Post-therapeutic positron emission tomography/computed tomography for early detection of non-small cell lung cancer recurrence
Introduction
Lung cancer comprises almost 25% of the total cancer deaths worldwide (1). Non-small cell lung cancer (NSCLC) accounts for 85% of all lung cancers (2).
Although surgical resection remains the optimal treatment for early stage NSCLC, approximately 40% of patients with stage I and 60% of patients with stage II NSCLC relapse and die within 5 years after curative resection (3).
Timely and accurate detection of recurrence in patients with NSCLC plays a crucial role with regard to the initiation of salvage therapies with the overall goal of increasing survival.
Positron emission tomography (PET) has shown superior sensitivity and specificity in detecting NSCLC lymph node metastasis compared to standard CT alone (4). PET scans have widely replaced bone scintigraphy for detection of bone metastasis and PET is superior to all other clinically available imaging techniques for the detection of distant metastasis, except for cerebral metastasis (5).
The implementation of integrated positron emission tomography/computed tomography (PET/CT) systems, matching detailed morphological information of CT and metabolic information of structures provided by PET, has further improved accuracy compared to PET or CT alone and has therefore already become an integral imaging modality for diagnosis, staging and response assessment in NSCLC patients (6-9). PET/CT is now emerging as a follow-up imaging modality in these patients. In a study in 2004, reported overall sensitivities, specificities and positive and negative predictive values of integrated PET/CT for diagnosis of NSCLC recurrence were 96%, 82%, 89% and 93%, respectively, compared to 96%, 53%, 75% and 90%, respectively, for PET alone in patients with suspected recurrence who had previously undergone surgical therapy, surgery combined with chemo- or radiotherapy or combined chemo-radiotherapy alone (10).
This review focuses on the value of integrated PET/CT as a state-of-the-art technique in the detection of recurrence of NSCLC after curative surgery, (chemo-) radiotherapy as well as radiofrequency ablation and discusses the cost-effectiveness of PET/CT for recurrence detection.
NSCLC recurrence patterns
Recurrence of NSCLC may be classified as loco-regional recurrence or distant metastasis (Figure 1). Distant metastases are the most common form of NSCLC recurrence. Depending on the stage of disease at primary diagnosis and treatment administered, metastatic recurrence comprises 39% to 65.5% of all recurrences (11). About 30% of NSCLC recurrences are reported to be loco-regional. Loco-regional recurrence is located within the treated hemithorax and usually presents with nodules involving the resection staple line or the area that was treated with radiotherapy or RFA, as well as the bronchial stump, pleura, chest wall and lymph nodes (2).

In addition to recurrences, new primary lung cancer is also reported in 1% to 2% of NSCLC patients per year following initial radical therapy (12).
Technical aspects
Performing an integrated PET/CT scan, CT can either be run as low-dose CT, used predominantly for attenuation correction and solely approximate anatomical mapping, or CT is used for both attenuation correction and diagnostic purposes, being then performed with a standard radiation dose and i.v. and oral contrast material (13).
The two main advantages gained with the use of integrated PET/CT are on the one hand detection of lesions initially not seen on CT or PET alone, and on the other hand a more precise allocation of metabolic activity to an anatomic structure resulting in a better characterization of the lesion as benign or malignant (7,14). However, sensitivity of PET is decreased in tumors <1 cm, partly due to respiratory motion which can be reduced by respiratory triggered acquisitions at the expense of longer scan times and lower signal-to-noise-ratio (7). Furthermore, PET sensitivity is decreased in the brain. As the most common tracer used for PET scans is 2-deoxy-2-(18F)fluoro-D-glucose (FDG), a radioactively labeled glucose molecule, and the naturally high avidity of brain parenchyma for glucose leads to the problem that cerebral metastases can be obscured (5).
