Targeting EML4-ALK driven non-small cell lung cancer (NSCLC)
Introduction
Recently, due to key discoveries relating to the molecular biology of many cancers and the development of effective and specific targeted treatments, the ability to personalize cancer therapy based on individual patient genotypes has become a reality in clinical practice (1). Some examples of this genotype-specific approach to anti-cancer therapeutics are BCR-ABL targeted therapy in chronic myelogenous leukemia, C-KIT inhibition in gastrointestinal stromal tumors, the use of Kristen rat sarcoma (KRAS) to negatively select EGFR inhibitors in colon cancer, HER2-directed therapy in breast cancer, and BRAF inhibitors in melanoma (2-13). Several other therapies are currently under investigation in clinical trials and will likely soon broaden this list further.
We have learned that there are different subsets of lung cancers that can be molecularly defined, targeted-treated and which exhibit differential outcomes in terms of response and survival when compared with tumors not harboring any specific mutations. The discovery of EGFR mutations in lung cancer represented the first event that marked this tremendous change in our understanding and management of lung cancer. Moreover, the discovery of the implications of Anaplastic Lymphoma Kinase (ALK) rearrangements in lung cancer has changed the paradigm of how we treat different subgroups of non-small cell lung cancer (NSCLC) patients (11,14).
ALK inhibitors are able to disrupt the signaling cascade related to cell survival, producing an apoptotic response (15,16). Crizotinib, an oral ALK inhibitor, has demonstrated a clinical benefit in this subset of patients that exceeds the usual expectations for this disease (13). Therefore, the inclusion of ALK screening in the molecular diagnosis of lung cancer is mandatory, considering that the frequency of ALK alterations has been reported to range from 2% to 25% of lung cancer patients between different series (1,2,17-24).
Some questions still remain a matter of debate. Firstly, which technique is most suitable to detect ALK alterations? Secondly, which patients should be included in screening programs? Thirdly, how should the sequence of available therapies be administered to these patients and, lastly, how can we understand the mechanisms of resistance that all patients invariably ultimately develop to ALK inhibitors?
ALK in lung cancer
Although ALK mutations do occur, the majority of ALK-positive tumors induce the aberrant signal through the formation of fusion genes. ALK rearrangements were initially identified in anaplastic large cell lymphoma. Since then, this alteration has been described in other tumors such as inflammatory myofibroblatic tumors, neuroblastoma and NSCLC, among others (11,25-29). These rearrangements induce a chimeric protein with ligand-independent tyrosine kinase activity that acts through different signaling pathways, such as RAS/MEK/ERK which are related to the proliferative effect, and PI3K/AKT y JAK3/STAT3 which are involved in cell survival (16,30,31).
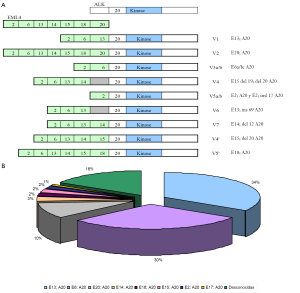
Up to eleven different variants of ALK chromosomic rearrangement have been described. Echinoderm microtubule associated protein like-4 (EML4) represents the most frequent partner for ALK in lung cancer. Figure 1 shows the general distribution of EML4-ALK rearrangement depending on different exons of EML4 present in the fusion forms. Other partners for ALK are TFG and KIF5B (30,32,33).
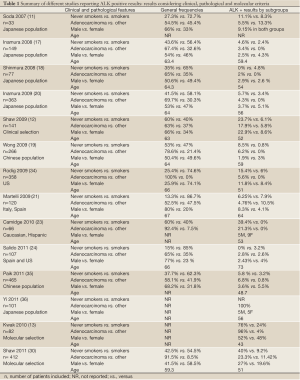
The presence of ALK rearrangements has more frequently been associated with certain clinical and pathological features, including adenocarcinoma histology (especially cribiform, signet-ring cells and solid patterns), never or light smoking history and male gender (Table 1). More importantly, wild type (WT) status for EGFR and KRAS mutations represents a more suitable criteria for ALK screening since simultaneous overlapping with other oncogenic driver mutations is uncommon (37,38). When considering these features, especially molecular selection, the likelihood of detecting an ALK rearrangement increases from 2-10% in the general population to 24-40% in this molecularly selected population, according to different series (see References and data in Table 1). Thus, the criteria for ALK screening should include the prior negative result of screening for EGFR and KRAS mutations, primarily avoiding the use of clinical and pathological characteristics (Figure 2A). Importantly, we should consider that frequencies of ALK rearrangements in other subgroup of patients, such as heavy smokers and other histology subtypes different to adenocarcinoma, are still only anecdotic.
