Prognostic value of lymph node ratio in patients with pathological N1 non-small cell lung cancer: a systematic review with meta-analysis
Introduction
Lung cancer causes about 1.4 million deaths per year world-wide (1). Non-small cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer. Only a fraction of NSCLC patients are diagnosed with localized, early-stage disease, when curative-intent surgical resection is possible (2,3). After surgery, the status of regional lymph node (LN) involvement is the most important prognostic factor (4). Pathologic nodal involvement (pN1-3) connotes a poor prognosis, but also predicts the likelihood of benefit from postoperative adjuvant therapy. Multiple investigators have demonstrated the heterogeneity in survival of patients with pN0 resections, suggesting the possibility that a significant proportion of these patients are understated, probably because LN metastasis is missed (5-8). However, the survival of patients with pathologic N1 is also heterogenous, ranging from a 5-year survival of 54% to 34% in the International Association for the Study of Lung Cancer (IASLC) Lung Cancer Staging Project database (4). The reported risk of recurrence for patients with pathologic Stage IIA-IIB disease ranges from 7% to 55% (9).
These results suggest a need to identify patterns of LN involvement that more accurately predict survival, particularly of patients with N1 disease (10,11). Because the thoroughness of nodal examination interacts with the likelihood of detecting nodal metastasis, the number of positive LNs may depend on by the number of LNs examined from the resection specimen. Therefore, the prognostic accuracy of the actual number of positive LNs is potentially restricted (12). The lymph node ratio (LNR)—the number of positive LNs divided by the number of LNs examined- has been suggested to be a more accurate prognostic indicator than the number of LNs with metastasis in different types of cancer including thyroid, gastric, colorectal, and cancer (13-19).
The prognostic value of the LNR in N1 NSCLC remains controversial. We conducted a meta-analysis of published reports in order to evaluate the LNR as a predictor of survival and recurrence in patients with pathological N1 NSCLC.
Methods
Search strategy
We comprehensively search PubMed and ISI Web of Science using an upper date limit of March 17, 2016, and no lower date limit. Search terms included: “lymph node ratio”, “LNR” “non-small cell lung cancer”, “NSCLC”, “N1 node”. References cited in the identified publications were also used to complete the search.
Inclusion criteria
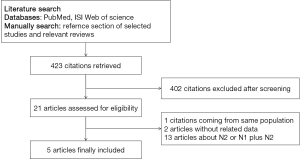
Two of the authors (Qian Li, Ping Zhan) independently determined the eligibility of the studies retrieved from the databases and bibliographies (Figure 1). Studies eligible for inclusion in this meta-analysis met the following criteria: include early stage NSCLC patients who underwent surgical resection; include the patients harboring pathological N1 disease; provide information on survival (studies investigating response rates only were excluded); have a follow-up time not less than two years; and for multiple publications reporting on the same patient population, only the most recent, or the most complete, report was included. Discordance among reviewers was resolved by mutual agreement after further discussion.
Exclusion criteria
Publications were excluded if they met any of the following criteria: (I) case series, case reports, reviews and conference reports; (II) duplicate publications; (III) studies based on overlapping cohorts from the same institution.
Data extraction and quality assessment
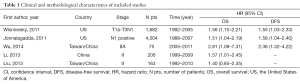
The final articles included were assessed independently by two reviewers (Qian Li and Ping Zhan) using the “Newcastle-Ottawa Scale for Assessing the Quality of Non-Random Studies in Meta-analyses” (20). Given the variability in the quality of cohort studies found in our initial literature search, we considered studies to be of high quality if they achieved a score of six or more on the Newcastle-Ottawa Scale. Data retrieved from the reports included first author, year of publication, country, lung cancer stage, number of patients, time, and survival data (Table 1).
Full table
Definition of outcomes
The primary outcome was overall survival (OS), measured from the date of surgery to the date of death or date of last follow-up; the secondary outcome was disease-free survival (DFS), measured from the date of surgery to the date of disease progression or date of last follow-up.