FDG uptake has been observed to vary between different NSCLC histologies, with adenocarcinomas generally being less FDG-avid than squamous cell carcinomas (15). Thus, detection of recurrence is extremely challenging for adenocarcinoma-in situ, minimally invasive adenocarcinoma and lepidic predominant adenocarcinoma since these tumors are often not FDG-avid and false-negative PET findings have been reported for bronchioloalveolar carcinoma recurrence in 40% of cases (16).
Iatrogenic causes of focal or diffuse FDG parenchymal uptake include: talc deposits after pleurodesis, percutaneous needle biopsy, mediastinoscopy and FDG microembolism (17).
PET/CT in current follow-up guidelines and in clinical practice
Current recommendations for follow-up imaging after NSCLC treatment are based on the knowledge about the high incidence of recurrence during the first 2 years following therapy. The National Comprehensive Cancer Network (NCCN) guidelines from 2010 suggest for patients at all stages of NSCLC routine history and physical examinations every 4 to 6 months in the first 2 years and then annually (18). In patients treated with curative intent in good performance an additional contrast-enhanced chest CT scan is recommended every 4 to 6 months postoperatively for 2 years, followed by a non-contrast-enhanced chest CT annually thereafter. Routinely screening with chest CT alone should be omitted, because many recurrences are extrathoracic (11). PET or brain magnetic resonance imaging (MRI) are currently not recommended for routine follow-up (18).
Yumuk et al. performed a survey and interviewed physicians from 38 centres of 12 different countries on which tests they were performing on asymptomatic patients during their post-treatment follow-up. Contradictory to the guidelines, the most commonly used test was a chest CT scan as well as a CT scan of the abdomen at 3 months post treatment (19). PET/CT and contrast enhanced MRI of the brain were done solely in symptomatic patients. These results suggest that a CT scan at 3 months after the end of radical treatment has become a standard in clinical practice with little high quality evidence.
PET/CT for follow-up after surgery
Lung cancer recurs after surgery in 30% to 75% of patients (20). Differentiation of recurrence from post-surgical changes is challenging with CT alone since many benign conditions, including atelectasis, consolidations, and radiation induced fibrosis, are difficult to distinguish from loco-regional recurrence (2).
PET/CT on the other hand, can yield false-positive results from active inflammation, particularly in the acute post-operative phase (21).
False-positive PET/CT results can be explained by an increase in glycolysis due to macrophage infiltration where inflammation is present, and a subsequently higher glucose demand and FDG uptake. In 2008, a British study retrospectively assessed FDG uptake in post-thoracotomy scars of NSCLC patients (22). Increased uptake was seen in 100% of the cases at 1-3 months, in 92% at 3-12 months, and still in 40% of the studies more than one year after surgery all in patients with no evidence of disease on follow-up. FDG uptake was observed to be diffuse in 67% of cases. Tumor recurrence in the scar was found in three cases, with focally increased uptake at 3-8 months after thoracotomy. The authors concluded that increased FDG uptake in post-thoracotomy scars is mainly diffuse, and decreases in incidence and intensity with time, with 60% of studies showing no scar uptake more than one year after surgery. Focally intense scar uptake was suggested to prompt biopsy for suspected recurrence.
These results contradict the usefulness of early post-surgical follow-up with PET/CT within the first three months, whereas usefulness of PET/CT in follow-up as from three months on is supported by these data.
A large prospective study by Choi et al., published in 2011, further evaluated the usefulness of PET/CT first performed one year after curative surgery (23). 358 patients having undergone complete resection of NSCLC were prospectively followed-up with PET/CT and conventional methods for recurrence of NSCLC at 3-month intervals for 2 years and after this at 6 month intervals for the next 3 years. Conventional methods comprised clinical, biochemical and radiographic assessment. Contrast-enhanced chest CT was done every 6 months whereas PET/CT was performed annually for 5 years after resection. Recurrence occurred in 31% of patients. In half of these patients, recurrence was detected with conventional methods. Concerning the other patients, recurrence was detected with both chest CT and PET/CT in 51% and solely with PET/CT in 37%. However, because PET/CT failed to detect 6 small or hypometabolic recurrent lesions, Choi et al. recommended as a screening algorithm annual PET/CT scans in combination with low-dose chest CT.