Full Table
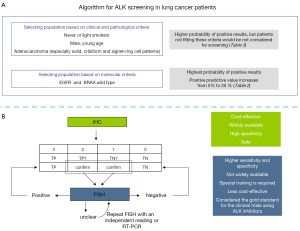
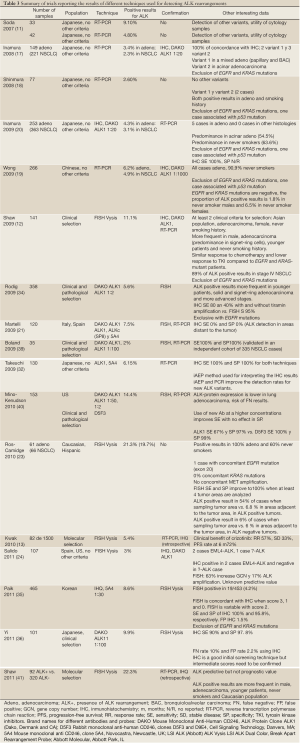
Currently, three different techniques are available for detecting ALK rearrangement, though which of these is the most convenient is still a matter of debate. Consideration needs to be given to the characteristics required for a diagnostic tool to become the technique of choice for large scale screening programs, such as high sensitivity and especially high specificity to detect real true positive cases and thus avoid the need for additional procedures. Moreover, this technique needs to be cost-effective and widely available (Table 2). However, when considering the specific use of the ALK inhibitor crizotinib in ALK-positive patients, fluorescence in situ hybridization (FISH) has been considered to be the gold standard for detecting ALK rearrangements, using the ALK Vysis LSI ALK Dual Color Break Apart Rearrangement Probe (Abbott Molecular, Abbott Park, IL). Other regulatory agencies admit the use of other diagnostic techniques, as in Japan and Europe.

Full Table
FISH confers higher sensitivity and specificity when compared to real time-PCR (RT-PCR) and immunohistochemistry (IHC). However, FISH is not widely available and is less cost-effective than other techniques. The algorithm these authors propose would include the use of IHC for the first analysis; results scored as 0 and 3 could be considered as true negative and true positive, respectively. However, for results scored as 2 and 1, a confirmatory test should be performed since these two groups accumulate the highest rates of false negative and false positive results (Table 3). This algorithm includes confirmation by FISH and RT-PCR (Figure 2B).
Full Table
Current status of ALK inhibition in lung cancer: crizotinib trials (Table 4)
Full Table
Since clinical practice currently differs from country to country, it is necessary to review data from different clinical trials to understand these differences, in particular how access to different drugs depends on patients’ regional backgrounds.
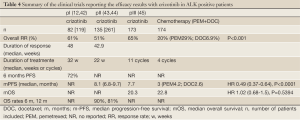
Crizotinib (PF-2341066; XALKori, Pfizer, New York, NY) is an oral small-molecule with tyrosine kinase inhibitor (TKI) properties of both MET and ALK (46). The fast approval of crizotinib in the US was based on the results of a phase I trial expansion cohort which included ALK-positive NSCLC patients (13) in which a total of 82 patients were treated. This trial demonstrated that crizotinib was an effective agent in this subset of patients with an overall response rate of 57% (56% confirmed partial responses and 33% stable disease). The estimated probability of 6 months progression-free survival (PFS) was 72%. Additionally, crizotinib was confirmed as a safe drug. The majority of adverse events were grade 1 and 2 gastrointestinal disorders (13). Based on these results, the FDA approved the use of crizotinib in NSCLC patients harboring ALK rearrangements independently of any prior treatment the patient had received. A more recent analysis of patients included in this expansion cohort (n=119) confirmed the previous findings: response rate was 61% and response occurred independently of clinical features such as age, gender, number of previous therapies and performance status. The median PFS was 10 months, and the estimated overall survival rates at 6 and 12 months were 90% and 81%, respectively (42).
