The role of interleukin-6 in malignant mesothelioma
Introduction
Malignant mesothelioma (MM) is a rare aggressive solid tumour that is invariably incurable. Current first-line chemotherapy regimens have minimal impact on overall survival and poor outcomes are compounded by the lack of an established second-line or maintenance therapy. Moreover, this bleak outlook is typified by the disappointing results yielded from recent clinical trials of novel targeted and cytotoxic agents. In industrialized countries, the incidence of MM is expected to rise in the next 20 to 30 years by 10-15% per year (1). Males represent 70-80% of cases (2) and from studies in US, UK and Japan, the combined MM-related death toll is predicted to sum up to more than 260,000 by 2050 (1-3). Meanwhile in Australia alone, such cases will increase to about 18,000 by 2020 (4). The nomenclature for MM is typically based on the sites of origin, which in order of prevalence are pleural (80%), peritoneal (10-20%), and pericardial (5%) (1). From a histological perspective, MM can be categorised into three subtypes; epithelioid (80%), sarcomatoid (10%), and biphasic or mixed (10%) (5). Previous reports have demonstrated greater variation of these statistics, namely 50%, 34% and 16% for epithelioid, biphasic and sarcomatoid respectively (6). The median overall survival (OS) rate of MM remains poor at approximately 10 months from the onset of symptoms (7). In a US study on pleural MM, 94% of patients died within 24 months from diagnosis and only less than 1% survived up to 5 years (8). Although the epithelioid subtype is consistently more prevalent, the non-epithelioid subtypes generally carry a worse prognosis (9).
The most significant aetiological factor of MM is asbestos exposure (10). After US, UK and France, Australia ranked 4th among the western world in the gross consumption of asbestos-cement products and is the highest on per capita basis (11). Indeed, the rising prevalence has also been attributed to non-occupational asbestos exposure (e.g., home renovation) causing a potential third wave of asbestos related disease. Moreover, the expected surge in MM diagnosis in the next few decades is due to the latency period between the first asbestos exposure and onset of symptoms which can last between 15-67 years (7). Other aetiological factors including exposure to minerals such as fibrous zeolite and fluoredenite amphibole (10). The onset of MM clinical symptoms most commonly present between ages 50-70 years (2). Most prevalent symptoms are chest/bone pain, dyspnoea, dysphagia and paraneoplastic syndrome namely thrombocytosis with an incidence of 30-40% (12). Other paraneoplastic phenomena may present such as endocrinopathies [e.g., syndrome of inappropriate antidiuretic hormone secretion (SIADH), hypoglycaemia, hypercalcaemia] and amyloid A amyloidosis (12-14). Radiological findings in this disease typically include pleural effusions and/or thickening (2). Poor prognostic factors of MM are non-epithelioid subtypes, male gender, >75 years of age, poor performance status as well as thrombocytosis (platelets ≥400×109/L), white blood cell ≥8.3×109/L and lactate dehydrogenase ≥500 IU/L (2). In addition, high levels of C-reactive protein (CRP), interleukin (IL)-4Rα and angiogenesis have also been established as poor prognostic factors (15-17).
Various cytotoxic agents have exhibited a modicum of efficacy in MM (Table 1) with a plethora of monotherapeutic and combinatorial trials showing superior efficacy with platinum-containing regimes in comparison with either single agent or non-platinum based therapies (28-30). However, a gold-standard first line treatment has only been established recently. In this setting for pleural MM, Vogelzang et al. investigated the comparative efficacy of a novel platinum-doublet consisting of cisplatin and pemetrexed (multifolate antagonist) against cisplatin monotherapy with an overall response rate (ORR) of 41.3% and 16.7% respectively (P<0.0001). Similarly, both median time to progression (5.7 vs. 3.9 months; P=0.001) and OS (12.1 vs. 9.3 months; P=0.02) favoured the combination arm which also had an acceptable safety profile (21). Although the authors reported toxicities including neutropenia, leukopenia, nausea, vomiting and fatigue, severe side effects could be effectively ameliorated with the administration of vitamin B12 and folic acid without compromising the anti-folate activity of pemetrexed (18). While no formal comparison with best supportive care (BSC) has been performed, it is estimated that this doublet regimen would confer an OS advantage of 3 months over BSC (31). With respect to peritoneal mesothelioma, there are a paucity of studies focusing on this particular subset of patients and current treatment strategies are based on data extrapolated from aforementioned trials with pleural MM. However, a study by Deraco et al. investigated the effect of perioperative systemic chemotherapy in patients who had undergone cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Unfortunately, there was no significant impact on OS and substantial post-operative morbidity was reflected in the considerable surgical and medical complications encountered (32).
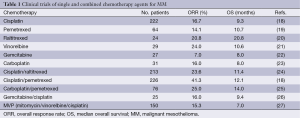
Full table
Depressingly, in over a decade since the Vozelgang study there has been no significant progress in systemic treatment for MM which is further exemplified by the absence of a widely accepted standard second-line therapy. This is in discordance with the management of most other solid tumours (33). Although recent successes have been forged with targeted therapies in a myriad of malignancies, this has not translated to the clinical treatment of MM (Table 2), with no appreciable extension in either progression free survival (PFS) or OS seen beyond that evident with cisplatin and pemetrexed. This lack of significant progress also extends to the sphere of radiotherapy and surgical management of MM. Although radiotherapy is often effective in palliating symptoms, it does not prolong OS (45). Furthermore, hemithoracic radiation alone has an associated 17% mortality rate while intensity modulated irradiation therapy (IMRT) is allied with significant pleural toxicity (2). With respect to surgery, the most common approaches include surgical pleurodesis via video assisted thoracoscopic surgery (VATS), surgical debulking and extra pleural pneumonectomy (EPP) (2). Debulking surgery has lower mortality rates (<5%) but the procedure seldom results in complete tumour resection. While EPP reduces local recurrence and may prolong OS, the morbidity rate was 60% (46,47).