Besides the question of optimal timing of the first follow-up scan, the controversy whether to screen NSCLC patients after potentially curative treatment regardless of clinically suspected recurrence or whether to perform PET/CT only in symptomatic patients is still debated (Figure 2).
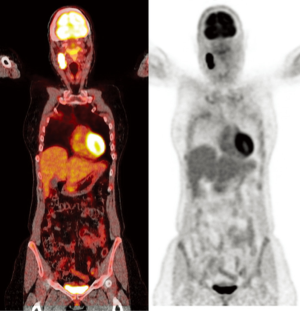
In this context, a Japanese study published in 2012 retrospectively evaluated the diagnostic accuracy of routinely performed PET/CT scans in post-operative asymptomatic NSCLC patients without suspicion of recurrence (24). A total of 101 NSCLC patients were followed-up for 5 years with a surveillance algorithm consisting of physical examination, chest radiograph, tumor marker, chest CT, PET/CT and brain MRI. Chest CT and PET/CT were performed in alternation every 6 months for the first 3 years. PET/CT was then performed every 12 months for the next 2 years. A total of 233 studies were acquired. The sensitivity, specificity, positive predictive value, negative predictive value and accuracy of PET/CT in recurrence evaluation were 94.4%, 97.6%, 89.5%, 98.8% and 97.0%, respectively. Recurrence rate in this asymptomatic patient cohort was 18%.
Another study with PET/CT in asymptomatic patients being performed at around 1 year after curative resection of NSCLC was conducted by the group of Cho et al. (25). The study enrolled 86 patients who had no suspicion of recurrence at the time of the PET/CT scan. 31.4% of the patients had recurrent disease in this cohort and 2 patients had extrathoracic double primary cancer. Six patients had extrathoracic recurrence without intrathoracic recurrence, contradicting the use of chest CT scans alone.
Jimenez-Bonilla et al. prospectively evaluated the contribution of PET/CT in patients with all stages of NSCLC with suspicion of recurrence in terms of sensitivity, specificity, impact on therapy and on survival (26). 59 suspicious lesions in 55 patients were investigated. PET/CT showed an overall sensitivity of 100% and 83% specificity. In 27 suspicious lesions where CT results were inconclusive, PET/CT showed 100% sensitivity and 78% specificity. PET/CT had an impact on patients’ treatment in 42 of all 59 cases of suspected recurrence. Overall survival of PET/CT diagnosed recurrence at 20 months and 5 years was 44% and 11%, respectively.
In comparison, a large retrospective study from 2009 analyzed post-recurrence survival rates in 123 stage I NSCLC patients who had received curative surgery between 1980 and 2000 (27). Patients either had local recurrence only or both local recurrence and distant metastases. The overall 1 and 2 year post-recurrence survival rates were 48.0% and 18.7%, respectively (27).
Comparing the survival rate observed in the PET/CT study by Jimenez-Bonilla at 20 months (44%) to the survival rate of the Hung study after 2 years (18.7%) especially when further taking into consideration, that Jimenez-Bonilla’s group also included patients at more advanced stages of NSCLC and not only stage I patients, these results are very encouraging: The outcome data of the study by Jimenez-Bonilla are suggesting a positive impact on survival using PET/CT for follow-up in the subgroup of symptomatic patients, with the limitation of the small number of patients enrolled.
Besides the high accuracy of PET/CT and its impact on treatment decisions and survival, another interesting issue—also with regard to cost-effectiveness—is the performance of PET/CT in detecting NSCLC recurrence compared to standard radiological examinations: two recent PET/CT studies prospectively enrolled patients that underwent NSCLC resection and assessed the accuracy of whole body PET/CT in recurrence detection in comparison to standard radiological examinations.