Similar results were obtained from patients included in the PROFILE 1005, a phase II single-arm study to evaluate the efficacy and safety of crizotinib in pretreated NSCLC patients harboring ALK rearrangements. A total of 136 patients received crizotinib in second line (9.6%), third line (27.2%) and forth line (27.2%). Thirty six percent of patients had received more than 4 previous lines of treatment. This study demonstrated an overall response rate of 50% for a heavily pretreated population. Except for Asian patients, no other clinical characteristics influenced response, with similar benefit regardless of smoking history, performance status and previous treatment exposure (43).
Notably, standard, second line, single-agent treatments for unselected patients with advanced NSCLC achieve an overall response rate of less than 10% and PFS of less than 3 months (47,48).
An up-to-date analysis for patients included in the PROFILE 1005 trial, in which more than 900 patients were treated, has been reported (44). The first 261 patients had received treatment with a median duration of 48 weeks and had been considered as mature population. The results were consistent with those previously reported. The overall response rate was 60% (54-66%) with median duration of response of 46 weeks (35-54 weeks) and PFS was 8.1 months (6.8-9.7 months). Fifteen percent of patients discontinued crizotinib and 10% had a dose reduction due to an adverse event. The most frequent adverse events were vision disorders (54%), nausea (51%), diarrhea (44%), vomiting (44%), and constipation (37%), which were mostly grade 1 and 2 (44).
Since most of ALK-positive patients currently receive crizotinib at some point during treatment, in the absence of data from a randomized controlled trial, the effect of this drug on overall survival remains unclear. Thus, a retrospective comparison to evaluate the impact of crizotinib on overall survival has been reported. Patients with advanced NSCLC from 3 patient cohorts were included in this analysis: 82 ALK-positive patients treated with crizotinib from the expansion cohort of a phase I trial of crizotinib, 36 ALK-positive controls who did not receive crizotinib and 253 ALK-negative/EGFR-negative patients. Among the ALK-positive patients treated with crizotinib, median overall survival from initiation of crizotinib was not reached and overall survival did not differ with age, gender, smoking exposure, or ethnic background. Overall survival in the ALK-positive crizotinib-naïve controls was similar to that in the entire cohort. However, overall survival was significantly improved in patients receiving crizotinib as second or third line therapy, compared with crizotinib-naïve patients receiving any other second line therapy (49).
Patient-reported outcomes of disease- and treatment-related symptoms, quality of life (QoL), and health status have been reported in the PROFILE 1005 trial (50). Data for symptom scores and QoL from the first 136 patients for whom efficacy and safety data are available have been presented (43,50,51). The results indicate that patients receiving crizotinib presented clinically meaningful and statistical (≥10-point change and P≤0.05, respectively) improvements in some symptoms from baseline. There were clinically meaningful improvements in pain, dyspnea, and cough from cycle 2, and in fatigue from cycle 5, and these improvements were maintained through subsequent cycles (49). Moreover, global QoL was maintained throughout treatment with crizotinib with clinically meaningful improvement at cycle 7 (51). Significant reductions in pain (50), dyspnea, cough, fatigue, insomnia, and alopecia symptom scales were maintained with therapy (51). Improvement in mean QoL was also reported but changes were not clinically significant, indicating that QoL was stable with more cycles of treatment (50). Clinical meaningful improvements were observed for physical, role and social functioning and for global QoL (51,52).
Recently, results for the PROFILE 1007 study have been reported (45). This large phase III trial (n=347) compared crizotinib vs. chemotherapy in ALK-positive patients previously treated with a prior chemotherapy regimen including a platinum-doublet. Patients were randomized to receive crizotinib or chemotherapy (pemetrexed or docetaxel, depending on the previous therapy). Those patients assigned to the chemotherapy arm were allowed to receive crizotinib when progression occurred. This crossover occurred in 62% of patients initially assigned to receive chemotherapy. The study met its primary endpoint, with a difference in PFS in favor of crizotinib [7.7 vs. 3 m, HR (95% CI), 0.49 (0.37-0.64), P<0.0001]. Response rate significantly favored crizotinib, with 65% of responses in the crizotinib arm vs. 20% in the chemotherapy arm (pemetrexed 29% and docetaxel 6.9%, P<0.0001). Interim analysis of overall survival (when 28% of survival events had occurred) showed no statistically significant difference between crizotinib and chemotherapy with a preliminary estimated median OS of 20.3 vs. 22.8 months; HR 3.02; 95% CI 0.68-1.5, P=0.5394), but not adjusted for crossover. The most frequent adverse events related to crizotinib were visual disturbances (59%), diarrhea (53%), nausea (52%), vomiting (44%), and elevated transaminases (36%). Frequent adverse events with chemotherapy were nausea (35%), fatigue (29%), decreased appetite (21%), and alopecia (20%). The incidence of grade 3-4 adverse events was similar in both arms (31%). Duration of treatment was longer for crizotinib vs. chemotherapy with a median number of administered cycles of 11 vs. 4, respectively (45). Crizotinib offered clinically meaningful and statistical (P<0.001) improvements in some symptoms from baseline. There were improvements in cough, dyspnea, fatigue, alopecia, insomnia, and pain. Moreover, global QoL as well as physical, role, emotional, cognitive and social functioning favored crizotinib over chemotherapy (P<0.001) (45).
