
Efficacy and safety of gemcitabine and capecitabine combination for patients with previously treated advanced primary pulmonary lymphoepithelioma-like carcinoma: a retrospective single-arm cohort study
Highlight box
Key findings
• A total of 16 patients with pretreated primary pulmonary lymphoepithelioma-like carcinoma (PLELC) were included in this study. The objective response rate and disease control rate were 50.00% and 87.50%, respectively. The median progression-free survival and overall survival were 9.3 and 41.5 months, respectively.
What is known and what is new?
• Reported studies about salvage treatment for pretreated PLELC are limited due to its rarity. Only a few case reports for previously treated PLELC have been documented. Positive interactions between gemcitabine and capecitabine have been reported in preclinical studies, and the clinical benefits of their combination have been reported for other malignancies.
• We explored the activity and safety of gemcitabine/capecitabine combination in pretreated PLELC, which could provide a novel treatment option for this unique cancer.
What is the implication, and what should change now?
• This study demonstrated the potential clinical benefit of gemcitabine plus capecitabine for pretreated PLELC. Further multicenter prospective studies are worthwhile.
Introduction
Primary pulmonary lymphoepithelioma-like carcinoma (PLELC) is a rare and unique pathological subtype of non-small cell lung cancer (NSCLC). In Asia, the incidence of PLELC is approximately 0.9% of NSCLC cases (1,2). Studies have reported on PLELC in Guangdong Province (3), Hong Kong (1), and Taiwan (4), but only a few cases have been described in reports from Western countries (5). PLELC tends to occur in younger, nonsmoking females (6). Most patients have early-stage or locally advanced disease at their first diagnosis, and radical resection is critical for resectable PLELC (7). PLELC was first reported in 1987 by Bégin et al., who described it as an Epstein-Barr virus (EBV)-associated epithelial neoplasm (8). In 2015, the World Health Organization (WHO) categorized PLELC as a subtype of other and unclassified carcinomas of lung cancer (9). The close relationship between PLELC with EBV infection has been well documented (7). Wang et al. reported that 42 out of 42 patients were positive for p63 and 34 out of 34 patients were negative for thyroid transcription factor-1 (TTF-1), indicating that PLELC may be similar to squamous cell carcinoma (10). Furthermore, PLELC presents histologically under an optical microscope as an undifferentiated carcinoma with lymphocytic infiltration, similar to an undifferentiated nasopharyngeal carcinoma (NPC) (11). In terms of the genetic characteristics of PLELC, it resembles NPC rather than other lung carcinomas (3).
Due to the low incidence of PLELC, evidence-based treatment guidelines generated from prospective clinical trials are scarce. Currently, the treatment strategy for PLELC follows the treatment guidelines for NSCLC. Chemotherapy plays an essential role in the treatment of unresectable PLELC owing to the low incidence of classic lung cancer driver gene mutations such as EGFR mutation and ALK rearrangement (10). In a panel consisting of 520 cancer-associated genes, Xie et al. found that the classic driver gene mutations of lung cancer were not detected, except for the mutation of KRAS and amplification of ERBB2 in 2 patients (12). Platinum-based doublets combined with immune checkpoint inhibitors (ICPIs) are recommended as frontline therapy. Lin et al. reported the results for patients with advanced PLELC treated with platinum-based chemotherapy regimens as first-line chemotherapy (13). The objective response rate was 32.3% with median progression-free survival (PFS) and overall survival (OS) of 7.7 and 36.7 months, respectively (13). The efficacy of platinum-based doublets plus ICPIs as first-line treatment for PLELC remains unclear. Although some patients with PLELC respond to primary treatment, recurrence in those patients is common (14). There are few studies of salvage systemic treatment and only a few case reports of capecitabine (CAP) and ICPIs such as nivolumab and pembrolizumab for pretreated PLELC (15-17). The exploration of second-line and beyond therapeutic strategy for PLELC will help to provide more treatment options and evidence for this unique cancer.
Gemcitabine (GEM) has been found to be an active agent in squamous cell carcinoma of the lung and also NPC (3,18). CAP essentially acts as a prodrug of 5-fluorouracil (5-FU), which is the rationale for its role as an alternative to 5-FU to treat NPC (19,20). Ho et al. reported that CAP had promising activity and good tolerability as salvage treatment in 5 patients (15). Among the 5 patients, there were 2 with stable disease (SD) and 1 with partial response (PR). Only 1 patient had moderately severe hand-foot syndrome, and another patient had grade 2 neutropenia (15). The combination of gemcitabine plus capecitabine (GEM/CAP) has shown a synergistic antitumor effect in preclinical studies (21). Additionally, the active efficacy and favorable toxicity profile of GEM/CAP have been reported in various studies, especially for pancreatic adenocarcinoma, biliary tract carcinoma, and thymic epithelial tumors (22-26). These findings provide support for the utility of GEM in combination with CAP. However, the effect and safety of GEM/CAP for pretreated advanced PLELC have not been reported.