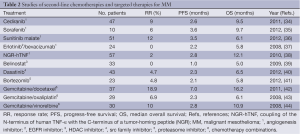
Full table
Indeed, a tri-modality treatment approach involving surgery, chemotherapy and radiation therapy has recently been compared with chemotherapy alone. The Mesothelioma and Radical Surgery (MARS) feasibility study compared three cycles of neoadjuvant chemotherapy followed by EPP and radical radiotherapy to chemotherapy alone (2). Poor outcomes and high morbidity were associated with tri-modality therapy alongside significant complications including such as grade 3 fatigue, pain, dyspnoea and paraplegia in 5 out of 8 patients who had undergone radical radiotherapy after EPP and chemotherapy (48). Interestingly, the one year survival was also higher in patients who did not receive EPP (73.1% vs. 52.2%) and in conclusion, the trimodality approach was not feasible (48). These poor treatment outcomes and lack of established second-line therapies indicate an unmet need for effective treatment strategies against MM. A potential approach to address this lies with identifying chief orchestrators of processes facilitating disease progression. In this regard, factors associated with inflammation are burgeoning areas of research. Indeed, the chronic inflammatory response triggered by prolonged asbestos exposure is thought to reduce anti-tumour immunity and subsequently enhance MM pathogenesis (49). This hypothesis was derived from the discovery of immunocompetent T-cells that produce the proinflammatory cytokine, IL-6, during asbestos exposure. The aetiological role of chronic inflammation in MM is also supported in a study by Hillegass et al. where elevation of inflammatory cytokines including IL-6 were observed (50). Furthermore, several other MM studies have consistently confirmed elevated IL-6 concentrations in serum and pleural fluid, suggesting a pivotal role for this cytokine in MM (51-55).
These observations are also mirrored in numerous malignancies such as breast, gastrointestinal, leukaemia, lymphoma, lung, melanoma, multiple myeloma, pancreatic, prostate, renal cell and gynaecological malignancies (56,57). Significantly, several reports have highlighted the integral role of IL-6 in facilitating key pathways and processes within the respective tumour microenvironments of these diseases (Table 3). Amidst other inflammatory cytokines and immunocompetent cells involved in MM development, there is compelling evidence to suggest that IL-6 has a significant role within the MM tumour microenvironment and may serve as a potential therapeutic target.

Full table
Functions of IL-6
IL-6 is a pleiotropic cytokine produced by various cells including macrophages, B cells, T cells, syncytiotrophoblasts, fibroblasts, epidermal keratinocytes, monocytes, endothelial cells and mesangial cells (86). Primary physiological functions involve induction of antibody production and acute phase reactions by stimulating B cells and hepatocyte respectively (87). It also plays a role in antigen-specific immune responses and inflammatory reactions (87). Besides mediating proliferation of T cells, thymocytes and synovial fibroblasts, IL-6 assists in the differentiation of cytotoxic T cell, macrophages, megakaryocytes and osteoclasts (87). In haematopoiesis, IL-6 promotes formation of multilineage blast cell colonies by acting synergistically with IL-3 (87). With respect to endothelial cells, it enhances expression of adhesion molecules and production of monocyte chemoattractant protein-1 (MCP-1) (87). Alongside its role in the recruitment of mesenchymal vascular cells and subsequent promotion of neoangiogenesis (87,88), there is ample evidence to support the significance of IL-6 in vital cellular processes.
IL-6 receptors and signalling
The IL-6 receptor consists of two polypeptide chains which exist in transmembrane and soluble forms (88). The α chain, known as gp80 (IL-6R), is an 80 kDa glycoprotein which binds specifically to IL-6 with high avidity (88). However its expression is restricted to hepatocytes and specialized subsets of leucocytes (monocytes, neutrophils, T-cells and B-cells). Unlike gp80, the 130kDa β chain; gp130, is ubiquitously expressed and mediates IL-6 signalling transduction (88). Alongside IL-6, gp130 binds to the additional cytokines namely IL-11, IL-27, leukaemia inhibitory factor, ciliary neurotrophic factor, oncostatin M, cardiotrophin-1 and neurotrophin-1 (88), which together comprise the IL-6 superfamily.
There are two predominant modes of IL-6 signalling. The binding of IL-6 to membrane bound gp80 and subsequent association with gp130 mediates ‘classical signalling’ (Figure 1) (89) through which IL-6 principally exerts its homeostatic functions.
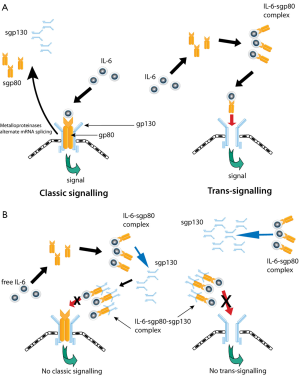
The soluble IL-6 receptors, sgp80 (or sIL-6R) and sgp130, result from either cleavage of the transmembranous proteins (via metalloproteinases) or translation from alternative spliced mRNA. Interestingly, unlike most soluble receptors, sgp80 behaves as an agonist, which contrasts with the inhibitory activity of sgp130 (89). Signalling as a result of IL-6/sgp80 complex binding to transmembranous gp130 is referred to as ‘trans-signalling’, which allows induction of IL-6 signalling in cells that lack membrane-bound gp80 (Figure 1) (89). This form of signal transduction is the fulcrum for tumourigenic processes attributed to IL-6 (89). Until recently, sgp130 was thought to inhibit only trans-signalling and not classical signalling as IL-6 does not interact with gp130 directly (89). However, a report has demonstrated that sgp130 also inhibits classical signalling indirectly by trapping IL-6/sgp80 complex hence eliminating free surrounding IL-6 which subsequently negates any binding to membrane-bound gp80 which would otherwise exert classical signalling induction (90).