Statistical methods
The hazard ratios (HRs) and their 95% confidence intervals (CIs) were combined to give the pooled effective value. Heterogeneity of the individual HRs was calculated with Chi-squared tests according to Peto’s method (21). Heterogeneity test with I2 statistic was performed. All the studies included were categorized by OS and DFS. Individual meta-analysis was conducted in each subgroup. If HRs were found to have acceptable homogeneity, a fixed effect model was used for secondary analysis; if not, a random effect model was used.
In this meta-analysis, Der Simonian Laird random effects analysis was used to estimate the effect of N1 LNR on survival (22). HR >1 indicates better survival of low N1 LNR, conversely, HR <1 implies worse survival for the group with high N1 LNR. The impact of N1 LNR on survival was considered to be statistically significant if the 95% CI did not overlap with 1. Horizontal lines represent 95% CIs. The HR point estimate was reflected in each box and the box area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (HR =1.0).
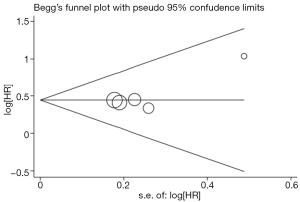
Existence of publication bias was evaluated by the methods of Begg et al. (23). Moreover, a contour-enhanced funnel plot was performed to aid in interpreting the funnel plot (24). Publication bias can lead to the asymmetry that studies appear to be missing in areas of low statistical significance, while it is less likely to cause the funnel asymmetry under the circumstance of studies missing in areas of high statistical significance. Intercept significance was determined by the t-test suggested by Egger (25). Statistical analysis was conducted using STATA version 11.0 (Stata Corporation, College Station, TX, USA).
Results
Study selection and characteristics
Five studies published between 2010 and 2014 were eligible for this systematic review with meta-analysis (26-30). All reported the prognostic value of LNR in patients with pathologic N1 NSCLC. The total number of patients included was 6,130, ranging from 75 to 4,004 patients per study. The major characteristics of the five eligible publications are reported in Table 1.
Meta-analysis
The results of the meta-analysis are reported in Figures 2,3 and 4. Overall, the pooled HR for all eligible studies evaluating the OS of high N1 LNR in resected early-stage NSCLC was 1.53 (95% CI: 1.22–1.85) using the fixed effects model (Figure 2). The heterogeneity between the reports was not significant (I2=0.0%, P=0.940). The HR indicates that in patients with pathologic N1 NSCLC, those with higher LNR had poor survival compared to those with lower LNR. Higher LNR was also associated with shorter DFS, as the pooled HR for DFS was 1.64 (95% CI: 1.19–2.09) (only 3 of the 5 studies had data available for DFS) (Figure 3). Again, the heterogeneity between the reports was not significant (I2=0.0%, P=0.591).
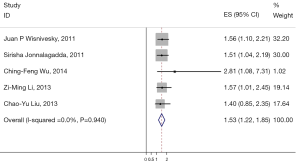
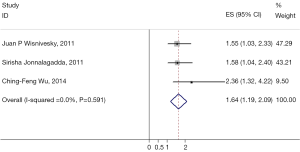
Meta-analysis of the association between N1 LNR and OS in patients with pathological N1 non-small cell lung cancer (NSCLC). Results are presented as the individual and summarized hazard ratios (HRs) with 95% confidence interval (CI). Each box represents the OR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line is set at the null value (OR =1.0). LNR, lymph node ratio; OS, overall survival; OR, odds ratios.
Begg’s funnel plot was performed to assess publication bias in the included literature. All five eligible studies investigating NSCLC patients yielded a Begg’s test score of P=0.806. Furthermore, according to the contour-enhanced funnel plot (Figure 4), no evidence of publication bias was found in all five studies.
Discussion
The status of LNs is a powerful determinant of survival in NSCLC and an essential component of the tumor, node, metastasis (TNM) classification for lung cancer (3,4). The TNM NSCLC staging system currently uses only the anatomic location of LNs to define N status. The LNR has been shown to be an important prognostic factor in several malignancies (13-19) and may overcome the limitation in the number of LNs sampled. Consistent with this notion Bria et al. showed an association between the LNR and lung cancer outcomes (12).