Given the rationality of the GEM/CAP combination and the clinical need for effective therapeutic strategies for patients with previously treated PLELC, we conducted this retrospective study to examine the activity and safety of GEM/CAP combination in pretreated advanced PLELC. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-256/rc).
Methods
Study population
In this retrospective, single-arm cohort study, patients with PLELC at Sun Yat-sen University Cancer Center between May 2013 and January 2021 were identified. Eligible patients included: (I) histologically diagnosed with PLELC; (II) progressive disease (PD) after at least 1 prior systemic therapy; (III) treated with GEM/CAP regimen as second-line therapy or beyond; (IV) having at least 1 assessable lesion; (V) Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–2; (VI) life expectancy of at least 3 months; and (VII) adequate bone marrow, liver, and kidney functions. Patients who had a history of NPC or were pregnant or lactating were excluded.
Diagnosis of PLELC was performed in accordance with the criteria described by the WHO classification (9). As we previously reported (7), undifferentiated carcinomas without lymphoid infiltrates and EBV-encoded RNA (EBER) staining were excluded in our study. Endoscopic examination of the nasopharynx or positron emission tomography (PET)-computed tomography (CT) scan was conducted to rule out lung metastases of NPC. All cases were restaged based on the 8th edition American Joint Committee on Cancer (AJCC) staging system [the 8th edition of the tumor-node-metastasis (TNM) classification for lung cancer] (27). Clinical and pathological characteristics, including age, gender, smoking status, ECOG PS, history of surgical operation, history of radiotherapy, prior systemic therapeutic regimens, distant metastatic sites, and status of driven gene mutation (EGFR mutation, ALK rearrangement, and ROS1 rearrangement), were collected from retrospective chart review and medical history. For explorative purposes, plasma levels of EBV DNA determined by quantitative real-time polymerase chain reaction (RT-PCR) were monitored in 3 patients before, during, and after treatment with GEM/CAP, based on the oncologist’s choice. Measurement of EBV DNA was performed along with tumor response evaluation. Patients who had smoked fewer than 100 cigarettes in their lifetime were defined as never-smokers. Patients were followed up through electronic medical records and telephone.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Sun Yat-sen University Cancer Center Research Ethics Board (No. B2020-404-01). Written informed consent was provided before data collection.
Treatment methods
Patients were treated with intravenous infusion GEM (1,000 mg/m2 on days 1 and 8) and oral CAP (1,000 mg/m2 twice daily on days 1–14) every 3 weeks. CAP could be administrated as maintenance therapy after 4 to 6 cycles of GEM/CAP, based on the decision of the physician and patient. Chest and upper abdominal CT, in addition to brain magnetic resonance imaging (MRI) if brain metastasis existed, were performed every 2 cycles. Tumor response was assessed by investigators in accordance with Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST 1.1) (28). Adverse events (AEs) were retrospectively collected from the chart and medical history and classified in accordance with Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0 (29).
Statistical analyses
All statistical analyses were performed by Statistical Product and Service Solutions (SPSS) software, version 25 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 8 software (GraphPad Software Inc., San Diego, CA, USA). Descriptive analyses of the clinical characteristics of the patients enrolled in this study were conducted. PFS was measured from the date of the first GEM/CAP administration until either the first documented PD or death, whichever occurred earlier. OS was calculated from the date of the first GEM/CAP administration to the date of death due to any cause or censoring at the date of data cutoff (Feb 3rd, 2021). Survival functions were estimated by the Kaplan-Meier method. Univariate analysis was performed by log-rank test. Two-sided significance level was defined as P<0.05.
Results
Demography and disease characteristics
A total of 16 PLELC patients received GEM/CAP as salvage chemotherapy at Sun Yat-sen University Cancer Center between May 2013 and January 2021. Characteristics of the patients are shown in Tables 1,2. Eight patients (50.00%) were male. The median age was 45.5 years (range, 35.0–65.0 years). Thirteen patients (81.25%) were stage IV, and 3 patients (18.75%) had recurrence after surgical treatment or definitive radiotherapy. There were 7, 7, 4, and 0 patients with pleura, liver, bone, and brain metastases, respectively. All patients had an ECOG PS of 0–1 (0, 56.25%; 1, 43.75%). Most patients (62.50%) had no history of smoking. Platinum-based therapy was the first-line treatment in all 16 patients, including pemetrexed, docetaxel, vinorelbine, or paclitaxel combined with platinum. Five of 16 (31.25%) patients had experienced 1 prior systemic chemotherapy regimen, 4 (25.00%) had received 2 prior regimens, 3 (18.75%) had received 4 prior regimens, and the remaining (25.00%) patients had 3 prior treatment regimens. Thirteen patients were tested for EGFR mutation, while the other 3 patients were not tested due to lack of tumor tissues, and only 1 patient was positive with EGFR exon20 insertion (Table 1). The rearrangements of ALK and ROS1 were wild type in 8 of 16 and 6 of 16 patients, respectively (Table 1).
