomes than for OS outcomes. LR incidence with death as a competing risk factor was our most reliable method to strictly evaluate LC at the level of individual tumors. For the total cohort, there was a robust increased LR hazard for SqCC compared to adenocarcinoma of 2.174 that approached statistical significance (P=0.061), which was even more pronounced in the SYN (SYN =3.03, P=0.016). While the metachronous subset in this analysis did not echo these findings, this was likely a result of inadequate sample size (metachronous n=42) and a lack of total local failure events in this subset, preventing us from drawing any reliable conclusions. In the lesion-specific multivariate analysis for the more lesion-specific outcome of LPFS, the relationship of SqCC histology to LPFS was unaffected after adjusting for most variables. Adjustment for prior surgery made the difference in LPFS by histology not statistically significant, which could be explained by the likelihood that patients who were able to undergo prior surgery had better performance status and less advanced disease at presentation. However, when multivariate analysis was performed for OS, an outcome intuitively more related to degree of total-body disease, the increased hazard of SqCC histology diminished after adjusting for other variables. The effect of SqCC histology on OS was even further weakened when analyses were repeated at the level of the patient instead of at the level each lesion.
Overall, our results suggest that, when compared to lung adenocarcinoma, SBRT-treated metastatic SqCC lesions have potentially reduced LC rates, as in early-stage NSCLC. Nonetheless, LC was high (over 80% at 2 years) for both histologies. Our findings align with clinical experience that pulmonary SqCC is associated with worse LC compared to pulmonary adenocarcinoma (29). Additionally, our results suggest that poorer clinical outcomes of SqCC histology compared to adenocarcinoma are more specific to individual tumor-based parameters like LC, and that the discrepancy in histology-based treatment response is more clinically meaningful in patients with lower overall lesion burden at time of treatment. It is possible that, oligometastatic or locally recurrent lung SqCC is more radioresistant than adenocarcinoma at the single-lesion level, leading to a discrepancy in post-SBRT performance for the two groups that is more pronounced with decreased total-body tumor load.
Our study is limited by a relatively small sample size and short follow-up time. Though the cohort considers a heterogenous group of patients, many studies of metastasis-directed radiotherapy have also included such diverse groups (3,4,18-20). Studies with larger sample sizes and more equal proportions of synchronous and metachronous disease are warranted to assess whether our findings of potentially reduced LC of SqCC are reproducible. Additionally, further research is warranted to specifically compare clinical outcomes of SBRT-treated advanced stage NSCLC with pulmonary metastases from other non-lung primaries. We also believe that evaluating SBRT fractionation schedules in treating pulmonary metastases is a pressing future direction for this research. At our center, we regular employ an unconventional SBRT schedule of one treatment per week. While there is scant literature investigating fractionation in SBRT-treatment of pulmonary metastatic lesions, we have found through clinical experience that most other centers deliver SBRT over shorter periods with treatments delivered daily or every other day. It is possible that our unique fractionation paradigm is responsible for the observed findings, and future studies should incorporate data from multiple centers to adjust for the effect of fractionation schedules on biologically effective dose and clinical outcomes.
We hope that our reporting of histology as a possible predictor of LC in SBRT-treated primary lung adenocarcinoma and SqCC pulmonary metastases, may serve as a risk stratification tool and guide to appropriate patient-selection in the treatment of pulmonary oligometastatic disease with SBRT.
Conclusions
This study suggests that, for SBRT-treated oligometastatic lung lesions, primary lung SqCC histology may be associated with worse clinical outcomes compared to primary lung adenocarcinoma. It is also possible that this discrepancy is more pronounced in localized disease and diminishes beyond a certain threshold of total-body disease burden.
