A randomized phase II study of pleurectomy/decortication preceded or followed by (neo-)adjuvant chemotherapy in patients with early stage malignant pleural mesothelioma (EORTC 1205)
Background
The aim of radical surgery in early stage malignant pleural mesothelioma (MPM) is gross resection of all macroscopically visible tumor (R1 resection), as a complete resection (R0) seems unattainable due to microscopic disease remaining at section margins. Two surgical procedures are commonly performed: the first is the extrapleural pneumonectomy (EPP), a rather standardized procedure in which ipsilateral lung, parietal and visceral pleura, pericardium and diaphragm are resected en bloc, with reconstruction of pericardium and/or diaphragm, through a single extended posterolateral thoracotomy (1). This operation was devised in the 1970s, albeit with a high (>30%) postoperative mortality (2), which is nowadays reduced to 3.4% in experienced hands (3).
The second procedure is the pleurectomy/decortication (PD), which consists of stripping the whole parietal, diaphragmatic, mediastinal and visceral pleura, leaving the lung in place, although multiple wedge resections, a segmentectomy or even a lobectomy may be necessary to acquire R1 resection. Resection of diaphragm and/or pericardium are optional depending on their gross aspect. When more than the pleural blades are removed, the procedure is called extended pleurectomy/decortication (e-PD). However, the latter procedure is not being performed uniformly worldwide (4), although several centers have extensive experience in performing this procedure in a standardized way (5-7).
Which of the above surgical procedures is superior has not convincingly been established, as no RCTs directly comparing both procedures are available, and the decision which surgical procedure to perform is more influenced by surgeon’s preference and expertise than data convincingly supporting one procedure over another. Unrandomized comparisons and pooled data from large registries suggests that (e-)PD may be the more feasible procedure, due to its lower perioperative mortality and morbidity (8) and better short- and long-term quality of life reported (9), including in elderly patients (10), due to its lung-sparing technique. In spite of (or thanks to) its less radical approach, overall survival (OS) of (e-)PD is reported to be similar or even better compared to EPP (11,12) and surgeons switching from EPP to e-PD did not report worse outcomes (13).
As both surgical procedures lead to incomplete resection, they are currently preferably performed as part of multimodality treatment, including neo-adjuvant or adjuvant chemotherapy, postoperative radiotherapy (PORT) or more laborious interventions like photodynamic therapy (PDT) or hyperthermic intraoperative chemotherapy (HIPEC), or combinations of these. A retrospective series of 384 patients (11) compared (very heterogeneous) multimodality treatment to surgery alone (EPP or PD) and found a doubling of median survival from 10 to 20 months with multimodality treatment.
The improved survival with multimodality treatment compared to surgery alone, may also be explained as an effect caused by the non-surgical modalities and patient selection, rather than as a benefit of combination. Six phase II studies (14-19) treated a total of 257 patients with early stage MPM with neo-adjuvant chemotherapy, followed by EPP (in 73–84%) and PORT (in 57–71%), with median survival ranging from 17 to 25.5 months, which indeed is longer than the 11.4 to 12.1 months reported in chemotherapy trials for unresectable MPM (20,21), but unresectability suggests more advanced stage or poorer performance status, which are associated with shorter survival anyway. The recent multimodality trial with preoperative radiotherapy and adjuvant chemotherapy in case of ypN2 (SMART) reported a median OS of 36 months (22).
In addition, the MARS trial compared (neo-adjuvant) chemotherapy followed by EPP to no EPP and showed better survival in the no EPP arm (even compared to some historical EPP series), with persistent worse quality of life in the EPP arm during the 2-year follow-up period (23). Although this trial was criticized for being severely underpowered (randomizing only 50 patients, where 670 were required for significance according to the authors) (24), it lead to a decline in EPP practice, with the 2018 British Thoracic Society guideline stating ‘do not offer EPP in MPM’ with a grade B recommendation (25).
Whether the less radical (e-)PD may lead to better results as part of multimodality treatment (in this case without PORT) is being explored by the MARS2 trial (NCT02040272), currently recruiting in the UK. The results are eagerly awaited. Published surgical multimodality series have reported a median OS of 32–36 months, with a 30-day mortality of 0–3% (5,6).