Table 1
| Variable | Data |
|---|---|
| Age (years) | |
| Median | 45.5 |
| Range | 35.0–65.0 |
| Gender, n (%) | |
| Male | 8 (50.00) |
| Female | 8 (50.00) |
| ECOG PS, n (%) | |
| 0 | 9 (56.25) |
| 1 | 7 (43.75) |
| History of smoking, n (%) | |
| Yes | 6 (37.50) |
| No | 10 (62.50) |
| Pleura metastases, n (%) | |
| Yes | 7 (43.75) |
| No | 9 (56.25) |
| Liver metastases, n (%) | |
| Yes | 7 (43.75) |
| No | 9 (56.25) |
| Bone metastases, n (%) | |
| Yes | 4 (25.00) |
| No | 12 (75.00) |
| Brain metastases, n (%) | |
| Yes | 0 |
| No | 16 (100.00) |
| EGFR mutation, n (%) | |
| Positive | 1 (6.25) |
| Negative | 12 (75.00) |
| Untested | 3 (18.75) |
| ALK rearrangement, n (%) | |
| Positive | 0 |
| Negative | 8 (50.00) |
| Untested | 8 (50.00) |
| ROS1 rearrangement, n (%) | |
| Positive | 0 |
| Negative | 6 (37.50) |
| Untested | 10 (62.50) |
| No. of prior chemotherapy, n (%) | |
| 1 | 5 (31.25) |
| 2 | 4 (25.00) |
| 3 | 4 (25.00) |
| 4 | 3 (18.75) |
| Cycles of GEM/CAP combination | |
| Median | 5.5 |
| Range | 2.0–12.0 |
| Cycles of CAP maintenance | |
| Median | 6 |
| Range | 1–35 |
ECOG, Eastern Cooperative Oncology Group; PS, performance status; No., number; GEM, gemcitabine; CAP, capecitabine.
Table 2
| Pt No. | Sex | Age (years) | Prior chemotherapy regimen | Disease extension | Line of GEM/CAP therapy | No. of cycles | Best response | CAP maintenance | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 46 | PEM/DDP/BEV, TAX/BEV | Pleura; abdominal, cervical, thoracic, supraclavicular LNs | 3 | 2 | PD | No | 4.0 | 17.3 |
| 2 | M | 65 | DOC/NDP, GEM/NDP, TAX/NDP | Liver; lung; pleura; abdominal, thoracic, supraclavicular LNs | 4 | 6 | SD | Yes | 8.8 | 9.2+ |
| 3 | M | 62 | PEM/DDP, DOC/BEV, icotinib | Liver; lung; pleura; bone; thoracic, supraclavicular LNs | 4 | 12 | PR | No | 16.7 | 41.5 |
| 4 | F | 51 | VIN/DDP, TAX/DDP | Thoracic LNs | 3 | 4 | SD | No | 11.5 | 33.4+ |
| 5 | F | 52 | PEM/DDP, TAX/CBP | Pleura; lung; supraclavicular, thoracic LNs | 3 | 8 | PR | No | 9.3 | 17.9 |
| 6 | M | 39 | PEM/DDP, DOC/BEV | Thoracic LNs | 3 | 4 | SD | No | 4.6+ | 73.5+ |
| 7 | F | 51 | TAX/DDP, DOC/GEM, clinical trial | Pleura; lung; supraclavicular, thoracic LNs | 4 | 4 | SD | No | 6.9 | 27.7+ |
| 8 | F | 45 | TAX/DDP, TAX/DDP, PEM/CBP/BEV | Liver; lung; pleura; bone; abdominal, thoracic, supraclavicular LNs | 4 | 9 | PR | No | 8.8 | 26.6+ |
| 9 | F | 48 | TAX/NDP | Lung; abdominal, thoracic, supraclavicular LNs | 2 | 4 | PR | Yes | 18.3 | 52.9+ |
| 10 | M | 43 | TAX/DDP, PEM, DOC/GEM, TAX/CAP | Thoracic LNs | 5 | 5 | SD | No | 21.5 | 33.9+ |
| 11 | F | 38 | TAX/NDP | Liver; bone; abdominal, thoracic, supraclavicular LNs | 2 | 6 | PR | Yes | 6.4 | 19.6 |
| 12 | M | 43 | TAX/NDP, GEM/DDP, tegafur, etoposide | Liver; thoracic, supraclavicular LNs | 5 | 4 | PR | Yes | 6.6 | 16.0 |
| 13 | M | 56 | TAX/DDP | Abdominal, thoracic LNs | 2 | 6 | PR | Yes | 29.3+ | 29.3+ |
| 14 | M | 37 | PEM/DDP/BEV, TAX/CBP/BEV, DOC/BEV, PEM/CBP/BEV | Liver; lung; pleura; thoracic LNs | 5 | 3 | PD | No | 2.0 | 4.1 |
| 15 | F | 35 | PEM/NDP | Thoracic LNs | 2 | 6 | SD | Yes | 20.7+ | 20.7+ |
| 16 | F | 45 | TAX/CBP | Liver; spleen; bone; abdominal, thoracic, supraclavicular LNs | 2 | 6 | PR | Yes | 11.0 | 21.6+ |
+, alive at data cutoff. Pt, patient; No., number; GEM, gemcitabine; CAP, capecitabine; PFS, progression-free survival; OS, overall survival; M, male; PEM, pemetrexed; DDP, cisplatin; BEV, bevacizumab; TAX, paclitaxel; LNs, lymph nodes; PD, progressive disease; DOC, docetaxel; NDP, nedaplatin; SD, stable disease; PR, partial response; F, female; VIN, vinorelbine; CBP, carboplatin.