Acknowledgments
We wish to thank Nathaniel Minnick, Krista Booth, and Trip Smith for their assistance. Without their logistical support and instruction, this endeavor would not have been possible.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-538/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-538/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-538/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-538/coif). RO reports that his spouse is employed by GE healthcare. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Riverside Regional Medical Center Institutional Review Board and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Dillekås H, Rogers MS, Straume O. Are 90% of deaths from cancer caused by metastases? Cancer Med 2019;8:5574-6. [Crossref] [PubMed]
- Chmura SJ, Salama JK, Weichselbaum RR. Stereotactic radiotherapy for pulmonary metastases. Semin Thorac Cardiovasc Surg 2013;25:292-9. [Crossref] [PubMed]
- Zhang Y, Xiao JP, Zhang HZ, et al. Stereotactic body radiation therapy favors long-term overall survival in patients with lung metastases: five-year experience of a single-institution. Chin Med J (Engl) 2011;124:4132-7. [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol 2020;38:2830-8. [Crossref] [PubMed]
- Ruers T, Punt C, Van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619-26. [Crossref] [PubMed]
- Gomez DR, Tang C, Zhang J, et al. Local Consolidative Therapy Vs. Maintenance Therapy or Observation for Patients With Oligometastatic Non-Small-Cell Lung Cancer: Long-Term Results of a Multi-Institutional, Phase II, Randomized Study. J Clin Oncol 2019;37:1558-65. [Crossref] [PubMed]
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018;36:446-53. [Crossref] [PubMed]
- Kowalchuk RO, Waters MR, Richardson KM, et al. Stereotactic body radiation therapy in the treatment of ovarian cancer. Radiat Oncol 2020;15:108. [Crossref] [PubMed]
- Kowalchuk RO, Waters MR, Dutta SW, et al. Recursive Partitioning Analysis for Local Control Achieved With Stereotactic Body Radiation Therapy for the Liver, Spine, or Lymph Nodes. Adv Radiat Oncol 2021;6:100612. [Crossref] [PubMed]
- Sahgal A, Atenafu EG, Chao S, et al. Vertebral compression fracture after spine stereotactic body radiotherapy: a multi-institutional analysis with a focus on radiation dose and the spinal instability neoplastic score. J Clin Oncol 2013;31:3426-31. [Crossref] [PubMed]
- Kowalchuk RO, Johnson-Tesch BA, Marion JT, et al. Development and Assessment of a Predictive Score for Vertebral Compression Fracture After Stereotactic Body Radiation Therapy for Spinal Metastases. JAMA Oncol 2022;8:412-9. [Crossref] [PubMed]
- Nguyen QN, Chun SG, Chow E, et al. Single-Fraction Stereotactic vs Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol 2019;5:872-8. [Crossref] [PubMed]
- Sahgal A, Myrehaug SD, Siva S, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol 2021;22:1023-33. [Crossref] [PubMed]
- Kowalchuk RO, Waters MR, Richardson M, et al. Low-dose hilar and mediastinal stereotactic body radiation therapy for non-small cell lung cancer: Analysis of outcomes in patients receiving one or multiple courses of treatment. Thorac Cancer 2020;11:2005-13. [Crossref] [PubMed]
- Kowalchuk RO, Waters MR, Richardson KM, et al. Stereotactic Body Radiation Therapy for Salvage Treatment of Recurrent Non-Small Cell Lung Cancer. Pract Radiat Oncol 2020;10:e475-e484. [Crossref] [PubMed]
- Cao C, Wang D, Tian DH, et al. A systematic review and meta-analysis of stereotactic body radiation therapy for colorectal pulmonary metastases. J Thorac Dis 2019;11:5187-98. [Crossref] [PubMed]
- Takeda A, Kunieda E, Ohashi T, et al. Stereotactic body radiotherapy (SBRT) for oligometastatic lung tumors from colorectal cancer and other primary cancers in comparison with primary lung cancer. Radiother Oncol 2011;101:255-9. [Crossref] [PubMed]
- McCammon R, Schefter TE, Gaspar LE, et al. Observation of a dose-control relationship for lung and liver tumors after stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 2009;73:112-8. [Crossref] [PubMed]
- Siva S, Bressel M, Mai T, et al. Single-Fraction vs Multifraction Stereotactic Ablative Body Radiotherapy for Pulmonary Oligometastases (SAFRON II): The Trans Tasman Radiation Oncology Group 13.01 Phase 2 Randomized Clinical Trial. JAMA Oncol 2021;7:1476-85. [Crossref] [PubMed]
- Wang Z, Kong QT, Li J, et al. Clinical outcomes of cyberknife stereotactic radiosurgery for lung metastases. J Thorac Dis 2015;7:407-12. [PubMed]
- Hoerner-Rieber J, Duma M, Blanck O, et al. Stereotactic body radiotherapy (SBRT) for pulmonary metastases from renal cell carcinoma-a multicenter analysis of the German working group "Stereotactic Radiotherapy". J Thorac Dis 2017;9:4512-22. [Crossref] [PubMed]
- Antonoff MB, Sofocleous CT, Callstrom MR, et al. The roles of surgery, stereotactic radiation, and ablation for treatment of pulmonary metastases. J Thorac Cardiovasc Surg 2022;163:495-502. [Crossref] [PubMed]
- Okunieff P, Petersen AL, Philip A, et al. Stereotactic Body Radiation Therapy (SBRT) for lung metastases. Acta Oncol 2006;45:808-17. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol 2009;27:1579-84. [Crossref] [PubMed]
- Hörner-Rieber J, Bernhardt D, Dern J, et al. Histology of non-small cell lung cancer predicts the response to stereotactic body radiotherapy. Radiother Oncol 2017;125:317-24. [Crossref] [PubMed]
- Abel S, Hasan S, White R, et al. Stereotactic ablative radiotherapy (SABR) in early stage non-small cell lung cancer: Comparing survival outcomes in adenocarcinoma and squamous cell carcinoma. Lung Cancer 2019;128:127-33. [Crossref] [PubMed]
- Ricardi U, Filippi AR, Guarneri A, et al. Stereotactic body radiation therapy for lung metastases. Lung Cancer 2012;75:77-81. [Crossref] [PubMed]
- Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol 2005;77:83-7. [Crossref] [PubMed]
- McAleese J, Taylor A, Walls GM, et al. Differential Relapse Patterns for Non-small Cell Lung Cancer Subtypes Adenocarcinoma and Squamous Cell Carcinoma: Implications for Radiation Oncology. Clin Oncol (R Coll Radiol) 2019;31:711-9. [Crossref] [PubMed]