Within the sphere of the tumour microenvironment, the malignant repertoire of IL-6 appears to be prominently manifested through Signal Transducers and Activators of Transcription (STAT) signalling. In response to IL-6 binding, gp130 activates Janus Kinase (JAK)1 and JAK2 which subsequently phosphorylates a tyrosine residue of STAT3 (91). STAT3 dimerizes then translocates into the nucleus from the cytoplasm. The phosphorylated STAT3 dimer then binds to the IFN-γ activated sequence (GAS) element which induces expression of apoptotic regulatory genes (Bcl-xL, XIAP, Mcl-1, Fas, and c-myc). In addition, STAT3 binds to p53 to further impede apoptosis regulation. This signalling is terminated by suppressor of cytokine signalling (SOCS) and protein inhibitor of activated STAT (PIAS). IL-6 is also known to signal via additional pathways including RAS/MAPK/ERK, AP1/JNK, Cox-2, PI3K/AKT, Notch3/Jagged-1 and Wnt (91).
IL-6 and MM
Clinical symptoms
Elevated concentrations of circulating IL-6 in MM have been reported to confer clinical features commonly observed in MM patients. A study by Bielefeldt-Ohmann et al. found that high serum levels of IL-6 were associated with cachexia, liver damage, diarrhoea and abdominal distension in addition to cell depletion and functional depression in the peripheral lymphoid organs (52). Notably, most of these clinical symptoms were effectively abrogated in an in vivo mouse model treated with anti-IL-6 monoclonal antibody (mAb) and recombinant human (rh) IFN-α. However, there was no direct effect on retarding tumour cell proliferation (52). Other biological processes associated with increased levels of IL-6 include fever, chronic inflammation, thrombocytosis and Amyloid A amyloidosis (13,31,53,55). Interestingly, an in vivo ovarian cancer study discovered that the underlying mechanism of paraneoplastic thrombocytosis revolves around the production of hepatic thrombopoietin which is facilitated by tumour derived IL-6 (92). Hence, this could feasibly support a similar function for IL-6 in thrombocytosis associated with MM.
Autocrine growth factor
Despite the clinical associations with IL-6, there appear to be conflicting reports with respect to its role as an autocrine growth factor in MM. Schmitter et al. have previously concluded that IL-6 was not an autocrine growth factor as addition of rhIL-6 to MM cell lines did not induce DNA synthesis in the cells (54). These results are mirrored in the aforementioned in vivo study by Bielefeldt-Ohmann et al. whereby anti-IL-6 therapy had negligible effects on tumour growth. Contrastingly, Adachi et al. conducted the first study in MM that described a putative role for IL-6 as an autocrine growth factor; an effect mediated via STAT3 signalling (93). This report emphasized the crucial role of sIL-6R for signalling in MM cell lines in vitro as cells lacking these receptors did not stimulate cell proliferation in response to IL-6 exposure compared to those treated with both IL-6 and rhsIL-6. Furthermore, this growth mediated by IL-6/sIL-6R was effectively inhibited with humanized anti-IL-6R antibody (93). Such observations further consolidate the notion of IL-6 mediating tumorigenic processes through transignalling (89). There is also a suggestion that IL-6 exerts autocrine functioning indirectly through the high affinity receptor for alpha melanocyte stimulating hormone (α-MSH); melanocortin 1 receptor (MC1R). Catania et al. demonstrated that MC1R enhances MM cell line secretion of IL-6 in addition to IL-8 and TGF-β. Interestingly, MC1R inhibition with synthetic α-MSH significantly impeded cell proliferation (94).
Angiogenesis
IL-6 is a well-established proangiogenic factor in a variety of tumour types (57,95,96). Hence it follows that a causal relationship exists between IL-6 and angiogenesis within the MM tumour microenvironment. The aforementioned Adachi study confirmed that stimulation of MM cells in vitro by IL-6/sIL-6R increased vascular endothelial growth factor (VEGF) expression via JAK2/STAT3 signalling (93). Moreover, inhibition of IL-6 using an anti-IL-6R mAb abrogated VEGF expression stimulated by IL-6/sIL-6R (93,97). As with the effects on cell proliferation, this study also highlights the significance of sIL-6R for VEGF induction in MM. Significant increases in the concentrations of VEGF in MM is further supported in a study by Kao et al. (98). They demonstrated strong correlations between circulating VEGF levels and OS. In a Phase II study in MM patients treated with thalidomide alone or in combination with cisplatin and gemcitabine, subjects with high VEGF levels (> median levels) which decreased to < median levels within 8 weeks of therapy had significantly prolonged survival compared to patients who had increased VEGF (P<0.05). Despite this, no such correlations with survival were witnessed with IL-6 and sIL-6 in this study. Hence, in light of the aforementioned preliminary data, future studies will be required to elucidate whether the potent proangiogenic effects of IL-6 established in other tumour types are also evident in MM (98).
