Characterization of PD-L1 protein expression and CD8+ tumor-infiltrating lymphocyte density, and their associations with clinical outcome in small-cell lung cancer
Introduction
Small-cell lung cancer (SCLC) accounts for approximately 15% of all lung cancers (1,2). The high aggressiveness and early widespread metastasis of SCLC result in the majority of patients being diagnosed with extensive-stage disease (ES-SCLC) (1-3). Therapeutic strategies have not substantially changed in more than 40 years. The median overall survival (OS) for early stage (I–III) SCLC is 15–20 months and for ES-SCLC is 9–11 months; the 5-year survival rate is 20–25% for early-stage SCLC and only about 2–6% for ES-SCLC (4-7). In spite of a high response rate with initial platinum-based chemotherapy, almost all patients with ES-SCLC will subsequently relapse after a short period of response (1-4).
Immune checkpoint inhibitors such as cytotoxic T lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1) and its ligand (PD-L1) could significantly enhance the antitumor immunity (8-11). A number of clinical trials demonstrated that anti-PD-1/PD-L1 antibodies monotherapy could only show a response rate of nearly 20% in non-small cell lung cancer (NSCLC) (12-16), but this strategy only showed very limited efficacy in pre-treated SCLC (17). The combination of anti-CTLA-4 and anti-PD-1 antibody showed promising results with the 2-year OS rate of ~30% (18,19). Moreover, a recent randomized phase III trial (IMpower133) demonstrated a markedly prolonged progression-free survival (PFS) and OS with atezolizumab plus etoposide and carboplatin than with placebo plus etoposide and carboplatin (20), which has become a new standard of care in the first-line setting of ES-SCLC. Nevertheless, immune checkpoints inhibitors alone or combinations is still challenging due to their modest antitumor activities in SCLC.
Emerging evidence suggest that PD-L1 expression and pre-existing anti-tumor immunity [such as CD8+ tumor-infiltrating lymphocytes (TILs)] play a significant role in the clinical activity of anti-PD-1/PD-L1 immunotherapy (11,21,22). Although a large number of studies characterize PD-L1 expression and CD8+ TILs in NSCLC, the reported data on SCLC is limited. Furthermore, published results are inconsistent on the prevalence and prognostic significance of PD-L1 expression and CD8+ TILs in SCLC. The investigation of PD-L1 expression and CD8+ TILs characterization in relation to clinicopathological features and clinical outcomes in SCLC may guide the development of new treatment strategies, by providing novel stratification parameters for therapeutic selection and the future design of clinical trials with immune checkpoints inhibitors. Therefore, we conducted this study with 56 surgically resected SCLC and a meta-analysis of 15 publications with 1,505 patients to systematically characterize PD-L1 expression and CD8+ TIL, and their impacts on clinical outcome in patients with SCLC.
Methods
Patients’ selection
We retrospectively screened patients who underwent surgical resection, palliative operation or open biopsy due to the histologically-confirmed SCLC, between 2012 and 2015, at three hospitals. Patient clinicopathological parameters including age, gender, smoking history, tumor location, pathological stage, pathological lymph nodal factors, pleural invasion, lymphatic invasion, and vascular invasion were recorded. Pathological staging was performed using the 7th edition of the TNM Classification of Malignant Tumors (23,24). A person who has smoked fewer than 100 cigarettes during their lifetime was defined as never smoker. For postoperative chemotherapy (POCT), cisplatin/carboplatin plus etoposide was administrated (4–6 cycles). Postoperative thoracic irradiation (PORT) with a total dose of 50–60 Gy with 1.8–2.0 Gy per fraction for 5 days per week was administered. For patients without brain metastasis identified by brain magnetic resonance imaging (MRI) prior to prophylactic cranial irradiation (PCI), a total dose of 25 Gy with 2.5 Gy per fraction, or a total dose of 30 Gy with 3.0 Gy per fraction was administrated. The exclusion criteria included histologically-confirmed mixed SCLC, patients with inadequate samples for PD-L1 and CD8 staining or who disagreed with the research protocols. This study was approved by the ethics committee of Shanghai Pulmonary Hospital (FK-17-0113) and conducted in line with the provisions of the Declaration of Helsinki.
PD-L1 protein expression analysis
PD-L1 protein expression was evaluated in patients with SCLC by immunohistochemistry (IHC) as described in our previous studies (25,26). Briefly, tumor sections of formalin-fixed and paraffin-embedded (FFPE) samples were cut at widths of 4–5 µm, dewaxed with xylene, and rehydrated through a graded series of ethanol. Next, the sections were incubated with 3% H2O2 (10 minutes), blocked with 5% goat serum, and incubated with an anti-human PD-L1 antibody (diluted 1:100; #13684, clone E1L3N, Cell Signaling Technology). Then, a peroxidase-labeled secondary antibody was applied to the sections (30 minutes) at room temperature. All immunohistochemical images were assessed by two pathologists (Z Dong and L Hou). The cut-off point for PD-L1 positive/negative expression was 5% (25-28).
