Treating epidermal growth factor receptor-mutated non-small cell lung cancer—is dacomitinib the winner?
Non-small cell lung cancer (NSCLC) (80–85% of all lung cancers) continues to be one of the major causes of cancer related deaths around the world (1). The development of molecularly targeted therapies (small molecules and monoclonal antibodies) has, however, significantly improved outcomes in the metastatic setting for NSCLC patients harbouring activated oncogenes such as epidermal growth factor receptor (EGFR) and translocated anaplastic lymphoma kinase (ALK) (2). By targeting the main pathways of NSCLC signal transduction, these drugs dramatically improved progression-free survival (PFS) and quality of life (QoL) in this highly selected subgroup of NSCLC patients and thereby sparing them from toxic chemotherapy approaches (3).
Since high tumour response rates have been achieved with first-line EGFR tyrosine kinase inhibitors (TKIs), these drugs are approved as standard first-line therapy for advanced or metastatic NSCLC harbouring EGFR mutations or the ALK rearrangement. However, disease progression in a majority of patients after 9 to 13 months of treatment is common and is mainly due to the occurrence of additional mutations (e.g., T790M) or amplification (e.g., c-MET) (4).
Currently, three different EGFR TKIs [gefitinib (Iressa®, AstraZeneca), erlotinib (Tarceva®, Roche), and afatinib (Gilotrif®, Boehringer)] are approved for the treatment of NSCLC patients harbouring common activating EGFR mutations, however, in terms of comparison of these drugs only results generated by indirect meta-analyses have been reported which were not always clear and convincing (5,6). In these patients, different randomised trials confirmed the significant superiority of EGFR TKIs versus standard platinum-based chemotherapy in first-line settings in terms of PFS, QoL and safety profile, but no randomised clinical trials evaluating erlotinib, gefitinib, or afatinib showed a statistical improvement of overall survival (OS) for patients treated with EGFR TKIs, when considered individually and based on the overall population (7,8). Although these trials seem to be very similar, exploring the same indications and end-points with different EGFR TKIs, they revealed many differences about study design, patient population and statistical analysis.
Although these three agents are established as first-line treatment options in this setting, there is still a lack of prospective randomised head-to-head comparisons of first- and second-generation TKIs to help guide treatment decisions. Other than the recent CTONG-0901 trial which compared gefitinib with erlotinib and found no difference in efficacy and safety (9), LUX-Lung-7 is the first published trial to compare an irreversible pan-ErbB family blocker, afatinib, with a reversible EGFR TKI, gefitinib, in treatment-naive patients with advanced NSCLC harbouring a common EGFR mutation (10,11). This multicentre, international, open-label, exploratory, randomised controlled phase IIb trial (NCT01466660) enrolled treatment-naive patients (N=319) with stage IIIB or IV NSCLC. Of note, no cross-over of patients was permitted according to the protocol.
PFS was found to be 11.0 months (95% CI: 10.6–12.9) with afatinib versus 10.9 months (95% CI: 9.1–11.5) with gefitinib (HR, 0.73; 95% CI: 0.57–0.95; P=0.017). After a median follow-up of 42.6 months, median OS with afatinib versus gefitinib was 27.9 versus 24.5 months (HR, 0.86; 95% CI: 0.66–1.12; P=0.258), a finding that was generally consistent across key patient subgroups, including those based on gender, ethnicity (Asian versus non-Asian), and EGFR mutation type (exon 19 deletion versus L858R) (Table 1).

Full table
Dacomitinib (Pfizer) is another small molecule targeting EGFR (erbB1, erbB2, and erbB4) that had been tested in a head-to-head comparison with gefitinib (12). The drug binds irreversibly to cysteine-797 and has been initially evaluated in two earlier phase III trials (Table 2). In the initial ARCHER-1017 study, a single-arm phase II trial (N=89), treatment-naïve advanced or metastatic NSCLC patients with activating EGFR mutations were treated with dacomitinib until progression or unacceptable toxicity. ORR was found to be 75.6% and median PFS was 18.2 months (15).