Chemoresistance
Chemotherapeutic responses in MM are often short lived and tumour progression can often present within a year of treatment completion. In a study of pleural MM, within 12 months, 59% of the tumours developed chemoresistance to vinorelbine, 31% against gemcitabine and 27% against cisplatin (99). Furthermore, epithelioid tumours were more chemoresistant compared to the non-epithelioid subtypes (33% vs. 18%), which appears counterintuitive in view of the poorer prognosis associated with the latter histotype. An additional study with pleural MM cell lines demonstrated varying degrees of chemoresistance towards standard first-line chemotherapy with cisplatin and pemetrexed; as monotherapies or in combination (100). A study by McLaren et al. found that the reduction of the cell growth correlated with a decrease in IL-6 levels even at low doses of cytotoxic agents (101). At increasing doses, however, a surge of IL-6 was observed. The authors associated this phenomenon with the temporary exacerbation of toxicities commonly seen in patients undergoing treatment and suggested the side effects would eventually be abated since IL-6 undergoes rapid plasma clearance. However, a further study observed a gradual reduction of IL-6 concentrations until 14 days following cisplatin and irinotecan treatment which was subsequently followed by a resurgence of IL-6. Hypothetically, this increase could mediate tumour progression (53).
Although the link between IL-6 and chemoresistance has been commonly attributed to its anti-apoptotic functions, within the realms of MM research there are few studies which focus on this aspect. Indeed, Adachi et al. concluded that IL-6/sIL-6R does not prevent apoptosis of MM cell lines induced by chemotherapy agents (93); a conclusion inferred from the observation that apoptotic cells were barely visible in the medium lacking IL-6/sIL-6R. This was linked to other studies which had demonstrated induction of apoptosis in MM cell lines by regimens such as cisplatin, progesterone and lovastatin. However, this contradicts the evidence of IL-6 behaving as a survival factor against drug-induced apoptosis in other tumour types as summarized in Table 3. Moreover, two separate in vitro and in vivo studies of pleural MM had demonstrated down-regulation of anti-apoptotic factors (e.g., Bcl-xl and Mcl-2) downstream of IL-6, leading to cisplatin- and TNF-α-induced apoptosis of the MM cells (102,103). Cytoplasmic or nuclear expression of another anti-apoptotic factor induced by IL-6, survivin, was also shown to be elevated in peritoneal MM patients and survivin gene knockdown had enhanced both spontaneous and drug-induced apoptosis (104). Interestingly, in pleural MM the high expression of survivin was found to correlate with higher level of apoptosis and proliferation of tumour cells (105). While Hmeljak et al. reported higher survivin expression in patients who responded to chemotherapy than those who had stable or progressed disease, Cregan et al. established that knockdown of survivin gene did not affect sensitivity of the pleural MM cell lines against cisplatin in vitro (105,106).
In addition, Fischer et al. have recently demonstrated that inhibition of PI3K signalling, which plays a role in regulating cellular drug trafficking, had reduced the chemoresistant population of MM cells and increased their sensitivity to pemetrexed (107). Furthermore, Giovannetti et al. reported that vandetanib, an EGFR/VEGFR-2/RET inhibitor that blocks Akt phosphorylation, had enhanced carboplatin and pemetrexed cytotoxicity as well as inducing apoptosis in pleural MM cell lines (108). In view of PI3K/Akt representing a prominent IL-6 signalling pathway, these studies suggest a possible indirect role for IL-6 mediated chemoresistance in MM.
IL-6 and MM prognosis
Although elevated serum concentrations of IL-6 has been implicated as a poor prognostic factor for advanced non-small cell lung cancer and metastatic breast carcinoma (80,109), such a role in MM has not been firmly established. However, there is certainly evidence associating IL-6 with established poor prognostic factors of MM including thrombocytosis, elevated CRP and IL-4Rα. This is exemplified by a study carried out by Nakano et al. that demonstrated significant correlations between IL-6, elevated platelet counts and CRP levels (53). Burt et al. also concluded that although IL-4Rα is a poor prognostic factor in MM, IL-4 has no direct effect on apoptosis or proliferation of the MM tumours (16). Administration of IL-4 increases the production of IL-6 significantly in pleural MM cell lines which may contribute to its poor prognosis. Conversely, when observing the relationship between plasma levels of IL-6 and patient survival, no significant difference has been reported (53,98). Hence, it is a possibility that IL-6 per se does have a detrimental effect on survival and is not an independent prognostic factor in MM. However, sIL-6R could potentially be assessed as a poor prognostic indicator due to its significant role in promoting cell proliferation.
Future directions
Amongst all solid malignancies, undoubtedly MM is viewed as one of the bleakest diseases in terms of its inherent chemoresistance which results in poor survival rates and the vastly disappointing responses to novel agents which have shown some promising activity in a selection of other tumour types. Taking these facts into consideration, there is an obvious urge to refresh the approach to developing systemic therapies that will forge new horizons in effective clinical management. This review has synthesized literature to support the validity of targeting the inflammatory cytokine IL-6 in an attempt to achieve this goal. IL-6 exhibits pleiotropy within the MM microenvironment by promoting cell proliferation, chemoresistance and clinical symptoms such as cachexia, thrombocytosis and immunosuppression. However, it must be stressed that the failure of monotherapeutic targeted salvage therapy (Table 2) would certainly preclude adopting similar approaches with anti-IL-6/anti-IL-6R mAb in future clinical trials for this disease. Although the biology of MM is indeed complex, perhaps the lack of success with novel therapeutics could also be explained by the paucity of studies looking at appropriate combinations of such drugs with inhibitors of targets responsible for inducing their intrinsic and acquired resistance. Interestingly, IL-6 is emerging as a potential mediator of resistance to standard cytotoxic agents used in MM (101). Furthermore, it has a role in the development of anti-angiogenic therapy resistance in numerous malignancies (96). Hence there is a sound rationale for developing trials with anti-IL-6 therapies utilised as adjunctive therapies to chemotherapy and anti-angiogenic agents either in combinatorial or maintenance settings. Appropriate stratification of patients likely to gain benefit through targeting IL-6 also requires further investigation. For example, both thrombocytosis and CRP are predominantly induced by IL-6 and could feasibly represent surrogate markers for IL-6 bioactivity. Whether these respective levels are robust predictors of response to anti-IL-6 therapies remains to be seen, but further basic research is a necessity to enable efficient translation of this approach.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: Facts, Myths, and Hypotheses. J Cell Physiol 2012;227:44-58. [PubMed]
- Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis 2008;3:34. [PubMed]
- Murayama T, Takahashi K, Natori Y, et al. Estimation of future mortality from pleural malignant mesothelioma in Japan based on an age-cohort model. Am J Ind Med 2006;49:1-7. [PubMed]
- Leigh J, Driscoll T. Malignant mesothelioma in Australia, 1945-2002. International Journal of Occupational and Environmental Health 2003;9:206-17. [PubMed]
- Corbishley CM. Pathology of malignant mesothelioma. In: Syrigos KN, Nutting CM, Roussos C. eds. Tumors of the Chest: Biology, Diagnosis and Management. Mannheim: Springer Berlin Heidelberg, 2006;493-501.