Treatment
As of the last follow-up, the median therapeutic cycle of GEM/CAP was 5.5 cycles (range, 2.0–12.0 cycles). Seven patients received oral CAP as the maintenance regimen after finishing GEM/CAP treatment, with a median of 6 cycles (range, 1–35 cycles), based on the decision of the physician and patient (Table 1). Subsequent post progression regimens included GEM/CAP rechallenge with or without bevacizumab, afatinib, clinical trials, docetaxel with or without CAP, docetaxel with cisplatin, ICPI, nanoparticle albumin-bound paclitaxel with or without bevacizumab, nanoparticle albumin-bound paclitaxel plus platinum with nimotuzumab or ICPI, osimertinib, palliative radiotherapy, pemetrexed/platinum with or without bevacizumab, taxanes combined with platinum, tegafur, and vinorelbine plus nimotuzumab (Figure 1).

Three patients (No. 1, 3, and 11) who received GEM/CAP for 2, 12, and 6 cycles, respectively, had disease progression after discontinuation of treatment with GEM/CAP. One had PD after rechallenge of 2 cycles of GEM/CAP and then received GEM/CAP with bevacizumab after the second progression. The other 2 patients received rechallenge GEM/CAP with bevacizumab. Among these 3 patients, 1 had 18% tumor shrinkage with a response of SD, and the other 2 patients achieved PR.
Evaluation of response
All 16 patients who had at least 2 cycles of chemotherapy were evaluable for the best response. There were 8 (50.00%) patients with PR, 6 (37.50%) with SD, 2 (12.50%) with PD, and no complete response (CR) in accordance with RECIST 1.1. Images of 2 patients are shown in Figure 2. The best overall response rate (ORR) was 50.00%, and the disease control rate (DCR) was 87.50% (Table 3).
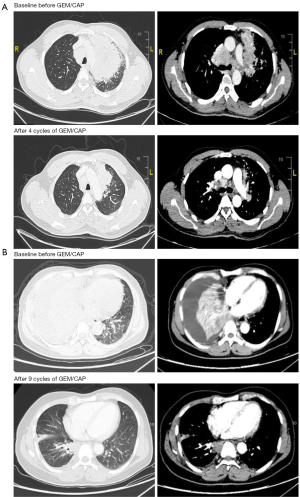
Table 3
| Best response | Number | Percentage |
|---|---|---|
| Complete response | 0 | 0.00% |
| Partial response | 8 | 50.00% |
| Stable disease | 6 | 37.50% |
| Progressive disease | 2 | 12.50% |
| Objective response rate | 8 | 50.00% |
| Disease control rate | 14 | 87.50% |
GEM, gemcitabine; CAP, capecitabine; PLELC, primary pulmonary lymphoepithelioma-like carcinoma.
The association between changes of concentration of EBV DNA with response
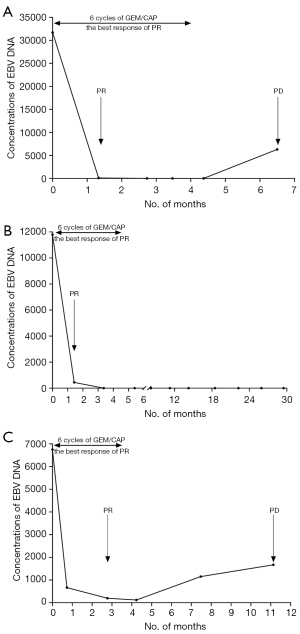
The concentration of plasma EBV DNA before, during, and after treatment with GEM/CAP were collected in 3 patients (No. 11, 13, and 16) to explore the relationship between changes in EBV DNA level with response to GEM/CAP treatment (Figure 3). EBV DNA concentration decreased significantly after GEM/CAP treatment in the 3 patients, and even descended to zero in 2 of them. The concentration of EBV DNA in 1 of these 2 patients remained zero without tumor progression until the time of data cutoff (Feb 3rd, 2021), with PFS of 29.3 months. All 3 patients achieved PR. The level of EBV DNA then elevated significantly in 2 of the 3 patients when further tumor progression occurred, with PFS of 6.4 and 11 months, respectively.