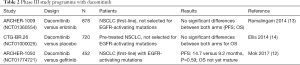
Full table
Due to the encouraging clinical activity as initial systemic treatment, dacomitinib was then further evaluated in a subsequent phase III trial. Most recently, the results of this multinational, multicentre, randomised, open-labelled, phase III trial (ARCHER-1050; NCT01774721) in terms of efficacy and safety of treatment with dacomitinib (45 mg/d) versus gefitinib (250 mg/d) in patients (N=452) with locally advanced or metastatic NSCLC with EGFR-activating mutations were reported (12). The primary endpoint was PFS per blinded independent review (IRC), and secondary endpoints included OS, ORR, and safety. Again, no cross-over of patients was permitted.
In terms of adverse events (AEs), there was more toxicity observed in the dacomitinib arm than in the gefitinib arm. Gastrointestinal all-grade AEs were more common in the dacomitinib arm compared with the gefitinib arm, including diarrhea (87.2% versus 55.8%, respectively) and decreased appetite (30.8% versus 24.6%). More patients in the dacomitinib arm compared with the gefitinib arm also experienced paronychia (61.7% versus 20.1%), dermatitis acneiform (48.9% versus 28.6%), and stomatitis (43.6% versus 17.9%). However, increases in liver enzymes levels were more frequently observed in the gefitinib arm (39.3%) compared with the dacomitinib arm (19.4%). No new safety signals were identified.
ORRs per IRC were similar between arms [75% (95% CI: 69–80%) for dacomitinib and 72% (95% CI: 65–77) for gefitinib (P=0.39)]. PFS per IRC was 14.7 months (95% CI: 11.1–16.6) versus 9.2 months (95% CI: 9.1–11.0) (P<0.0001) (HR, 0.59; 95% CI: 0.47–0.74). OS is not yet mature and will be reported separately.
The increased toxicity for dacomitinib may be due, at least in part, to the chemical nature of the drug (an irreversible TKI) and dose modifications for the drug were relatively common, with 150 patients in the dacomitinib arm receiving a dose reduction (66.1%), with a median time to dose reduction of 2.8 months, compared with 18 patients (8.0%) and a median time to dose reduction of 3.3 months in the gefitinib arm.
In this study dacomitinib was found to be the first second-generation TKI which demonstrated a statistically significant and clinically meaningful improvement of PFS compared with the first-generation drug gefitinib and therefore might be the new first-line option for NSCLC patients with activating EGFR mutations.
However, it should be noted that dacomitinib’s efficacy was accompanied by an increase in skin and gastrointestinal toxicities, therefore, the efficacy versus toxicity balance (“therapeutic window”) will be important for the selection of the best TKI for an individual patient. Moreover, it is important to note that tolerability also plays a determining role in the selection and dosing of a TKI. The tolerability profiles between erlotinib, gefitinib, afatinib, and dacomitinib are different and the selection of the therapy will still be based on the individual clinical decision.
In addition, it is also critical to see whether treatment with dacomitinib will result in an OS benefit which then would add weight to the proposal that the drug will be the new first-line option for NSCLC patients with EGFR-activating mutations since a more toxic therapy should be accompanied by an expectation of substantially greater OS benefit to provide a clinically meaningful outcome to patients.
OS, defined as the time from randomisation to death from any cause, is a direct measure of clinical benefit to a NSCLC patient suggesting that OS offers the greatest clinical benefit, provided that QoL is not compromised. In addition, it should be noted that clinical trials can also demonstrate clinically meaningful outcomes even without affecting OS, such as trials that demonstrate non-inferiority compared with existing therapies with significantly less toxicity as published in an earlier Editorial in this journal (16).
Frankly, the goals of any new TKI treatment for NSCLC patients are to allow the patient to live longer and to live better. Therefore, clinical trials in NSCLC have two important endpoints: OS and the QoL of that survival. All other endpoints should be considered intermediate, becoming surrogates to those important two endpoints only if formally validated. Uncertainty remains about whether an improvement in PFS represents a clinical benefit in patients with NSCLC in the same way that prolongation of survival or an improvement in symptoms and QoL does. Furthermore, to date the relationship between PFS and OS has not been established in advanced NSCLC following TKI treatment and remains to be controversial (17).