Takenaka et al. prospectively compared whole-body PET/CT and standard radiological follow-up examinations in the assessment of recurrence in post-operative NSCLC patients (28). A total of 92 consecutive patients with complete resection were enrolled. The standard radiological examination for distant metastasis assessment performed during the initial and the follow-up examinations and for local recurrence after surgery included contrast-enhanced MRI of the brain, contrast-enhanced whole-body CT and bone scintigraphy. Final diagnosis of recurrence was based on the results of more than 1 year of follow-up and/or pathological examinations. ROC curves were used to compare the diagnostic capability of the two methods for assessment of post-operative recurrence on a per-patient basis. Sensitivity, specificity and accuracy were determined as well. There were no statistically significant differences in the area under the curve of sensitivity, specificity and accuracy between PET/CT and standard radiological examinations (P>0.05). Hence, the authors concluded that PET/CT can be used for assessment of post-operative recurrence in NSCLC patients with an accuracy as good as that of standard radiological examinations; yet, with the non-negligible advantage of only one examination for the patient instead of three. This factor might play a crucial role for an efficient workflow of large departments that follow-up large patient cohorts.
Onishi et al. investigated in a prospective study in 2011 the value of qualitative as well as of quantitative PET/CT for the assessment of post-operative intra- and extrathoracic recurrence in NSCLC patients compared to standard radiological examinations (29). 121 patients who had undergone complete resection were followed-up. Again, ROC analysis was used to compare the methods in their assessment of post-operative recurrence on a per-patient basis. Additionally, optimal cut-off values for FDG uptake measurement at a suspicious site detected on the basis of qualitative PET/CT were determined. Analogous to Takenaka’s results, areas under the curve for accuracy of qualitative PET/CT and standard radiological examinations showed no significant differences (P>0.05). At an optimal cut-off value of 2.5, specificity and accuracy of combined quantitative and qualitative PET/CT were significantly higher than of qualitative PET/CT and standard radiological examinations alone (P<0.05). Accuracy in the evaluation of post-operative intra- and extrathoracic recurrence in NSCLC patients by qualitative and/or quantitative PET/CT was consequently rated equivalent to or higher than that of standard radiological examinations.
Kanzaki et al. retrospectively examined the clinical value of PET/CT in a large cohort of 241 patients with NSCLC after potentially curative surgery and even proposed that conventional imaging for the detection of extrathoracic metastases in patients who underwent potentially curative surgery for NSCLC can be completely omitted (with the exception of brain MRI) if PET/CT performed at least 6 months after surgery is negative, due to its high negative predictive value (30). 490 PET/CT studies were evaluated in this study. PET/CT correctly diagnosed recurrence in 34 of 35 patients and provided true negative findings in 198 of 206 patients who had no evidence of recurrence (sensitivity, specificity, accuracy, positive predictive value, and negative predictive value of 97%, 96%, 96%, 81%, and 99%, respectively), indicating a high diagnostic performance in this patient group.
Follow-up of NSCLC after non-surgical treatment
The field of non-surgical therapies of primary lung cancer has grown rapidly in recent years. The use of external beam radiotherapy alone as a curative approach to therapy has been abandoned due to the high local recurrence rate of up to 70% (31). In contrary, minimally invasive image-guided therapies using thermal energies such as radiofrequency ablation, microwave ablation or cryoablation, and as the most common one stereotactic body radiation therapy (SBRT) have emerged as non-surgical treatment options (32). Yet, as the tumor is not resected, surveillance of recurrence and especially of tumor margins is crucial and challenging due to post-interventional parenchymal changes.
PET/CT in NSCLC follow-up after (chemo-) radiotherapy
SBRT has become the standard therapeutic approach for inoperable stage I NSCLC. SBRT induces parenchymal damage leading to fibrosis. It can be difficult to differentiate local recurrence from radiation-induced lung opacity. Radiation-induced fibrosis can appear more than 1 year after the end of therapy (33). Furthermore, secondary radiation-induced pneumonitis has been reported within 9 months after SBRT (32).
A small study by Hoopes et al. observed on PET scans in a patient cohort of inoperable stage I NSCLC after SBRT treatment a moderately hypermetabolic activity up to 2 years after SBRT (34). This persistent uptake is being attributed to a more persistent inflammation and fibrosis after SBRT compared to fractionated radiotherapy (7).