Safety
All 16 patients were evaluable for toxicity. In general, treatment was well tolerated. The majority of therapy-related AEs were grade 1–2. The most common hematological and nonhematological adverse reactions at any grade were neutropenia and hand-foot syndrome (31.25% and 43.75%, respectively). Only 2 patients had grade 3–4 AEs. One patient had grade 3 hand-foot syndrome, and the other had grade 4 febrile neutropenia and recovered after treatment. There were no chemotherapy-related deaths (Table 4).
Table 4
| Adverse event | Any grade, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) | Grade 4, n (%) |
|---|---|---|---|---|---|
| Neutropenia | 5 (31.25) | 2 (12.50) | 2 (12.50) | 0 | 1 (6.25) |
| Anemia | 1 (6.25) | 0 | 1 (6.25) | 0 | 0 |
| Thrombocytopenia | 1 (6.25) | 1 (6.25) | 0 | 0 | 0 |
| Nausea | 1 (6.25) | 1 (6.25) | 0 | 0 | 0 |
| Vomiting | 1 (6.25) | 0 | 1 (6.25) | 0 | 0 |
| Anorexia | 1 (6.25) | 1 (6.25) | 0 | 0 | 0 |
| Diarrhea | 2 (12.50) | 1 (6.25) | 1 (6.25) | 0 | 0 |
| Constipation | 3 (18.75) | 2 (12.50) | 1 (6.25) | 0 | 0 |
| Fatigue | 1 (6.25) | 1 (6.25) | 0 | 0 | 0 |
| Stomatitis | 5 (31.25) | 4 (25.00) | 1 (6.25) | 0 | 0 |
| Alopecia | 2 (12.50) | 2 (12.50) | 0 | 0 | 0 |
| Hand-foot syndrome | 7 (43.75) | 5 (31.25) | 1 (6.25) | 1 (6.25) | 0 |
PLELC, primary pulmonary lymphoepithelioma-like carcinoma.
Survival analysis
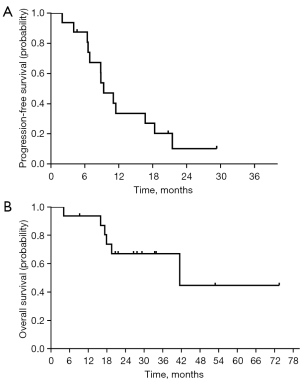
At the time of data cutoff (Feb 3rd, 2021), the survival data of the 16 patients were evaluated. Seven patients were still alive, 6 patients had died, and 3 patients were lost to follow-up. At a median follow-up of 29.3 months [95% confidence interval (CI): 20.3–38.3 months], the median PFS was 9.3 months (95% CI: 6.5–12.1 months) (Figure 4A). The median OS was 41.5 months (95% CI: 3.1–79.8 months) (Figure 4B). There were no statistically significant differences in patients with or without CAP maintenance therapy for PFS (11.0 vs. 9.3 months, P=0.292) and OS (not reached vs. 41.5 months, P=0.705) by log-rank test.
Discussion
PLELC is considered a unique subtype of NSCLC and has a low incidence (7). The majority of cases of PLELC reported in the literature are from Southern China such as Guangdong Province (3,11), Hong Kong (1,15), Taiwan (4,30), and Southeast Asia (31,32). The incidence in Asia is approximately 0.9% of NSCLC cases (1,2,4), whereas data from Western countries are lacking, and the rare reporting of cases in papers suggests a much lower incidence (1). In our cohort, PLELC was mostly associated with young nonsmokers with a median age of 45.5 years (range, 35.0–65.0 years), which was consistent with other reports (7,33). Lin et al. reported that the median age of PLELC was 47 years, and the age was on average 10 years younger than that of other NSCLCs (33).
In addition, the frequency of driver gene mutations of PLELC is rather low compared with other types of pulmonary cancer (4,10,34,35). The use of next-generation sequencing for 27 tumor tissues of PLELC patients showed no mutations of the classic driver genes of lung cancer were detected, except for mutation of KRAS gene and ERBB2 gene amplification in 2 patients (12). Therefore, chemotherapy plays an important role in patients with advanced or metastatic PLELC (4). Moreover, PLELC is similar to NPC in somatic mutation spectrum, mutation rates, and changes in signal transduction pathways (3), indicating chemotherapy drugs sensitive to NPC, such as 5-FU, GEM, paclitaxel, and platinum, may be effective in PLELC (36-39). Favorable efficacy and acceptable cytotoxicity of GEM/CAP were reported for other tumors, including pancreatic adenocarcinoma, biliary cancer, and thymic epithelial tumors (22-26). Preclinical research findings have shown a positive interaction between GEM and CAP (21). Given the need for proper and effective therapeutic regimens for patients with heavily pretreated PLELC, we conducted this retrospective study to explore the activity and toxicity of GEM/CAP for previously treated PLELC.