- Hillerdal G. Malignant mesothelioma 1982 - review of 4710 published cases. Br J Dis Chest 1983;77:321-43. [PubMed]
- British Thoracic Society Standards of Care Committee. BTS statement on malignant mesothelioma in the UK, 2007. Thorax 2007;62 Suppl 2:ii1-ii19. [PubMed]
- Ceresoli GL, Locati LD, Ferreri AJ, et al. Therapeutic outcome according to histologic subtype in 121 patients with malignant pleural mesothelioma. Lung Cancer 2001;34:279-87. [PubMed]
- Mineo TC, Ambrogi V. Malignant Pleural Mesothelioma: Factors Influencing the Prognosis. Oncology 2012;26:1164-75. [PubMed]
- Gibbs GW, Berry G. Mesothelioma and asbestos. Regul Toxicol Pharmacol 2008;52:S223-31. [PubMed]
- Olsen NJ, Franklin PJ, Reid A, et al. Increasing incidence of malignant mesothelioma after exposure to asbestos during home maintenance and renovation. Med J Aust 2011;195:271-4. [PubMed]
- Rusch VW. Clinical features and current treatment of diffuse malignant pleural mesothelioma. Lung Cancer 1995;12 Suppl 2:S127-46. [PubMed]
- Motoyama T, Honma T, Watanabe H, et al. Interleukin 6-producing malignant mesothelioma. Virchows Arch B Cell Pathol Incl Mol Pathol 1993;64:367-72. [PubMed]
- Doval DC, Pande SB, Sharma JB, et al. Haematologic Paraneoplastic Syndromes in Pleural Mesothelioma-A report of two cases and review of the literature. Indian J Chest Dis Allied Sci 2009;51:185-8.
- Ghanim B, Hoda MA, Winter MP, et al. Pretreatment Serum C-Reactive Protein Levels Predict Benefit From Multimodality Treatment Including Radical Surgery in Malignant Pleural Mesothelioma A Retrospective Multicenter Analysis. Ann Surg 2012;256:357-62. [PubMed]
- Burt BM, Bader A, Winter D, et al. Expression of interleukin-4 receptor alpha in human pleural mesothelioma is associated with poor survival and promotion of tumor inflammation. Clin Cancer Res 2012;18:1568-77. [PubMed]
- Edwards JG, Cox G, Andi A, et al. Angiogenesis is an independent prognostic factor in malignant mesothelioma. British Journal of Cancer 2001;85:863-8. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Scagliotti GV, Shin DM, Kindler HL, et al. Phase II study of pemetrexed with and without folic acid and vitamin B-12 as front-line therapy in malignant pleural mesothelioma. J Clin Oncol 2003;21:1556-61. [PubMed]
- Baas P, Ardizzoni A, Grossi F, et al. The activity of raltitrexed (Tomudex (R)) in malignant pleural mesothelioma: an EORTC phase II study (08992). Eur J Cancer 2003;39:353-7. [PubMed]
- Steele JP, Shamash J, Evans MT, et al. Phase II study of vinorelbine in patients with malignant pleural mesothelioma. J Clin Oncol 2000;18:3912-7. [PubMed]
- van Meerbeeck JP, Bass P, Debruyne C, et al. A phase II study of gemcitabine in patients with malignant pleural mesothelioma. Cancer 1999;85:2577-82. [PubMed]
- Raghavan D, Gianoutsos P, Bishop J, et al. Phase-II trial of carboplatin in the management of malignant mesothelioma. J Clin Oncol 1990;8:151-4. [PubMed]
- van Meerbeeck JP, Gaafar R, Manegold C, et al. Randomized phase III study of cisplatin with or without raltitrexed in patients with malignant pleural mesothelioma: An intergroup study of the European Organisation for Research and Treatment of Cancer Lung Cancer Group and the National Cancer Institute of Canada. J Clin Oncol 2005;23:6881-9. [PubMed]
- Castagneto B, Botta M, Aitini E, et al. Phase II study of pemetrexed in combination with carboplatin in patients with malignant pleural mesothelioma (MPM). Ann Oncol 2008;19:370-3. [PubMed]
- van Haarst JM, Baas P, Manegold C, et al. Multicentre phase II study of gemcitabine and cisplatin In malignant pleural mesothelioma. Br J Cancer 2002;86:342-5. [PubMed]
- Andreopoulou E, Ross PJ, O’Brien ME, et al. The palliative benefits of MVP (mitomycin C, vinblastine and cisplatin) chemotherapy in patients with malignant mesothelioma. Ann Oncol 2004;15:1406-12. [PubMed]