Takeda et al. retrospectively assessed the additional value of dual-time-point maximum standardized uptake values (SUVmax) in PET/CT for detection of local recurrence after SBRT of NSCLC in 214 scans of 154 patients (33). Tri-monthly follow-up CT scans were acquired and PET/CT scans were done one year after SBRT or when recurrence was clinically suspected. On early and late images, optimal SUVmax thresholds were identified as 3.2 and 4.2. Using these thresholds, sensitivity and specificity were 100% and 96-98%, respectively. The authors therefore stated that SUVmax on PET/CT could predict local recurrence after SBRT for localized NSCLC. In a similar study, Zhang et al. also investigated whether the additional assessment of SUVmax on PET/CT after SBRT could help to predict local recurrence in 128 patients with stage I NSCLC or isolated recurrent/secondary parenchymal NSCLC patients (35). The authors found a SUVmax greater than 5, especially more than 6 months after SBRT to be associated with a higher local recurrence rate, whereas SUVmax from PET/CT scans performed within 6 months of treatment were not correlated with local recurrence. With the cutoff SUVmax of 5, sensitivity for correct prediction of local recurrence was calculated as 100%, specificity was 91%, positive predictive value was 50% and a negative predictive value of 100% was observed. The authors concluded that quantitative PET/CT was helpful for distinguishing SBRT-induced consolidation from local recurrence.
In contrast, van Loon et al. hypothesized that early PET/CT scans 3 months after curative-intended (chemo-) radiotherapy could lead to early detection of progressive disease (PD) amenable for radical treatment (36). Therefore, 100 patients with NSCLC were prospectively evaluated. All patients underwent a planned PET/CT scan 3 months after the start of radiotherapy. 24 patients had PD 3 months post-treatment of whom 16 patients were symptomatic. Yet, no curative treatment could be offered to any of these patients, which limits the impact of PET/CT on treatment decisions in the specific population of symptomatic patients. To 3/8 asymptomatic patients who were diagnosed PD, radical treatment could be offered. Progression—according to the EORTC criteria for PET and the RECIST criteria for CT—potentially amenable for radical therapy was in this study solely detected with PET/CT, but not with CT alone (37,38). Thus, van Loon suggested that asymptomatic patients would profit the most from an early PET/CT scan. However, it has still to be proven that the detection and therapy of early recurrence or PD leads to an overall higher survival in this patient cohort.
PET/CT in NSCLC follow-up after radiofrequency ablation (RFA)
Patients with stage I NSCLC who do not undergo surgical treatment are—besides SBRT—predominantly treated with RFA. The most common pattern of recurrence after RFA is loco-regional recurrence (39). As for SRBT, RFA causes focal changes in the lung parenchyma such as ground glass opacities around the treated tumor site (40). So far, there is no consensus existing on a standard protocol for post-RFA follow up. However, after RFA, continuous follow-up imaging seems to be beneficial to the patients because recurrence has been reported to occur throughout the first 2 years post-treatment (39).
Eradat et al. proposes an algorithm of CT follow-up 1-2 months after RFA followed by a PET/CT scan at 3 months thereafter alternated by contrast-enhanced CT every 3 months for 2 years (32). Similarly, the group by Beland is proposing contrast-enhanced CT at 3 weeks and 3 months followed by PET/CT at 6 months; alternating CT and PET/CT examinations then performed every 3 months (39).
Cost effectiveness
In spite of the experiences of PET/CT as a helpful staging imaging modality in the treatment of NSCLC and encouraging results concerning the accuracy of PET/CT in detecting recurrence reported in the few follow-up studies performed so far, and mostly enrolling patients with follow-up after surgical therapy, the 2nd edition of the American College of Chest Physicians (ACCP) evidence-based guidelines on the follow-up and surveillance of lung cancer patients did not recommend PET/CT for standard surveillance. The reason given for this decision was a lack of evidence that follow-up PET/CT improves either survival rates or quality of life of NSCLC patients (11).