This data clearly establish that crizotinib is superior to standard second line chemotherapy, usually with docetaxel and pemetrexed which were the comparators in this trial. This superiority was confirmed in terms of prolonging PFS and improving response rate, as well as improving patient symptoms and QoL.
Results from the currently ongoing PROFILE 1014 study (Clinicaltrials.gov identifier NCT01154140) comparing first line crizotinib vs. chemotherapy are expected to elucidate whether, mirroring the experience with EGFR-TKIs in EGFR-mutant lung cancer, the ALK inhibitor is a better strategy when administered upfront (53-57).
Beyond crizotinib
Despite the good activity and tolerability profile of crizotinib for treating ALK-positive patients, several molecules have been being tested to evaluate newer regimens with a more desirable toxicity profile and more convenient administration schedules for patients, though without jeopardizing clinical activity. Moreover, patients with initial good responses to crizotinib invariably develop resistance. Therefore, further therapies are required when resistance occurs.
Based on the previous experience with EGFR-mutant NSCLC, mutations affecting the kinase domain of ALK were expected to mediate resistance to crizotinib. In fact, the first report of the presence of such mutations was published along with the first results of crizotinib activity in ALK-positive NSCLC (13,58). The presence of two different kinase domain mutations, L1196M and C1156Y, occurred in different clones from the same patient. Other resistant mutations have been reported to date (L1152R, G1269A, S1206Y, G1202R and 1151 Tins) with further mutations already identified. Collectively these mutations can mediate crizotinib resistance in ALK-positive tumors (59-61). These findings are in contrast with the experience in EGFR, in which resistance is mainly mediated by the emergence of a predominant mutation, T790M, and other secondary mutations are rare (62,63). Furthermore, different ALK mutations identified so far have shown a differential spectrum of sensitivity to crizotinib and other ALK inhibitors, suggesting that not all the newer ALK inhibitors may be equally effective in treating ALK-positive patients who develop resistance to crizotinib (60,64,65).
Other mechanisms implicated in ALK resistance have been described. These include, firstly, the copy number gain of the ALK gene fusion, which occurs simultaneously with resistant mutations (61,66). Secondly, the presence of other oncogenes that may become active via mutation or other mechanism and coexist with ALK, such as EGFR, HER2 or KIT (59-61,63). Thirdly the emergence of a separate clone that harbors other oncogenes different to ALK, such as EGFR or KRAS (61). Additionally, the underexposure of the Central Nervous System (CNS) to crizotinib may partly underlie this resistance and warrants consideration for the development of newer ALK inhibitors that can attain optimal concentration in the cerebrospinal fluid (67).
LDK378 is a next generation ALK inhibitor able to inhibit both ALK and the C1156Y variant. Results of the first in-human phase I trial have been recently reported (68). Fifty-six ALK-positive patients were included (50 patients with ALK-positive lung cancers). LDK378 was administered orally once-daily, starting at 50 mg/day. Of 47 patients evaluable for response, 24 (51%) responded and all responses were in ALK-positive NSCLC patients. Twenty one (81%) of 26 patients who had progressed to crizotinib and were treated at a dose level of ≥400 mg/day responded. The maximum tolerated dose was 750 mg/day. Dose limiting toxicities included diarrhea, vomiting, nausea, dehydration, and ALT elevation. The most frequent grade 3 side effect was diarrhea, which occurred in 5 (9%) patients. However, the most common side effects (all grades) were nausea (59%), vomiting (54%) and diarrhea (48%). Some activity has been reported in CNS metastases, which suggests good penetration in the cerebrospinal fluid.