In our study, the combination of GEM/CAP demonstrated an ORR of 50.00% and DCR of 87.50% in heavily pretreated PLELC patients, which were much more favorable than other recommend second-line therapeutic regimens in advanced NSCLC. The ORRs of second-line regimens were 7.10–20.00% for pretreated NSCLC in published studies (40-42). In terms of second-line therapy of advanced NPC, an ORR of 43.75%, 23.53%, and 20.50% were reported in advanced NPC treated with GEM, CAP, and toripalimab, respectively (18,43,44). Although a higher activity was demonstrated in our study, the small sample size and lack of head-to-head comparison of GEM/CAP with standard second-line therapy are limitations of our study. We hope that larger-scale clinical trials are conducted to further confirm the results of our study.
High expression of programmed cell death-ligand 1 (PD-L1) has been detected in PLELC, with 2 studies reporting a positive rate of 61.7% and 69.0%, respectively (12,45), indicating that ICPIs may potentially be feasible therapeutic agents for PLELC (30). One retrospective study showed that ORRs were 33.3% in the immunochemotherapy group and 28.6% in the immunotherapy group, with median PFS of 11.8 months in the immunochemotherapy group and 11.0 months in the immunotherapy group as front-line treatments for advanced PLELC (46). However, there are few case reports describing the response to ICPIs in patients with previously treated PLELC due to the rarity of PLELC (16,17). The comparison of the efficacy of chemotherapy with or without ICPIs in PLELC by randomized trial is worth further exploration.
In this retrospective study, the median PFS and OS were 9.3 and 41.5 months, respectively, while the reported median PFS and OS for pretreated NSCLC in published studies were 10.6 weeks to 3.5 months and 7.0 to 9.2 months, respectively (40-42). The survival data of patients treated with GEM/CAP were consistent with our previous research. Patients with PLELC had better prognosis when compared with other subtypes of NSCLC (7). The favorable clinical outcomes of the patients in our study may be related to the intrinsic nature of PLELC, as also reported previously (6).
Regarding AEs, this study showed tolerable toxicity of GEM/CAP as salvage therapy in advanced PLELC. The majority of AEs were grade 1 and 2. Hand-foot syndrome and neutropenia were the most common treatment-related side effects, which were consistent with previous studies of GEM/CAP for other malignancies (47-49).
The role of EBV infection has previously been assessed in the tumorigenesis and development of PLELC (7,45). In our study, plasma EBV DNA levels were monitored before, during, and after treatment with GEM/CAP combination in 3 patients for explorative purposes, and we found that dynamic changes in EBV DNA level during anticancer treatment were associated with the clinical outcome. PR and prolonged PFS were observed in all 3 patients after receiving GEM/CAP treatment, with consistently declining EBV DNA levels, similar to that reported by Ngan et al. (50,51). Xie et al. showed that patients with undetectable plasma EBV DNA concentration after radical surgery had better disease-free survival and OS than those with persistently detectable EBV DNA levels after radical resection for resectable PLELC (6). Further exploration of the clinical value of routine monitoring of postbaseline dynamic changes in plasma EBV DNA level as a tumor marker is worthwhile during anticancer treatment.
In summary, this is the first study to explore the efficacy and safety of GEM/CAP regimen as salvage chemotherapy for pretreated PLELC. However, this study is limited by the nature of retrospective studies, the small sample size, and the heterogeneity of the patient population and previous treatments. Multicenter prospective clinical trials are needed to further confirm the efficacy and safety of this regimen. In addition, evaluation of different chemotherapy regimens as well as ICPIs should be further conducted.
Conclusions
This study preliminarily showed the favorable activity and tolerable toxicity of GEM/CAP combination as second-line or beyond treatment in pretreated advanced PLELC. Further multicenter prospective studies are worthwhile.
Acknowledgments
The work was presented as a Mini Oral Presentation at the IASLC 2020 World Conference on Lung Cancer (WCLC 2020), January 28–31, 2021, Singapore. We would like to thank Diana Wei for her help with language improvement.