In the only cost-effectiveness study of NSCLC follow-up so far with 100 patients, van Loon et al. prospectively compared long-term cost-effectiveness of 3 different follow-up strategies, all starting 3 months after therapy. The authors either performed a PET/CT scan, a chest CT scan or conventional follow-up with a chest radiograph (41). Cost-effectiveness was expressed in incremental cost-effectiveness ratios (ICERs), calculating the incremental costs per quality adjusted life year (QALY) gained. Both PET/CT- and CT-based follow-up were calculated to be more costly but at the same time also more effective than a chest radiograph follow-up. CT-based follow-up resulted in an incremental cost-effectiveness ratio (ICER) of euro 264.033 per QALY gained compared to a chest radiograph, whereas for PET/CT-based follow-up, the ICER was euro 69.086 per QALY gained. A subgroup analysis of asymptomatic patients undergoing PET/CT resulted in an ICER of euro 42.265 per QALY gained compared to chest radiograph follow-up. Assuming a ceiling ratio of euro 80.000, PET/CT-based follow-up was calculated to have the highest probability of being cost-effective (73%). The authors therefore concluded that a PET/CT scan 3 months after curative-intended (chemo-) radiotherapy is a potentially cost-effective follow-up method, and is more cost-effective than CT alone. Performing PET/CT scans only in asymptomatic patients seems to be equally effective and even more cost-effective.
Conclusions
Current guidelines do not recommend the use of PET/CT for assessment of NSCLC recurrence. Recommendations of different authors concerning the initiation and frequency of follow-up with PET/CT scans are largely varying between post-surgical NSCLC follow-up and surveillance of patients treated with radiotherapy and radiofrequency ablation. Most studies on NSCLC follow-up were conducted in post-surgical stage I NSCLC patients and PET/CT was mostly performed annually, starting one year after surgical treatment.
Concerning follow-up after non-surgical potentially curative treatment of NSCLC patients, controversial results have been published on the optimal timing of the first PET/CT scans. Different algorithms from different working groups schedule the first PET/CT scan from 3 months on to one year in this patient cohort. Concerning follow-up after RFA, very few studies on follow-up of these patients have been published so far. In two existing follow-up algorithms, PET/CT is performed for the first time 3 months and 6 months after treatment, respectively.
The additional value of quantitative PET measurements in prediction of recurrence has been suggested in the evaluation of thoracotomy scars as well as in the surveillance of patients treated with SBRT.
Despite encouraging results of high accuracy of PET/CT for the assessment of NSCLC recurrence and reports of impact on changes in patient management, controversy exists about whether to follow-up symptom-based or whether to screen on a routinely basis independently of symptoms and clinical findings (10,36).
Currently, PET/CT is rather used in symptomatic patients with suspicion of recurrence. However, impact on therapeutic management was mainly reported for asymptomatic patients with regard to salvage therapies. Nevertheless, high quality evidence is still lacking that intensive surveillance programs and earlier detection of recurrence leads to a survival benefit and despite of one encouraging cost-effectiveness study, incremental costs of integrated PET/CT scanners might probably play a role in decisions for or against surveillance guidelines including PET/CT to come up (41-43).
In the future, large-scale randomized trials should predominantly focus on the impact of PET/CT on treatment outcome. Furthermore, optimal starting point and frequency of follow-up PET/CT scans should be determined, especially in patients treated with the emerging minimally-invasive image-guided therapies and lastly the utility of quantitative PET/CT measurements for recurrence detection has to be clarified.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Caulo A, Mirsadraee S, Maggi F, et al. Integrated imaging of non-small cell lung cancer recurrence: CT and PET-CT findings, possible pitfalls and risk of recurrence criteria. Eur Radiol 2012;22:588-606. [PubMed]
- Hyun SH, Choi JY, Kim K, et al. Volume-based parameters of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography improve outcome prediction in early-stage non-small cell lung cancer after surgical resection. Ann Surg 2013;257:364-70. [PubMed]
- Pieterman RM, van Putten JW, Meuzelaar JJ, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med 2000;343:254-61. [PubMed]
- Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology 1999;212:803-9. [PubMed]
- Chao F, Zhang H. PET/CT in the staging of the non-small-cell lung cancer. J Biomed Biotechnol 2012;2012:783739.