Multimodality treatment protocols have commonly consisted of chemotherapy, followed by surgery and PORT. Historically, adjuvant chemotherapy following either EPP or (e-)PD has been explored first in an attempt to eliminate microscopically residual disease, after resection of the tumor bulk (26). Currently the generally accepted chemotherapeutic regimen—also in the neo-adjuvant setting—is cisplatin plus pemetrexed, based on its superiority to cisplatin alone in unresectable MPM (20,21), although several other regimens are still in use, illustrated by cisplatin plus gemcitabine being the most used regimen in the MARS trial (23). In a retrospective analysis, Sharkey et al. did not find a difference in OS between adjuvant and neo-adjuvant chemotherapy (27). In a systematic review Cao et al. report a median OS of 23.1 months with the adjuvant chemotherapy group versus 27.8 in the neo-adjuvant chemotherapy group (28). Important to note here is that OS was measured from different starting points in the different trials, which undoubtedly affects the entire analysis. Also, the trials assessing adjuvant chemotherapy are older than the neo-adjuvant series and mostly of retrospective nature. The adjuvant chemotherapy regimens differed between the trials, so comparing them as a group to the more homogenous neo-adjuvant trials is presumptuous. From NSCLC we know that both approaches lead to a similar improvement in outcome (29). The phase II multimodality trials using the neo-adjuvant approach observed that 74–84% of patients managed to complete neo-adjuvant chemotherapy plus EPP, but only 52–65% received PORT; in comparison, in the MARS trial, of 24 patients treated with upfront chemotherapy and assigned to EPP, 16 underwent EPP and 8 underwent both EPP and PORT.
A 2018 Cochrane systematic review stated that there is a lack of available evidence to support the use of radical multimodality treatment in routine clinical practice; it should only be performed as part of a clinical trial (30).
It is important to consider that OS in unresectable MPM (in particular epithelioid histology) is also improving by advances in systemic therapy, such as addition of bevacizumab (median OS 18.8 vs. 16.1 months in the MAPS trial) (31) or nintedanib (median OS 20.6 vs. 15.2 months in the LUME-Meso trial) (32) to the standard chemotherapeutic regimen cisplatin plus pemetrexed. In addition, immunotherapy has shown promising results after progression with chemotherapy (mean OS 13.6 months with nivolumab and not reached with the combination of nivolumab and ipilimumab) in the MAPS2 trial (33) and is currently being explored in first line. Still, a median OS of 20 months remains considerably shorter (with more advanced disease as an obvious bias in this group) compared to the 32–36 months reached with surgical multimodality treatment in experienced centers, with a 30-day mortality rate which is not exceeding that of chemotherapy for NSCLC (34). Based on this evidence, the 2018 ASCO guideline recommends radical surgery in selected patients with early-stage disease (35).
Study design and inclusion criteria
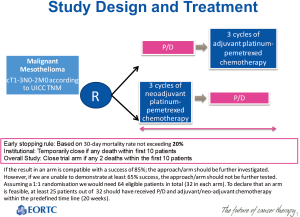
In order to ever compare EPP and (e-)PD in a randomized multimodality way, the latter procedure requires standardization and the optimal sequence of surgery and chemotherapy should be determined. EORTC 1205 (NCT02436733) is a phase II trial of the EORTC Lung Cancer Group (LCG), currently running in 6 centers in 4 countries (Belgium, Netherlands, Egypt and France), randomizing eligible patients in a 1:1 ratio between immediate surgery, followed by 3 cycles of chemotherapy) (arm A) and deferred surgery, following—if no progression—neo-adjuvant chemotherapy (arm B) (Figure 1). e-PD is the resection procedure in both arms; chemotherapy consists of 3 cycles of cisplatin 75 mg/m2 plus pemetrexed 500 mg/m2 IV on day 1 of a 21-day cycle. All patients receive vitamin B12 and folic acid, and standard prophylaxis for highly emetogenic chemotherapy is applied with cisplatin administration.
Eligible patients have pathologically proven MPM, irrespective of the histological subtype, of an early stage (cT1–3 N0–2 M0 according to the UICC TNM 7 classification system), and are fit for surgery and chemotherapy. Focal chest wall lesions are acceptable, but widespread chest wall or mediastinal invasion (cT4), contralateral (cN3), supraclavicular or coeliac lymph node involvement are not, based on assessment with PET-CT. No prior chemotherapy or radiotherapy of the lower neck, thorax or abdomen is allowed, including prophylactic track irradiation. Diagnostic VATS with talc pleurodesis is recommended, and if so, recommended to be performed before randomization.
Endpoints and statistical analysis
The primary end-point of EORTC 1205 is the successful completion of multimodality treatment within 20 weeks, defined as:
- Having received three cycles of chemotherapy plus the surgical intervention.
- Being alive, without signs of progressive disease and without persistent grade III–IV treatment-related adverse events.
Secondary end-points are surgical quality and uniformity indicators, progression free survival (PFS), OS, treatment-failure-free survival (TFFS), operative morbidity and mortality, toxicity and safety. Surgical quality and uniformity will be continuously assessed during the study. In order to ensure surgical quality, the following measures will apply:
- All procedures will be performed by expert and certified thoracic surgeons with experience in mesothelioma surgery, in particular PD. Surgeons from Ghent and Rotterdam first visited a referent surgeon in London, who has extensive experience with PD, and are responsible for cross visits during the pilot phase in other centers.