- Ellis P, Davies AM, Evans WK, et al. Lung Canc Dis Site Grp Canc Care O., The use of chemotherapy in patients with advanced malignant pleural mesothelioma: A systematic review and practice guideline. J Thorac Oncol 2006;1:591-601. [PubMed]
- Bertino P, Carbone M, Pass H. Chemotherapy of malignant pleural mesothelioma. Expert Opin Pharmacother 2009;10:99-107. [PubMed]
- Vogelzang NJ. Chemotherapy for malignant pleural mesothelioma. Lancet 2008;371:1640-2. [PubMed]
- Nowak AK. Chemotherapy for malignant pleural mesothelioma: a review of current management and a look to the future. Ann Cardiothorac Surg 2012;1:508-15. [PubMed]
- Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1093-100. [PubMed]
- Zucali PA, Simonelli M, Michetti G, et al. Second-line chemotherapy in malignant pleural mesothelioma: Results of a retrospective multicenter survey. Lung Cancer 2012;75:360-7. [PubMed]
- Garland LL, Chansky K, Wozniak AJ, et al. Phase II Study of Cediranib in Patients with Malignant Pleural Mesothelioma SWOG S0509. J Thorac Oncol 2011;6:1938-45. [PubMed]
- Papa S, Popat S, Shah R, et al. Phase 2 study of sorafenib in malignant mesothelioma previously treated with platinum-containing chemotherapy. J Thorac Oncol 2013;8:783-7. [PubMed]
- Nowak AK, Millward MJ, Creaney J, et al. A Phase II Study of Intermittent Sunitinib Malate as Second-Line Therapy in Progressive Malignant Pleural Mesothelioma. J Thorac Oncol 2012;7:1449-56. [PubMed]
- Jackman DM, Kindler HL, Yeap BY, et al. Erlotinib plus bevacizumab in previously treated patients with malignant pleural mesothelioma. Cancer 2008;113:808-14. [PubMed]
- Gregorc V, Zucali PA, Santoro A, et al. Phase II Study of Asparagine-Glycine-Arginine-Human Tumor Necrosis Factor alpha, a Selective Vascular Targeting Agent, in Previously Treated Patients With Malignant Pleural Mesothelioma. J Clin Oncol 2010;28:2604-11. [PubMed]
- Ramalingam SS, Belani CP, Ruel C, et al. Phase II Study of Belinostat (PXD101), a Histone Deacetylase Inhibitor, for Second Line Therapy of Advanced Malignant Pleural Mesothelioma. J Thorac Oncol 2009;4:97-101. [PubMed]
- Dudek AZ, Pang H, Kratzke RA, et al. Phase II study of dasatinib in patients with previously treated malignant mesothelioma (cancer and leukemia group B 30601): a brief report. J Thorac Oncol 2012;7:755-9. [PubMed]
- Fennell DA, McDowell C, Busacca S, et al. Phase II Clinical Trial of First or Second-Line Treatment with Bortezomib in Patients with Malignant Pleural Mesothelioma. J Thorac Oncol 2012;7:1466-70. [PubMed]
- Tourkantonis I, Makrilia N, Ralli M, et al. Phase II study of gemcitabine plus docetaxel as second-line treatment in malignant pleural mesothelioma: a single institution study. Am J Clin Oncol 2011;34:38-42. [PubMed]
- Xanthopoulos A, Bauer TT, Blum TG, et al. Gemcitabine combined with oxaliplatin in pretreated patients with malignant pleural mesothelioma: an observational study. J Occup Med Toxicol 2008;3:34. [PubMed]
- Zucali PA, Ceresoli GL, Garassino I, et al. Gemcitabine and vinorelbine in pemetrexed-pretreated patients with malignant pleural mesothelioma. Cancer 2008;112:1555-61. [PubMed]
- Ball DL, Cruickshank DG. The treatment of malignant mesothelioma of the pleura: review of a 5-year experience, with special reference to radiotherapy. Am J Clin Oncol 1990;13:4-9. [PubMed]
- Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant pleural mesothelioma. J Thorac Cardiovasc Surg 2001;122:788-95. [PubMed]
- Sugarbaker DJ, Jaklitsch MT, Bueno R, et al. Prevention, early detection, and management of complications after 328 consecutive extrapleural pneumonectomies. J Thorac Cardiovasc Surg 2004;128:138-46. [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [PubMed]
- Matsuzaki H, Maeda M, Lee S, et al. Asbestos-induced cellular and molecular alteration of immunocompetent cells and their relationship with chronic inflammation and carcinogenesis. J Biomed Biotechnol 2012;2012:492608.