In this meta-analysis, we have combined five published studies including 6,130 NSCLC patients with pathologic N1 NSCLC to evaluate the prognostic value of the LNR. Our results show high N1 LNR is significantly associated with worse OS [HR 1.53 (95% CI: 1.22–1.85)] and DFS [HR 1.64 (95% CI: 1.19–2.09)]. Therefore, the LNR should be considered a predictor of survival and recurrence in patients with pathologic N1 NSCLC. The higher LNR, the worse the prognosis.
Surgical resection is the key curative treatment modality for patients with N1 NSCLC (31). However, patients with pathologic N1 disease have heterogeneous outcomes (4,9,32). For example, in the IASLC’s Lung Cancer Staging Project database, intercontinental pathologic N1 5-year survival rates ranged from 54% in Asia to 34% in Europe (4). Some have proposed that this survival heterogeneity is partly driven by heterogeneity in staging accuracy caused by heterogeneity in the thoroughness of hilar and intrapulmonary LN retrieval (33-35). The value of the LNR may be in partially adjusting for this heterogeneity in thoroughness of nodal evaluation.
Current guidelines on lung cancer surgery do not specify the number of LNs that should be sampled for adequate staging. Several large population-based studies have suggested that more than ten LNs should be examined in the resection specimens of patients categorized as pathologic node-negative (5-8). There has been less emphasis on the need for thorough N1 nodal examination in patients with pN1 disease. However, the burden of metastatic disease, reflected by the number or proportion of LNs with metastasis, may have great prognostic significance. The total number of N1 nodes detected with metastasis is, theoretically, limited by the total number of LNs examined. Our results support using the LNR as an independent means of risk-stratifying patients with pN1, and potentially identifying patients who might benefit from more intense postoperative adjuvant therapy, might need closer surveillance, or might be targeted for enrollment into clinical trials of novel adjuvant therapies.
Potential limitations of our study include the limitation to articles published in the English language, the possibility of a publication bias, since we could not include studies that may not have been published because of negative results, and the small number of eligible studies in this meta-analysis. Despite these limitations, our study shows that a high LNR connotes a poor prognosis in patients with resected N1 NSCLC. The LNR should be considered in determining post-operative management of patients with pN1, because it provides a more accurate assessment of prognosis.
Acknowledgements
We apologize to all researchers whose relevant contributions were not cited due to space limitations.
Funding: This study was supported by the Natural Science Foundation of Jiangsu Province, China (No. BK20140736), the Standard Diagnosis and Treatment Program of Key Disease in Jiangsu Province (No. BL2013026), and the National Natural Science Foundation of China (No. 81370172).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin 2015;65:5-29. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Asamura H, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:1675-84.
- Ludwig MS, Goodman M, Miller DL, et al. Postoperative survival and the number of lymph nodes sampled during resection of node-negative non-small cell lung cancer. Chest 2005;128:1545-50. [Crossref] [PubMed]
- Varlotto JM, Recht A, Nikolov M, et al. Extent of lymphadenectomy and outcome for patients with stage I nonsmall cell lung cancer. Cancer 2009;115:851-8. [Crossref] [PubMed]
- Ou SH, Zell JA. Prognostic significance of the number of lymph nodes removed at lobectomy in stage IA non-small cell lung cancer. J Thorac Oncol 2008;3:880-6. [Crossref] [PubMed]
- Osarogiagbon RU, Ogbata O, Yu X. Number of lymph nodes associated with maximal reduction of long-term mortality risk in pathologic node-negative non-small cell lung cancer. Ann Thorac Surg 2014;97:385-93. [Crossref] [PubMed]
- Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11-year experience with 975 patients. Cancer 2009;115:5218-27. [Crossref] [PubMed]