Finally, given the recent development of third-generation EGFR TKIs such as osimertinib (Tagrisso®, AstraZeneca), ASP8273 (Astellas), and olmutinib (Olita®, Hanmi Pharmaceuticals: approved in South Korea only), which are highly effective against T790M mutation-positive tumours (18) improved understanding of, and screening for, mechanisms of acquired resistance to first-line EGFR-targeted agents will also help to determine the most appropriate and effective sequence of treatments for EGFR mutation-positive NSCLC patients. In addition, preclinical development on the discovery of fourth-generation EGFR TKIs and U to Y allosteric strategies to combat the C797S EGFR resistance problem (leading mechanism of resistance to the third-generation inhibitors) is also underway and may hold promise for the future therapeutic landscape of EGFR mutation-positive NSCLCs (19).
In this regard, the results from the SOLAR trial (ASP8273 versus gefitinib or erlotinib; NCT02588261) and the FLAURA trial (osimertinib versus gefitinib or erlotinib, NCT02296125) (Table 1) are eagerly awaited to further clarify the role of third-generation TKIs as first-line treatment for NSCLC patients harbouring EGFR activating mutations. Both trials are ongoing, but no longer recruiting patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ramalingam S, Belani C. Systemic chemotherapy for advanced non-small cell lung cancer: recent advances and future directions. Oncologist 2008;13:5-13. [Crossref] [PubMed]
- Batson S, Mitchell SA, Windisch R, et al. Tyrosine kinase inhibitor combination therapy in first-line treatment of non-small-cell lung cancer: systematic review and network meta-analysis. Onco Targets Ther 2017;10:2473-82. [Crossref] [PubMed]
- Dempke WC. Targeted Therapy for NSCLC--A Double-edged Sword? Anticancer Res 2015;35:2503-12. [PubMed]
- Zhang K, Yuan Q. Current mechanism of acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitors and updated therapy strategies in human nonsmall cell lung cancer. J Cancer Res Ther 2016;12:C131-7. [Crossref] [PubMed]
- Lee CK, Wu YL, Ding PN, et al. Impact of Specific Epidermal Growth Factor Receptor (EGFR) Mutations and Clinical Characteristics on Outcomes After Treatment With EGFR Tyrosine Kinase Inhibitors Versus Chemotherapy in EGFR-Mutant Lung Cancer: A Meta-Analysis. J Clin Oncol 2015;33:1958-65. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Yang JJ, Zhou Q, Yan HH, et al. A phase III randomised controlled trial of erlotinib vs gefitinib in advanced non-small cell lung cancer with EGFR mutations. Br J Cancer 2017;116:568-74. [Crossref] [PubMed]
- Paz-Ares L, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol 2017;28:270-7. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Mok T, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib for the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer (ARCHER 1050): A randomized, open-label phase III trial. J Clin Oncol 2017;35:abstr LBA9007. [Crossref]
- Jänne PA, Ou SH, Kim DW, et al. Dacomitinib as first-line treatment in patients with clinically or molecularly selected advanced non-small-cell lung cancer: a multicentre, open-label, phase 2 trial. Lancet Oncol 2014;15:1433-41. [Crossref] [PubMed]
- Ramalingam SS, Jänne PA, Mok T, et al. Dacomitinib versus erlotinib in patients with advanced-stage, previously treated non-small-cell lung cancer (ARCHER 1009): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:1369-78. [Crossref] [PubMed]
- Ellis PM, Shepherd FA, Millward M, et al. Dacomitinib compared with placebo in pretreated patients with advanced or metastatic non-small-cell lung cancer (NCIC CTG BR.26): a double-blind, randomised, phase 3 trial. Lancet Oncol 2014;15:1379-88. [Crossref] [PubMed]
- Fenchel K, Sellmann L, Dempke WC. Overall survival in non-small cell lung cancer-what is clinically meaningful? Transl Lung Cancer Res 2016;5:115-9. [PubMed]
- Booth CM, Eisenhauer EA. Progression-free survival: meaningful or simply measurable? J Clin Oncol 2012;30:1030-3. [Crossref] [PubMed]
- Juan O, Popat S. Treatment choice in epidermal growth factor receptor mutation-positive non-small cell lung carcinoma: latest evidence and clinical implications. Ther Adv Med Oncol 2017;9:201-16. [Crossref] [PubMed]
- Patel H, Pawara R, Ansari A, et al. Recent updates on third generation EGFR inhibitors and emergence of fourth generation EGFR inhibitors to combat C797S resistance. Eur J Med Chem 2017;142:32-47. [Crossref] [PubMed]