- Hillegass JM, Shukla A, Lathrop SA, et al. Inflammation precedes the development of human malignant mesotheliomas in a SCID mouse xenograft model. Ann N Y Acad Sci 2010;1203:7-14. [PubMed]
- Higashihara M, Sunaga S, Tange T, et al. Increased secretion of interleukin-6 in malignant mesothelioma cells from a patient with marked thrombocytosis. Cancer 1992;70:2105-8. [PubMed]
- Bielefeldt-Ohmann H, Marzo AL, Himbeck RP, et al. Interleukin-6 involvement in mesothelioma pathobiology: inhibition by interferon alpha immunotherapy. Cancer Immunol Immunother 1995;40:241-50. [PubMed]
- Nakano T, Chahinian AP, Shinjo M, et al. Interleukin 6 and its relationship to clinical parameters in patients with malignant pleural mesothelioma. Br J Cancer 1998;77:907-12. [PubMed]
- Schmitter D, Lauber B, Fagg B, et al. Hematopoietic growth factors secreted by seven human pleural mesothelioma cell lines: interleukin-6 production as a common feature. Int J Cancer 1992;51:296-301. [PubMed]
- Monti G, Jaurand MC, Monnet I, et al. Intrapleural production of interleukin-6 during mesothelioma and its modulation by gamma-interferon treatment. Cancer Res 1994;54:4419-23. [PubMed]
- Trikha M, Corringham R, Klein B, et al. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: A review of the rationale and clinical evidence. Clin Cancer Res 2003;9:4653-65. [PubMed]
- Coward JI, Kulbe H. The role of interleukin-6 in gynaecological malignancies. Cytokine Growth Factor Rev 2012;23:333-42. [PubMed]
- Jourdan M, De Vos J, Mechti N, et al. Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: interleukin-6, interferon-alpha and insulin-like growth factor 1. Cell Death Differ 2000;7:1244-52. [PubMed]
- Ishikawa H, Tsuyama N, Liu SQ, et al. Accelerated proliferation of myeloma cells by interleukin-6 cooperating with fibroblast growth factor receptor 3-mediated signals. Oncogene 2005;24:6328-32. [PubMed]
- Voorhees PM, Chen Q, Kuhn DJ, et al. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clin Cancer Res 2007;13:6469-78. [PubMed]
- Pu YS, Hour TC, Chuang SE, et al. Interleukin-6 is responsible for drug resistance and anti-apoptotic effects in prostatic cancer cells. Prostate 2004;60:120-9. [PubMed]
- Nakashima J, Tachibana M, Horiguchi Y, et al. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res 2000;6:2702-6. [PubMed]
- Akimoto S, Okumura A, Fuse H. Relationship between serum levels of interleukin-6, tumor necrosis factor-alpha and bone turnover markers in prostate cancer patients. Endocr J 1998;45:183-9. [PubMed]
- Santer FR, Malinowska K, Culig Z, et al. Interleukin-6 trans-signalling differentially regulates proliferation, migration, adhesion and maspin expression in human prostate cancer cells. Endocr Relat Cancer 2010;17:241-53. [PubMed]
- Rojas A, Liu G, Coleman I, et al. IL-6 promotes prostate tumorigenesis and progression through autocrine cross-activation of IGF-IR. Oncogene 2011;30:2345-55. [PubMed]
- Borsellino N, Belldegrun A, Bonavida B. Endogenous interleukin-6 is a resistance factor for cis-diamminedichloroplatinum and etoposide-mediated cytotoxicity of human prostate carcinoma cell-lines. Cancer Res 1995;55:4633-9. [PubMed]
- Tempfer C, Zeisler H, Sliutz G, et al. Serum evaluation of interleukin 6 in ovarian cancer patients. Gynecol Oncol 1997;66:27-30. [PubMed]
- Berek JS, Chung C, Kaldi K, et al. Serum interleukin-6 levels correlate with disease status in patients with epithelial ovarian cancer. Am J Obstet Gynecol 1991;164:1038-42; discussion 1042-3. [PubMed]
- Kallio JP, Tammela TL, Marttinen AT, et al. Soluble immunological parameters and early prognosis of renal cell cancer patients. J Exp Clin Cancer Res 2001;20:523-8. [PubMed]
- Mizutani Y, Bonavida B, Koishihara Y, et al. Sensitization of human renal-cell carcinoma-cells to cis-diaminedichloroplatinum(II) by anti-interleukin-6 monoclonal-antibody or anti-interleukin-6 receptor monoclonal-antibody. Cancer Res 1995;55:590-6. [PubMed]
- Suchi K, Fujiwara H, Okamura S, et al. Overexpression of Interleukin-6 Suppresses Cisplatin-induced Cytotoxicity in Esophageal Squamous Cell Carcinoma Cells. Anticancer Res 2011;31:67-75. [PubMed]
- Brozek W, Bises G, Girsch T, et al. Differentiation-dependent expression and mitogenic action of interleukin-6 in human colon carcinoma cells: Relevance for tumour progression. Eur J Cancer 2005;41:2347-54. [PubMed]
- Foran E, Garrity-Park MM, Mureau C, et al. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol Cancer Res 2010;8:471-81. [PubMed]
- Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell 2008;13:7-9. [PubMed]
- Lu C, Kerbel RS. Interleukin-6 undergoes transition from paracrine growth inhibitor to autocrine stimulator during human melanoma progression. J Cell Biol 1993;120:1281-8. [PubMed]
- Silvani A, Ferrari G, Paonessa G, et al. Down-regulation of interleukin-6 receptor-alpha chain in interleukin-6 transduced melanoma-cells causes selective resistance to interleukin-6 but not to oncostatin-m. Cancer Res 1995;55:2200-5. [PubMed]
- Shi Z, Yang WM, Chen LP, et al. Enhanced chemosensitization in multidrug-resistant human breast cancer cells by inhibition of IL-6 and IL-8 production. Breast Cancer Res Treat 2012;135:737-47. [PubMed]
- Sasser AK, Sullivan NJ, Studebaker AW, et al. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. Faseb Journal 2007;21:3763-70. [PubMed]
- Studebaker AW, Storci G, Werbeck JL, et al. Fibroblasts Isolated from Common Sites of Breast Cancer Metastasis Enhance Cancer Cell Growth Rates and Invasiveness in an Interleukin-6-Dependent Manner. Cancer Res 2008;68:9087-95. [PubMed]
- Zhang GJ, Adachi I. Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer Res 1999;19:1427-32. [PubMed]
- Yabuhara A, Komiyama A. Castlemans disease and interleukin-6. Leukemia & Lymphoma 1990;2:369-80.