- Marra A, Hillejan L, Zaboura G, et al. Pathologic N1 non-small cell lung cancer: correlation between pattern of lymphatic spread and prognosis. J Thorac Cardiovasc Surg 2003;125:543-53. [Crossref] [PubMed]
- Tanaka F, Yanagihara K, Otake Y, et al. Prognostic factors in patients with resected pathologic (p-) T1-2N1M0 non-small cell lung cancer (NSCLC). Eur J Cardiothorac Surg 2001;19:555-61. [Crossref] [PubMed]
- Bria E, Milella M, Sperduti I, et al. A novel clinical prognostic score incorporating the number of resected lymph-nodes to predict recurrence and survival in non-small-cell lung cancer. Lung Cancer 2009;66:365-71. [Crossref] [PubMed]
- Lee HY, Choi HJ, Park KJ, et al. Prognostic significance of metastatic lymph node ratio in node-positive colon carcinoma. Ann Surg Oncol 2007;14:1712-7. [Crossref] [PubMed]
- Berger AC, Sigurdson ER, LeVoyer T, et al. Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 2005;23:8706-12. [Crossref] [PubMed]
- Peschaud F, Benoist S, Julié C, et al. The ratio of metastatic to examined lymph nodes is a powerful independent prognostic factor in rectal cancer. Ann Surg 2008;248:1067-73. [Crossref] [PubMed]
- Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg 2007;245:543-52. [Crossref] [PubMed]
- Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol 2013;20:1906-11. [Crossref] [PubMed]
- Allaix ME, Arezzo A, Cassoni P, et al. Metastatic lymph node ratio as a prognostic factor after laparoscopic total mesorectal excision for extraperitoneal rectal cancer. Surg Endosc 2013;27:1957-67. [Crossref] [PubMed]
- Chen S, Zhao BW, Li YF, et al. The prognostic value of harvested lymph nodes and the metastatic lymph node ratio for gastric cancer patients: results of a study of 1,101 patients. PLoS One 2012;7:e49424. [Crossref] [PubMed]
- Liu Y, Yuan D, Ye W, et al. Prognostic value of circulating endothelial cells in non-small cell lung cancer patients: a systematic review and meta-analysis. Transl Lung Cancer Res 2015;4:610-8. [PubMed]
- Yusuf S, Peto R, Lewis J, et al. Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 1985;27:335-71. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Peters JL, Sutton AJ, Jones DR, et al. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J Clin Epidemiol 2008;61:991-6. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Wisnivesky JP, Arciniega J, Mhango G, et al. Lymph node ratio as a prognostic factor in elderly patients with pathological N1 non-small cell lung cancer. Thorax 2011;66:287-93. [Crossref] [PubMed]
- Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724-31. [Crossref] [PubMed]
- Wu CF, Wu CY, Fu JY, et al. Prognostic value of metastatic N1 lymph node ratio and angiolymphatic invasion in patients with pathologic stage IIA non-small cell lung cancer. Medicine (Baltimore) 2014;93:e102. [Crossref] [PubMed]
- Li ZM, Ding ZP, Luo QQ, et al. Prognostic significance of the extent of lymph node involvement in stage II-N1 non-small cell lung cancer. Chest 2013;144:1253-60. [Crossref] [PubMed]
- Liu CY, Hung JJ, Wang BY, et al. Prognostic factors in resected pathological N1-stage II nonsmall cell lung cancer. Eur Respir J 2013;41:649-55. [Crossref] [PubMed]
- Fujimoto T, Cassivi SD, Yang P, et al. Completely resected N1 non-small cell lung cancer: factors affecting recurrence and long-term survival. J Thorac Cardiovasc Surg 2006;132:499-506. [Crossref] [PubMed]
- Caldarella A, Crocetti E, Comin CE, et al. Prognostic variability among nonsmall cell lung cancer patients with pathologic N1 lymph node involvement. Epidemiological figures with strong clinical implications. Cancer 2006;107:793-8. [Crossref] [PubMed]
- Osarogiagbon RU, Darling GE. Towards optimal pathologic staging of resectable non-small cell lung cancer. Transl Lung Cancer Res 2013;2:364-71. [PubMed]
- Smeltzer MP, Faris N, Yu X, et al. Missed Intrapulmonary Lymph Node Metastasis and Survival After Resection of Non-Small Cell Lung Cancer. Ann Thorac Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Osarogiagbon RU, Decker PA, Ballman K, et al. Survival Implications of Variation in the Thoroughness of Pathologic Lymph Node Examination in American College of Surgeons Oncology Group Z0030 (Alliance). Ann Thorac Surg 2016. [Epub ahead of print]. [Crossref] [PubMed]