Funding: This work was funded by the Medical Scientific Research Foundation of Guangdong Province of China (No. A2017186).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-256/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-256/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-256/coif). All authors report that the study was supported by the Medical Scientific Research Foundation of Guangdong Province of China (No. A2017186). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by Sun Yat-sen University Cancer Center Research Ethics Board (No. B2020-404-01). Written informed consent was provided before data collection.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ho JC, Wong MP, Lam WK. Lymphoepithelioma-like carcinoma of the lung. Respirology 2006;11:539-45. [Crossref] [PubMed]
- Han AJ, Xiong M, Zong YS. Association of Epstein-Barr virus with lymphoepithelioma-like carcinoma of the lung in southern China. Am J Clin Pathol 2000;114:220-6. [Crossref] [PubMed]
- Hong S, Liu D, Luo S, et al. The genomic landscape of Epstein-Barr virus-associated pulmonary lymphoepithelioma-like carcinoma. Nat Commun 2019;10:3108. [Crossref] [PubMed]
- Chang YL, Wu CT, Shih JY, et al. Unique p53 and epidermal growth factor receptor gene mutation status in 46 pulmonary lymphoepithelioma-like carcinomas. Cancer Sci 2011;102:282-7. [Crossref] [PubMed]
- Pazos M, Eze C, Kahnert K, et al. Novel Multimodal Management of Post-Partum Synchronous Metastatic Pulmonary EBV-Associated Lymphoepithelioma-Like Carcinoma (LELC)-A Case Report. Diagnostics (Basel) 2021;11:2072. [Crossref] [PubMed]
- Xie M, Wu X, Wang F, et al. Clinical Significance of Plasma Epstein-Barr Virus DNA in Pulmonary Lymphoepithelioma-like Carcinoma (LELC) Patients. J Thorac Oncol 2018;13:218-27. [Crossref] [PubMed]
- Liang Y, Wang L, Zhu Y, et al. Primary pulmonary lymphoepithelioma-like carcinoma: fifty-two patients with long-term follow-up. Cancer 2012;118:4748-58. [Crossref] [PubMed]
- Bégin LR, Eskandari J, Joncas J, et al. Epstein-Barr virus related lymphoepithelioma-like carcinoma of lung. J Surg Oncol 1987;36:280-3. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Wang L, Lin Y, Cai Q, et al. Detection of rearrangement of anaplastic lymphoma kinase (ALK) and mutation of epidermal growth factor receptor (EGFR) in primary pulmonary lymphoepithelioma-like carcinoma. J Thorac Dis 2015;7:1556-62. [PubMed]
- Wang L, Long W, Li PF, et al. An Elevated Peripheral Blood Monocyte-to-Lymphocyte Ratio Predicts Poor Prognosis in Patients with Primary Pulmonary Lymphoepithelioma-Like Carcinoma. PLoS One 2015;10:e0126269. [Crossref] [PubMed]
- Xie Z, Liu L, Lin X, et al. A multicenter analysis of genomic profiles and PD-L1 expression of primary lymphoepithelioma-like carcinoma of the lung. Mod Pathol 2020;33:626-38. [Crossref] [PubMed]
- Lin Z, Fu S, Zhou Y, et al. First-line platinum-based chemotherapy and survival outcomes in locally advanced or metastatic pulmonary lymphoepithelioma-like carcinoma. Lung Cancer 2019;137:100-7. [Crossref] [PubMed]
- Kumar V, Dave V, Harris J, et al. Response of advanced stage recurrent lymphoepithelioma-like carcinoma to nivolumab. Immunotherapy 2017;9:955-61. [Crossref] [PubMed]
- Ho JC, Lam DC, Wong MK, et al. Capecitabine as salvage treatment for lymphoepithelioma-like carcinoma of lung. J Thorac Oncol 2009;4:1174-7. [Crossref] [PubMed]
- Kim C, Rajan A, DeBrito PA, et al. Metastatic lymphoepithelioma-like carcinoma of the lung treated with nivolumab: a case report and focused review of literature. Transl Lung Cancer Res 2016;5:720-6. [Crossref] [PubMed]
- Wu Z, Xian X, Wang K, et al. Immune Checkpoint Blockade Therapy May Be a Feasible Option for Primary Pulmonary Lymphoepithelioma-like Carcinoma. Front Oncol 2021;11:626566. [Crossref] [PubMed]
- Zhang L, Zhang Y, Huang PY, et al. Phase II clinical study of gemcitabine in the treatment of patients with advanced nasopharyngeal carcinoma after the failure of platinum-based chemotherapy. Cancer Chemother Pharmacol 2008;61:33-8. [Crossref] [PubMed]
- Chua D, Wei WI, Sham JS, et al. Capecitabine monotherapy for recurrent and metastatic nasopharyngeal cancer. Jpn J Clin Oncol 2008;38:244-9. [Crossref] [PubMed]
- Ishitsuka H. Capecitabine: preclinical pharmacology studies. Invest New Drugs 2000;18:343-54. [Crossref] [PubMed]
- Hess V, Salzberg M, Borner M, et al. Combining capecitabine and gemcitabine in patients with advanced pancreatic carcinoma: a phase I/II trial. J Clin Oncol 2003;21:66-8. [Crossref] [PubMed]
- Palmieri G, Merola G, Federico P, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Ann Oncol 2010;21:1168-72. [Crossref] [PubMed]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Knox JJ, Hedley D, Oza A, et al. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol 2005;23:2332-8. [Crossref] [PubMed]