- The study will be limited to a number of credentialed centers.
- During surgery photographic documentation of crucial areas of interest is mandatory as a proof of macroscopic completeness of resection. Reporting adverse events, monitoring of postoperative pleural effusions and documenting the timing of removal of chest drains is required. Surgical reports and pictures will be cross-read by an independent surgical quality board for accurateness and completeness.
In order to standardize the procedure, the following instructions will apply:
- The minimal procedure to which all participating thoracic surgeons must conform is complete parietal and visceral pleurectomy to remove all tumor. Optional procedures according to the surgeon’s perioperative decision are defined into the QA surgery guidelines. Hyperthermic lavage and prophylactic track irradiation are not allowed.
- All procedures will be performed by open thoracotomy; VATS is not allowed. In case of uncontrolled pleural fluid, a pleurodesis at least 4 weeks prior to the procedure is mandatory.
- Participating surgeons will refer to the recommended operative technique provided by the referent surgeon and a copy of the operative report for central analysis of the intraoperative findings and extent of surgery will be requested from the participating sites.
In addition, biomarkers for the evaluation of response to neoadjuvant chemotherapy and tumor tissue for molecular profiling will be collected. Randomization will be performed centrally with stratification for center.
The inclusion of 32 patients in every treatment arm is required for statistical significance, of which 25 should complete treatment within the allocated time of 20 weeks for feasibility. If the result in an arm is compatible with a success of 85% in the studied population, the approach/arm should be further investigated. However, if we are unable to demonstrate a success in the studied population in at least 65%, the approach/arm should be rejected from further testing. In the neoadjuvant arm, progression before surgery will be considered a failure with respect to the primary endpoint. The exact type I and type II errors for each test are respectively 0.082 and 0.096 (i.e., the study is powered at 90% level).
The study will be stopped in case the 30-day mortality rate exceeds 20% (early stopping rule). A collaborating site will be temporarily closed in case of any death within the first 10 patients treated. A study arm will be closed in case of 2 deaths within the first 10 patients treated.
Future perspectives
The results of this study will allow the EORTC LCG to take the superior arm, if any, to a follow up study comparing e-PD to either no surgery or to EPP, based on the results of the MARS2 trial, currently running in the UK (36), which compares neo-adjuvant chemotherapy followed by (e-)PD or no (e-)PD, in analogy with its predecessor the MARS trial.
In addition, details of the surgical quality audit will allow to describe the variation in the e-PD procedure and standardize it for further trials and implementation.
By randomizing the patients upfront, before the first intervention and analyzing by intention-to-treat, an exact starting point for survival to event estimation will be available, avoiding the trap of immortal time bias and addressing the issue of optimal sequencing of chemotherapy in its different aspects of efficacy and toxicity.
Conclusions
EORTC 1205 is an important trial in mesothelioma, which can help to shed light on the role of e-PD and the optimal sequencing of chemotherapy in a multimodality protocol, in addition to fostering collaboration between major surgical oncological centers and preparing for the next generation of multimodality trials in mesothelioma.
Acknowledgements
This work was supported by the European Organisation for Research and Treatment of Cancer.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wolf AS, Daniel J, Sugarbaker DJ. Surgical techniques for multimodality treatment of malignant pleural mesothelioma: Extrapleural pneumonectomy and pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:132-48. [Crossref] [PubMed]
- Butchart EG, Ashcroft T, Barnsley WC, et al. Pleuropneumonectomy in the management of diffuse malignant mesothelioma of the pleura. Experience with 29 patients. Thorax 1976;31:15-24. [Crossref] [PubMed]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [Crossref] [PubMed]
- Rice D, Rusch V, Pass H, et al. Recommendations for uniform definitions of surgical techniques for malignant pleural mesothelioma. J Thorac Oncol 2011;6:1304-12. [Crossref] [PubMed]
- Lang-Lazdunski L, Bille A, Papa S, et al. Pleurectomy/decortication, hyperthermic pleural lavage with povidone-iodine, prophylactic radiotherapy, and systemic chemotherapy in patients with malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 2015;149:558-65; discussion 565-6. [Crossref] [PubMed]
- Friedberg JS, Simone CB, Culligan MJ, et al. Extended pleurectomy-decortication-based treatment for advanced stage epithelial mesothelioma yielding a median survival of nearly three years. Ann Thorac Surg 2017;103:912-9. [Crossref] [PubMed]