CH5424804 is a next generation ALK inhibitor able to inhibit ALK as well as the C1156Y and L1196M variants. Recently communicated results of a phase I/II trial demonstrated very promising activity in crizotinib-naïve ALK-positive NSCLC with a response rate of 85% and range of duration of treatment from 2-46 weeks. Thirty four patients were enrolled in the trial and CH5424804 was administered at 300 mg twice-daily. The majority of patients remain on treatment at the time of this communication. The main treatment-related adverse events were ALT, AST and bilirrubin elevation (7, 6 and 3 patients, respectively), neutropenia (5 patients, 2 grade 3), rash (4 patients), nausea (4 patients), and myalgia (3 patients) which were mostly grade 1 except for neutropenia (2 cases were grade 3). Only one patient presented a treatment-related eye disorder and was grade 1. No dose reductions were necessary due to side effects. Activity in CNS metastases was shown (69).
AP26113 is a novel, synthetic, orally-active TKI that inhibits mutant forms of ALK and EGFR, as well as TKI-resistant forms such as L1196M (ALK) and T790M (EGFR) (66). This drug does not inhibit the native form of EGFR. Results of the first in-human phase 1/2 trial have been recently reported (70). A total of 34 patients were included in the dose-finding phase, starting at a dose of 30 mg/day. Twenty-seven patients had lung cancer (11 ALK-positive patients, 11 EGFR-mutant patients and 5 WT for ALK and EGFR). Nine ALK-positive patients were crizotinib-resistant, while 2 were crizotinib-naïve. Among the ALK patients, 8 partial responses were recorded, 6 among the crizotinib-resistant patients and 2 among crizotinib-naïve patients. The initial doses of 60 and 90 mg/day were sufficient to achieve some of these partial responses. The more frequent side effects were nausea (32%), diarrhea (18%, 3% of grade 3), loss of appetite (12%), fatigue (26%, 3% of grade 3), and vomiting (12%). Four (12%) patients presented pneumonia, in all cases grade 3. Notably, no rash or visual disturbances were reported. Similarly to previous next generation ALK inhibitors, activity in CNS disease has been reported. The phase 2 expansion will include 4 cohorts: ALK-positive lung cancers naïve to crizotinib, crizotinib-resistant ALK-positive lung cancers, EGFR mutant lung cancers resistant to reversible TKIs, and other cancers harboring ALK abnormalities.
Another strategy to try to overcome ALK resistance consists of targeting the chaperone pathway. Results of Heat-Shock-Protein 90 (HSP90) inhibition in a cohort of ALK-positive patients have been reported (71). AUY992 is a potent, non-geldanamycin, HSP90 inhibitor. Its activity as a once-weekly, 1-hour infusion has been tested in a specific cohort of 22 ALK-positive lung cancer patients. The overall response rate was 32%, with a disease control rate of 59% and an estimated PFS at 18 weeks of 35.8%. The overall response rate in ALK-positive crizotinib-naïve patients (8) was 50%, with a disease control rate of 100% and an estimated PFS of 62.5% at 18 weeks. The most frequent treatment related side effects were eye disorders (74%), diarrhea (68%), nausea (39%), vomiting (26%), and fatigue (21%). Grade 3-4 side effects included eye disorders (7%), diarrhea (6%), and fatigue (4%). AUY922 had an acceptable safety profile. Activity was demonstrated both in crizotinib-naïve and crizotinib-resistant patients.
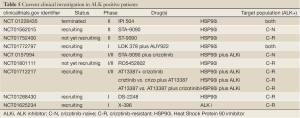
Other ALK inhibitors, as well as HSP90 inhibitors and different combinations are being currently tested in clinical trials to evaluate the safety profile and the activity in patients harboring ALK rearrangement (Table 5).
Full Table
Conclusions
Lung cancer harboring ALK rearrangements has emerged as a relevant subtype of this disease, based both on its particular natural history and on the success of crizotinib in efficaciously treating this specific population. However, some challenges remain, such as a how to better manage adverse events related to treatment, more convenient therapeutic schedules for our patients, how to effectively treat CNS disease and overcome or delay the emergence of resistance. Newer strategies including next generation ALK inhibitors or novel drugs may help to address some of these questions.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Hayden EC. Personalized cancer therapy gets closer. Nature 2009;458:131-2.
- Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCRABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med 2001;344:1038-42.
- Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80.
- Khambata-Ford S, Garrett CR, Meropol NJ, et al. Expression of epiregulin and amphiregulin and K-ras mutation status predict disease control in metastatic colorectal cancer patients treated with cetuximab. J Clin Oncol 2007;25:3230-7.