- Cho JY, Paik YH, Chang YS, et al. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer 2005;104:2753-8. [Crossref] [PubMed]
- Koeberle D, Saletti P, Borner M, et al. Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol 2008;26:3702-8. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Freites-Martinez A, Santana N, Arias-Santiago S, et al. Using the Common Terminology Criteria for Adverse Events (CTCAE - Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr 2021;112:90-2. (Engl Ed). [Crossref] [PubMed]
- Chang YL, Yang CY, Lin MW, et al. PD-L1 is highly expressed in lung lymphoepithelioma-like carcinoma: A potential rationale for immunotherapy. Lung Cancer 2015;88:254-9. [Crossref] [PubMed]
- Simoni Y, Becht E, Li S, et al. Partial absence of PD-1 expression by tumor-infiltrating EBV-specific CD8(+) T cells in EBV-driven lymphoepithelioma-like carcinoma. Clin Transl Immunology 2020;9:e1175. [Crossref] [PubMed]
- Archwamety A, Ruangchira-Urai R, Akewanlop C, et al. Primary pulmonary lymphoepithelioma-like carcinoma treated with immunotherapy: A case report and literature review. Thorac Cancer 2022;13:2539-41. [Crossref] [PubMed]
- Lin Z, Situ D, Chang X, et al. Surgical treatment for primary pulmonary lymphoepithelioma-like carcinoma. Interact Cardiovasc Thorac Surg 2016;23:41-6. [Crossref] [PubMed]
- Tam IY, Chung LP, Suen WS, et al. Distinct epidermal growth factor receptor and KRAS mutation patterns in non-small cell lung cancer patients with different tobacco exposure and clinicopathologic features. Clin Cancer Res 2006;12:1647-53. [Crossref] [PubMed]
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33. [Crossref] [PubMed]
- Boussen H, Cvitkovic E, Wendling JL, et al. Chemotherapy of metastatic and/or recurrent undifferentiated nasopharyngeal carcinoma with cisplatin, bleomycin, and fluorouracil. J Clin Oncol 1991;9:1675-81. [Crossref] [PubMed]
- Zhang L, Huang Y, Hong S, et al. Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 2016;388:1883-92. [Crossref] [PubMed]
- Liu SL, Sun XS, Li XY, et al. Liposomal paclitaxel versus docetaxel in induction chemotherapy using Taxanes, cisplatin and 5-fluorouracil for locally advanced nasopharyngeal carcinoma. BMC Cancer 2018;18:1279. [Crossref] [PubMed]
- Tang LQ, Chen DP, Guo L, et al. Concurrent chemoradiotherapy with nedaplatin versus cisplatin in stage II-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 2018;19:461-73. [Crossref] [PubMed]
- Shepherd FA, Dancey J, Ramlau R, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 2000;18:2095-103. [Crossref] [PubMed]
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Chua DT, Sham JS, Au GK. A phase II study of capecitabine in patients with recurrent and metastatic nasopharyngeal carcinoma pretreated with platinum-based chemotherapy. Oral Oncol 2003;39:361-6. [Crossref] [PubMed]
- Wang FH, Wei XL, Feng J, et al. Efficacy, Safety, and Correlative Biomarkers of Toripalimab in Previously Treated Recurrent or Metastatic Nasopharyngeal Carcinoma: A Phase II Clinical Trial (POLARIS-02). J Clin Oncol 2021;39:704-12. [Crossref] [PubMed]
- Chen B, Zhang Y, Dai S, et al. Molecular characteristics of primary pulmonary lymphoepithelioma-like carcinoma based on integrated genomic analyses. Signal Transduct Target Ther 2021;6:6. [Crossref] [PubMed]
- Xiao Y, He J, Luo S, et al. Comparison of Immunotherapy, Chemotherapy, and Chemoimmunotherapy in Advanced Pulmonary Lymphoepithelioma-Like Carcinoma: A Retrospective Study. Front Oncol 2022;12:820302. [Crossref] [PubMed]
- Scheithauer W, Schüll B, Ulrich-Pur H, et al. Biweekly high-dose gemcitabine alone or in combination with capecitabine in patients with metastatic pancreatic adenocarcinoma: a randomized phase II trial. Ann Oncol 2003;14:97-104. [Crossref] [PubMed]
- Stathopoulos GP, Syrigos K, Polyzos A, et al. Front-line treatment of inoperable or metastatic pancreatic cancer with gemcitabine and capecitabine: an intergroup, multicenter, phase II study. Ann Oncol 2004;15:224-9. [Crossref] [PubMed]
- Cunningham D, Chau I, Stocken DD, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513-8. [Crossref] [PubMed]
- Ngan RK, Yip TT, Cheng WW, et al. Circulating Epstein-Barr virus DNA in serum of patients with lymphoepithelioma-like carcinoma of the lung: a potential surrogate marker for monitoring disease. Clin Cancer Res 2002;8:986-94. [PubMed]
- Ngan RK, Yip TT, Cheng WW, et al. Clinical role of circulating Epstein-Barr virus DNA as a tumor marker in lymphoepithelioma-like carcinoma of the lung. Ann N Y Acad Sci 2004;1022:263-70. [Crossref] [PubMed]