- Sharkey AJ, Bilancia R, Tenconi S, et al. Extended pleurectomy decortication for malignant pleural mesothelioma in the elderly: the need for an inclusive yet selective approach. Interact Cardiovasc Thorac Surg 2017;25:696-702. [Crossref] [PubMed]
- Burt BM, Cameron RB, Mollberg NM, et al. Malignant pleural mesothelioma and the society of thoracic surgeons database: an analysis of surgical morbidity and mortality. J Thorac Cardiovasc Surg 2014;148:30-5. [Crossref] [PubMed]
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: A harmful procedure. Lung Cancer 2012;77:151-5. [Crossref] [PubMed]
- Williams T, Duraid H, Watson S, et al. Extended pleurectomy and decortication for malignant pleural mesothelioma is an effective and safe cytoreductive surgery in the elderly. Ann Thorac Surg 2015;100:1868-74. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: Results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6. [Crossref] [PubMed]
- Flores RM, Riedel E, Donington JS, et al. Frequency of use and predictors of cancer directed surgery in the management of malignant pleural mesothelioma in a community-based (Surveillance, Epidemiology, and End Results [SEER]) population. J Thorac Oncol 2010;5:1649-54. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Weder W, Kestenholz P, Taverna C, et al. Neoadjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. J Clin Oncol 2004;22:3451-7. [Crossref] [PubMed]
- Weder W, Stahel R, Bernhard J, et al. Swiss Group for Clinical Cancer Research. Multicenter trial of neo-adjuvant chemotherapy followed by extrapleural pneumonectomy in malignant pleural mesothelioma. Ann Oncol 2007;18:1196-202. [Crossref] [PubMed]
- Rea F, Marulli G, Bortolotti L, et al. Induction chemotherapy, extrapleural pneumonectomy (EPP) and adjuvant hemi-thoracic radiation in malignant pleural mesothelioma (MPM): feasibility and results. Lung Cancer 2007;57:89-95. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Trimodality treatment of malignant pleural mesothelioma. J Thorac Oncol 2008;3:499-504. [Crossref] [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [Crossref] [PubMed]
- Van Schil PE, Baas P, Gaafar R, et al. European Organisation for Research and Treatment of Cancer (EORTC) Lung Cancer Group. Trimodality therapy for malignant pleural mesothelioma: results from an EORTC phase II multicentre trial. Eur Respir J 2010;36:1362-9. [Crossref] [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: an intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [Crossref] [PubMed]
- de Perrot M, Feld R, Leighl NB, et al. Accelerated hemithoracic radiation followed by extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2016;151:468-73. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Weder W, Stahel RA, Baas P, et al. The MARS feasibility trial: conclusions not supported by data. Lancet Oncol 2011;12:1093-4. [Crossref] [PubMed]
- Woolhouse I, Bishop L, Darlison L, et al. British Thoracic Society guideline for the investigation and management of malignant pleural mesothelioma. Thorax 2018;73:i1-i30. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, Extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Sharkey AJ, O'Byrne KJ, Nakas A, et al. How does the timing of chemotherapy affect outcome following radical surgery for malignant pleural mesothelioma? Lung Cancer 2016;100:5-13. [Crossref] [PubMed]
- Cao C, Tian D, Manganas C, et al. Systematic review of trimodality therapy for patients with malignant pleural mesothelioma. Ann Cardiothorac Surg 2012;1:428-37. [PubMed]
- NSCLC Meta-analysis Collaborative Group. Preoperative chemotherapy for non-small-cell lung cancer: a systematic review and meta-analysis of individual participant data. Lancet 2014;383:1561-71. [Crossref] [PubMed]
- Abdel-Rahman O, Elsayed Z, Mohamed H, et al. Radical multimodality treatment for malignant mesothelioma. Cochrane Database Syst Rev 2018;1. [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomized, controlled, open-label phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Grosso F, Steele N, Novello S, et al. Nintedanib plus Pemetrexed/Cisplatin in patients with malignant pleural mesothelioma: phase II results from the randomized, placebo-controlled LUME-Meso trial. J Clin Oncol 2017;35:3591-600. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Greillier L, et al. Abstract LBA58_PR: Second or 3rd line nivolumab (Nivo) versus nivo plus ipilimumab (Ipi) in malignant pleural mesothelioma (MPM) patients: Updated results of the IFCT-1501 MAPS2 randomized phase 2 trial. Ann Oncol 2017;28:suppl_5.
- Wallington M, Saxon EB, Bomb M, et al. 30-day mortality after systemic anticancer treatment for breast and lung cancer in England: a population-based, observational study. Lancet Oncol 2016;17:1203-16. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of malignant pleural mesothelioma: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Lim EM. MARS 2: a feasibility study comparing (extended) pleurectomy decortication versus no pleurectomy decortication in patients with malignant pleural mesothelioma (MARS2). Available online: http://www.clinicaltrials.gov/show/NCT02040272. Accessed January 23, 2018.