- Lièvre A, Bachet JB, Boige V, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J Clin Oncol 2008;26:374-9.
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92.
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37.
- Sequist LV, Bell DW, Lynch TJ, et al. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol 2007;25:587-95.
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39.
- Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500.
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6.
- Shaw AT, Yeap BY, Kenudson MM, et al. Clinical Features and Outcome of Patients With Non Small-Cell Lung Cancer Who Harbor EML4-ALK. J Clin Oncol 2009;27:4247-53.
- Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010;363:1693-703.
- Soda M, Takada S, Takeuchi K, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A 2008;105:19893-7.
- Koivunen JP, Mermel C, Zejnullahu K, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res 2008;14:4275-83.
- McDermott U, Iafrate AJ, Gray NS, et al. Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 2008;68:3389-95.
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol 2008;3:13-7.
- Shinmura K, Kageyama S, Tao H, et al. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer 2008;61:163-9.
- Wong DW, Leung EL, So KK, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer 2009;115:1723-33.
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15.
- Martelli MP, Sozzi G, Hernandez L, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. Am J Pathol 2009;174:661-70.
- Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466-72.
- Camidge DR, Kono SA, Flacco A, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res 2010;16:5581-90.
- Salido M, Pijuan L, Martínez-Avilés L, et al. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J Thorac Oncol 2011;6:21-7.
- Le Beau MM, Bitter MA, Larson RA, et al. The t(2;5)(p23;q35): a recurring chromosomal abnormality in Ki-1-positive anaplastic large cell lymphoma. Leukemia 1989;3:866-70.
- Debelenko LV, Arthur DC, Pack SD, et al. Identification of CARS-ALK fusion in primary and metastatic lesions of an inflammatory myofibroblastic tumor. Lab Invest 2003;83:1255-65.
- Chen Y, Takita J, Choi YL, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature 2008;455:971-4.
- Murugan AK, Xing M. Anaplastic thyroid cancers harbor novel oncogenic mutations of the ALK gene. Cancer Res 2011;71:4403-11.
- van Gaal JC, Flucke UE, Roeffen MH, et al. Anaplastic lymphoma kinase aberrations in rhabdomyosarcoma: clinical and prognostic implications. J Clin Oncol 2012;30:308-15.
- Rikova K, Guo A, Zeng Q, et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007;131:1190-203.
- Souttou B, Carvalho NB, Raulais D, et al. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem 2001;276:9526-31.
- Takeuchi K, Choi YL, Togashi Y, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res 2009;15:3143-9.
- Webb TR, Slavish J, George RE, et al. Anaplastic lymphoma kinase: role in cancer pathogenesis and small-molecule inhibitor development for therapy. Expert Rev Anticancer Ther 2009;9:331-56.
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23.
- Paik JH, Choe G, Kim H, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol 2011;6:466-72.
- Yi ES, Boland JM, Maleszewski JJ, et al. Correlation of IHC and FISH for ALK gene rearrangement in non-small cell lung carcinoma: IHC score algorithm for FISH. J Thorac Oncol 2011;6:459-65.
- Wecikhardt AJ, Camide R. The therapeutic potential of anaplastic lymphoma kinase inhibitors in lung cancer: rationale and clinical evidence. Clin Invest 2011;1:1119-26.
- Kris MG, Jonson BE, Kwiatkowski DJ, et al. Identification of driver mutations in tumor specimens from 1,000 patients with lung adenocarcinoma: The NCI’s Lung Cancer Mutation Consortium (LCMC). J Clin Oncol 2011;29:abstr CRA7506.
- Boland JM, Erdogan S, Vasmatzis G, et al. Anaplastic lymphoma kinase immunoreactivity correlates with ALK gene rearrangement and transcriptional up-regulation in non-small cell lung carcinomas. Hum Pathol 2009;40:1152-8.
- Mino-Kenudson M, Chirieac LR, Law K, et al. A novel, highly sensitive antibody allows for the routine detection of ALK-rearranged lung adenocarcinomas by standard immunohistochemistry. Clin Cancer Res 2010;16:1561-71.
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12.
- Camidge DR, Bang Y, Kwak EL, et al. Progression-free survival (PFS) from a phase I study of crizotinib (PF-02341066) in patients with ALK-positive non-small cell lung cancer (NSCLC). J Clin Oncol 2011;29:abstr 2501.