- Cuaron J, Dunphy M, Rimner A. Role of FDG-PET scans in staging, response assessment, and follow-up care for non-small cell lung cancer. Front Oncol 2012;2:208. [PubMed]
- Kratochwil C, Haberkorn U, Giesel FL. PET/CT for diagnostics and therapy stratification of lung cancer. Radiologe 2010;50:684-91. [PubMed]
- De Wever W, Stroobants S, Coolen J, et al. Integrated PET/CT in the staging of nonsmall cell lung cancer: technical aspects and clinical integration. Eur Respir J 2009;33:201-12. [PubMed]
- Keidar Z, Haim N, Guralnik L, et al. PET/CT using 18F-FDG in suspected lung cancer recurrence: diagnostic value and impact on patient management. J Nucl Med 2004;45:1640-6. [PubMed]
- Rubins J, Unger M, Colice GL, et al. Follow-up and surveillance of the lung cancer patient following curative intent therapy: ACCP evidence-based clinical practice guideline (2nd edition). Chest 2007;132:355S-367S.
- Ponn RB. Lightning can strike twice: second primary lung cancers. Chest 2000;118:1526-9. [PubMed]
- Pfannenberg AC, Aschoff P, Brechtel K, et al. Low dose non-enhanced CT versus standard dose contrast-enhanced CT in combined PET/CT protocols for staging and therapy planning in non-small cell lung cancer. Eur J Nucl Med Mol Imaging 2007;34:36-44. [PubMed]
- Bar-Shalom R, Yefremov N, Guralnik L, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003;44:1200-9. [PubMed]
- Shim SS, Lee KS, Kim BT, et al. Focal parenchymal lung lesions showing a potential of false-positive and false-negative interpretations on integrated PET/CT. AJR Am J Roentgenol 2006;186:639-48. [PubMed]
- Heyneman LE, Patz EF. PET imaging in patients with bronchioloalveolar cell carcinoma. Lung Cancer 2002;38:261-6. [PubMed]
- Hany TF, Heuberger J, von Schulthess GK. Iatrogenic FDG foci in the lungs: a pitfall of PET image interpretation. Eur Radiol 2003;13:2122-7. [PubMed]
- Ettinger DS, Akerley W, Bepler G, et al. Non-small cell lung cancer. J Natl Compr Canc Netw 2010;8:740-801. [PubMed]
- Yumuk PF, Mohammed N, Maat AP, et al. How do lung cancer specialists follow their patients with stage III non-small cell lung cancer (NSCLC) after definitive treatment? A short report. Eur J Cancer 2012;48:2163-5. [PubMed]
- Inoue T, Kim EE, Komaki R, et al. Detecting recurrent or residual lung cancer with FDG-PET. J Nucl Med 1995;36:788-93. [PubMed]
- Opoka L, Szołkowska M, Podgajny Z, et al. Assessment of recurrence of non-small cell lung cancer after therapy using CT and Integrated PET/CT. Pneumonol Alergol Pol 2013;81:214-20. [PubMed]
- Gorenberg M, Bar-Shalom R, Israel O. Patterns of FDG uptake in post-thoracotomy surgical scars in patients with lung cancer. Br J Radiol 2008;81:821-5. [PubMed]
- Choi SH, Kim YT, Kim SK, et al. Positron emission tomography-computed tomography for postoperative surveillance in non-small cell lung cancer. Ann Thorac Surg 2011;92:1826-32; discussion 1832.