- Beck JT, Hsu SM, Wijdenes J, et al. Alleviation of systemic manifestations of castlemans disease by monoclonal anti-interleukin-6 antibody. N Engl J Med 1994;330:602-5. [PubMed]
- Hohaus S, Massini G, Giachelia M, et al. Anemia in Hodgkin’s Lymphoma: The Role of Interleukin-6 and Hepcidin. J Clin Oncol 2010;28:2538-43. [PubMed]
- Fayad L, Cabanillas F, Talpaz M, et al. High serum interleukin-6 levels correlate with a shorter failure-free survival in indolent lymphoma. Leukemia & Lymphoma 1998;30:563-71. [PubMed]
- Kanno H, Yasunaga Y, Iuchi K, et al. Interleukin-6-mediated growth enhancement of cell lines derived from pyothorax-associated lymphoma. Lab Invest 1996;75:167-73. [PubMed]
- Naka T, Nishimoto N, Kishimoto T. The paradigm of IL-6: from basic science to medicine. Arthritis Res 2002;4 Suppl 3:S233-42. [PubMed]
- Kishimoto T. The biology of interleukin-6. Blood 1989;74:1-10. [PubMed]
- Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther 2006;8 Suppl 2:S2. [PubMed]
- Scheller J, Ohnesorge N, Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 2006;63:321-9. [PubMed]
- Garbers C, Thaiss W, Jones GW, et al. Inhibition of Classic Signaling Is a Novel Function of Soluble Glycoprotein 130 (sgp130), Which Is Controlled by the Ratio of Interleukin 6 and Soluble Interleukin 6 Receptor. J Biol Chem 2011;286:42959-70. [PubMed]
- Guo Y, Xu F, Lu T, et al. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 2012;38:904-10. [PubMed]
- Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med 2012;366:610-8. [PubMed]
- Adachi Y, Aoki C, Yoshio-Hoshino N, et al. Interleukin-6 induces both cell growth and VEGF production in malignant mesotheliomas. Int J Cancer 2006;119:1303-11. [PubMed]
- Catania A, Colombo G, Carlin A, et al. Autocrine inhibitory influences of alpha-melanocyte-stimulating hormone in malignant pleural mesothelioma. J Leukoc Biol 2004;75:253-9. [PubMed]
- Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002;21:2000-8. [PubMed]
- Middleton K, Jones J, Lwin Z, et al. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol 2014;89:129-39. [PubMed]
- Adachi Y, Yoshio-Hoshino N, Aoki C, et al. VEGF Targeting in Mesotheliomas Using an Interleukin-6 Signal Inhibitor Based on Adenovirus Gene Delivery. Anticancer Res 2010;30:1947-52. [PubMed]
- Kao SC, Harvie R, Paturi F, et al. The predictive role of serum VEGF in an advanced malignant mesothelioma patient cohort treated with thalidomide alone or combined with cisplatin/gemcitabine. Lung Cancer 2012;75:248-54. [PubMed]
- Mujoomdar AA, Tilleman TR, Richards WG, et al. Prevalence of in vitro chemotherapeutic drug resistance in primary malignant pleural mesothelioma: Result in a cohort of 203 resection specimens. J Thorac Cardiovasc Surg 2010;140:352-5. [PubMed]
- Kitazono-Saitoh M, Takiguchi Y, Kitazono S, et al. Interaction and cross-resistance of cisplatin and pemetrexed in malignant pleural mesothelioma cell lines. Oncol Rep 2012;28:33-40. [PubMed]
- McLaren BR, Robinson BW, Lake RA. New chemotherapeutics in malignant mesothelioma: effects on cell growth and IL-6 production. Cancer Chemother Pharmacol 2000;45:502-8. [PubMed]
- Varin E, Denoyelle C, Brotin E, et al. Downregulation of Bcl-x(L) and Mcl-1 is sufficient to induce cell death in mesothelioma cells highly refractory to conventional chemotherapy. Carcinogenesis 2010;31:984-93. [PubMed]
- Fox SA, Loh SS, Dharmarajan AM, et al. Cisplatin and TNF-alpha downregulate transcription of Bcl-xL in murine malignant mesothelioma cells. Biochem Biophys Res Commun 2005;337:983-91. [PubMed]
- Zaffaroni N, Costa A, Pennati M, et al. Survivin is highly expressed and promotes cell survival in malignant peritoneal mesothelioma. Cell Oncol 2007;29:453-66. [PubMed]
- Hmeljak J, Erculj N, Dolzan V, et al. Is survivin expression prognostic or predictive in malignant pleural mesothelioma? Virchows Archiv 2013;462:315-21. [PubMed]
- Cregan IL, Dharmarajan AM, Fox SA. Mechanisms of cisplatin-induced cell death in malignant mesothelioma cells: Role of inhibitor of apoptosis proteins (IAPs) and caspases. Int J Oncol 2013;42:444-52. [PubMed]
- Fischer B, Frei C, Moura U, et al. Inhibition of phosphoinositide-3 kinase pathway down regulates ABCG2 function and sensitizes malignant pleural mesothelioma to chemotherapy. Lung Cancer 2012;78:23-9. [PubMed]
- Giovannetti E, Zucali PA, Assaraf YG, et al. Preclinical emergence of vandetanib as a potent antitumour agent in mesothelioma: molecular mechanisms underlying its synergistic interaction with pemetrexed and carboplatin. Br J Cancer 2011;105:1542-53. [PubMed]
- Songür N, Kuru B, Kalkan F, et al. Serum interleukin-6 levels correlate with malnutrition and survival in patients with advanced non-small cell lung cancer. Tumori 2004;90:196-200. [PubMed]