- CrinòL, Kim D, Riely GJ, et al. Initial phase II results with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC): PROFILE 1005. J Clin Oncol 2011;29:abstr 7514.
- KimD, Ahn M, Yang P, et al. Updated results of a global phase II study with crizotinin in advanced ALK-positive Non-Small Cell Lung Cancer (NSCLC). Ann Oncol 2012; 23:Abstr 1230 PD.
- Shaw AT, Kim DW, Nakagawa K, et al. Phase III Randomized Study of Crizotinib Versus Pemetrexed or Docetaxel Chemotherapy in Advanced, ALK-Positive Non-Small Cell Lung Cancer (NSCLC). Ann Oncol 2012;23:LBA1.
- Cui JJ, Tran-Dubé M, Shen H, et al. Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK). J Med Chem 2011;54:6342-63.
- Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589-97.
- Shepherd FA, Rodrigues PJ, Ciuleanu T, et al. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123-32.
- Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in advanced NSCLC harboring anaplastic lymphoma kinase gene rearrangement: a retrospective analysis. Lancet Oncol 2011;12:1004-12.
- Blackhall FH, Petersen JA, Wilner K, et al. PROFILE 1005: preliminary patient-reported outcomes (PROs) from an ongoing phase 2 study of crizotinib (PF-02341066) in anaplastic lymphoma kinase (ALK)-positive advanced non-small cell lung cancer (NSCLC). Oral presentation at: 14th World Conference on Lung Cancer (WCLC); July 3-7, 2011; Amsterdam, The Netherlands.
- Kim DW, Blackhall F, Soria JC, et al. A global phase 2 study including efficacy, safety and patient-reported outcomes (PROs) with crizotinib in patients (Pts) with ALK-positive non-small cell lung cancer (NSCLC). Eur J Cancer 2011;47:S617.
- Blackhall FH, Evans TL, Han J, et al. Impact of Crizotinib Treatment on Patient-Reported Symptoms and Quality of Life in Advanced ALK-Positive Non-Small Cell Lung Cancer (NSCLC). Ann Oncol 2012;23:Abstr1231 PD.
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57.
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for Non-Small-Cell Lung Cancer with mutated EGFR. N Engl J Med 2010;362:2380-8.
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-28.
- Zhou C, Wu Y-L, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802). Lancet Oncol 2011;12:735-42.
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46.
- Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med 2010;363:1734-9.
- Sasaki T, Koivunen J, Ogino A, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res 2011;71:6051-60.
- Katayama R, Shaw AT, Khan TM, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med 2012;4:120ra17.
- Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res 2012;18:1472-82.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26.
- Balak MN, Gong Y, Riely GJ, et al. Novel D761Y and common secondary T790M mutations in epidermal growth factor receptor-mutant lung adenocarcinomas with acquired resistance to kinase inhibitors. Clin Cancer Res 2006;12:6494-501.
- Zhang S, Wang F, Keats J, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des 2011;78:999-1005.
- Heuckmann JM, Hölzel M, Sos ML, et al. ALK mutations conferring differential resistance to structurally diverse ALK inhibitors. Clin Cancer Res 2011;17:7394-401.
- Katayama R, Khan TM, Benes C, et al. Therapeutic strategies to overcome crizotinib resistance in non-small cell lung cancers harboring the fusion oncogene EML4-ALK. Proc Natl Acad Sci U S A 2011;108:7535-40.
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5.
- Shaw AT, Camidge DR, Felip E, et al. Results of a first-in-human phase I study of the ALK inhibitor LDK 378 in advanced solid tumors. Ann Oncol 2012;23:Abstr 440O.
- Nishio M, Kiura K, Nakagawa K, et al. A phase I/II study of ALK inhibitor CH5424802 in patients with ALK-positive NSCLC; safety and efficacy interim results of the phase II portion. Ann Oncol 2012; 23:Abstr 441O.
- Gettinger S, Weiss GJ, Salgia R, et al. A first-in-human dose-finding study of the ALK/EGFR inhibitir AP26113 in patients with advanced malignances. Ann Oncol 2012;23:Abstr 439O.
- Felip E, Carcereny E, Barlesi F, et al. Phase II activity of the HSP90 inhibitor AUY992 i patients with ALK –rearranged (ALK+) or EGFR- mutated Non-Small Cell lung cancer (NSCLC). Ann Oncol 2012;23:Abstr 438O.