- Toba H, Sakiyama S, Otsuka H, et al. 18F-fluorodeoxyglucose positron emission tomography/computed tomography is useful in postoperative follow-up of asymptomatic non-small-cell lung cancer patients. Interact Cardiovasc Thorac Surg 2012;15:859-64. [PubMed]
- Cho S, Lee EB. A follow-up of integrated positron emission tomography/computed tomography after curative resection of non-small-cell lung cancer in asymptomatic patients. J Thorac Cardiovasc Surg 2010;139:1447-51. [PubMed]
- Jiménez-Bonilla JF, Quirce R, Martínez-Rodríguez I, et al. Diagnosis of recurrence and assessment of post-recurrence survival in patients with extracranial non-small cell lung cancer evaluated by 18F-FDG PET/CT. Lung Cancer 2013;81:71-6. [PubMed]
- Hung JJ, Hsu WH, Hsieh CC, et al. Post-recurrence survival in completely resected stage I non-small cell lung cancer with local recurrence. Thorax 2009;64:192-6. [PubMed]
- Takenaka D, Ohno Y, Koyama H, et al. Integrated FDG-PET/CT vs. standard radiological examinations: comparison of capability for assessment of postoperative recurrence in non-small cell lung cancer patients. Eur J Radiol 2010;74:458-64. [PubMed]
- Onishi Y, Ohno Y, Koyama H, et al. Non-small cell carcinoma: comparison of postoperative intra- and extrathoracic recurrence assessment capability of qualitatively and/or quantitatively assessed FDG-PET/CT and standard radiological examinations. Eur J Radiol 2011;79:473-9. [PubMed]
- Kanzaki R, Higashiyama M, Maeda J, et al. Clinical value of F18-fluorodeoxyglucose positron emission tomography-computed tomography in patients with non-small cell lung cancer after potentially curative surgery: experience with 241 patients. Interact Cardiovasc Thorac Surg 2010;10:1009-14. [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1-11. [PubMed]
- Eradat J, Abtin F, Gutierrez A, et al. Evaluation of treatment response after nonoperative therapy for early-stage non-small cell lung carcinoma. Cancer J 2011;17:38-48. [PubMed]
- Takeda A, Kunieda E, Fujii H, et al. Evaluation for local failure by 18F-FDG PET/CT in comparison with CT findings after stereotactic body radiotherapy (SBRT) for localized non-small-cell lung cancer. Lung Cancer 2013;79:248-53. [PubMed]
- Hoopes DJ, Tann M, Fletcher JW, et al. FDG-PET and stereotactic body radiotherapy (SBRT) for stage I non-small-cell lung cancer. Lung Cancer 2007;56:229-34. [PubMed]
- Zhang X, Liu H, Balter P, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:1558-65. [PubMed]
- van Loon J, Grutters J, Wanders R, et al. Follow-up with 18FDG-PET-CT after radical radiotherapy with or without chemotherapy allows the detection of potentially curable progressive disease in non-small cell lung cancer patients: a prospective study. Eur J Cancer 2009;45:588-95. [PubMed]
- Young H, Baum R, Cremerius U, et al. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer 1999;35:1773-82. [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [PubMed]
- Beland MD, Wasser EJ, Mayo-Smith WW, et al. Primary non-small cell lung cancer: review of frequency, location, and time of recurrence after radiofrequency ablation. Radiology 2010;254:301-7. [PubMed]
- Bojarski JD, Dupuy DE, Mayo-Smith WW. CT imaging findings of pulmonary neoplasms after treatment with radiofrequency ablation: results in 32 tumors. AJR Am J Roentgenol 2005;185:466-71. [PubMed]
- van Loon J, Grutters JP, Wanders R, et al. 18FDG-PET-CT in the follow-up of non-small cell lung cancer patients after radical radiotherapy with or without chemotherapy: an economic evaluation. Eur J Cancer 2010;46:110-9. [PubMed]
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v103-15. [PubMed]
- Benamore R, Shepherd FA, Leighl N, et al. Does intensive follow-up alter outcome in patients with advanced lung cancer? J Thorac Oncol 2007;2:273-81. [PubMed]