CD8+ TIL density assessment
CD8+ TIL density assessment was performed according to the previous reports (25,26,29,30). IHC for CD8+ TIL density was conducted on the fully automated Bond-III system (Leica Microsystems, Newcastle-upon-Tyne, UK) by using onboard heat-induced antigen retrieval with epitope retrieval solution 2 for 10 minutes at 99 °C, and then, incubated with a mouse anti-CD8 monoclonal antibody (M7103, clone C8144B, DAKO, Denmark) for 30 minutes at room temperature. This automated system utilized a Refine polymer detection kit with horseradish peroxidase (HRP)-polymer as a secondary antibody and DAB. All immunohistochemical images were also evaluated by two senior pathologists (Z Dong and L Hou). The cut-off value for high/low CD8+ TIL density was 5%.
Systematic review with meta-analysis
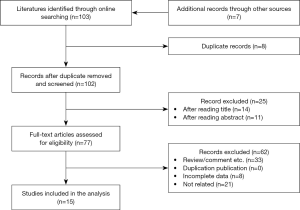
We then performed a literature review of publication search via the online databases including PubMed/Medline, Cochrane Library, EMBASE, Web of Science, and Google Scholar through June 2019, using “lung cancer” and “PD-L1”, and their corresponding keywords. Data on the association between PD-L1 expression and clinical outcomes, and clinicopathological features in patients with SCLC were identified from published articles meeting the inclusion criteria (Figure S1). The details of methodology are summarized in the Supplementary file 1.

Statistical analyses
Chi-square or Fisher’s exact tests were used to assess the associations between PD-L1 expression or CD8+ TILs density and clinicopathological characteristics. Continuous variables were analyzed by analysis of variance and Tukey’s multiple comparison tests. Kaplan-Meier curves were utilized to evaluate patients’ outcomes, and the log-rank tests were used to assess the significance of differences among groups. Cox proportional hazards models were leveraged for uni- and multivariate analyses to calculate the hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). The OS was calculated from the date of SCLC diagnosis to death from any cause or was censored at the last follow-up date. P<0.05 (two-sided) were considered statistically significant. Meta-analysis was performed using Stata version 14.0 (Stata Corporation, TX, USA). All statistical analyses were conducted using IBM SPSS Statistics v22.0 (IBM Corp., Armonk, NY, USA).
Results
Characterization of PD-L1 expression and CD8+ TIL density
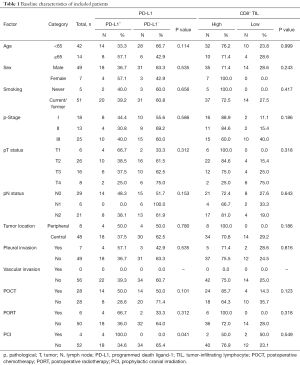
Fifty-six patients were finally analyzed. Most of them (75.0%) were <65 years old at initial diagnosis. Seven (12.5%) of them were female and 5 (8.9%) were never-smokers. Thirty-one (55.4%) patients were diagnosed with pathological stage I–II. Most (85.7%) of them had central tumor location. Seven (12.5%) of the patients had pleural invasion and none had vascular invasion. Twenty-eight (50.0%) patients received POCT, and 6 (10.7%) received PORT.
Representative images of PD-L1 protein expression and CD8+ TILs are listed in Figure 1A and Figure 1B. Twenty-two (39.3%) patients had positive PD-L1 expression (Figure 1C) and 42 (75.0%) had high CD8+ TIL density (Figure 1D). The clinicopathological features of all included patients are listed in Table 1. PD-L1 expression level was not associated with CD8+ TILs density (P=0.528; Figure 1E). No significant differences in PD-L1 expression including age (P=0.114), sex (P=0.535), smoking history (P=0.656), pathologic stages (I vs. II/III, P=0.586), lymph node metastasis (P=0.153), tumor location (peripheral vs. central, P=0.780), pleural invasion (P=0.535), POCT (P=0.101) and PORT (P=0.312) were observed. Of note, patients received PCI had higher proportion of positive PD-L1 expression than those without PCI (P=0.041). There were no significant differences in CD8+ TIL density in terms of all listed clinicopathological features (Table 1).

Full table
Prognostic value of PD-L1 protein expression and CD8+ TILs density
The median follow-up time was 618 days (range, 99–1,369 days). Kaplan-Meier curves indicated that positive PD-L1 expression was associated with a significantly longer OS (HR =0.37, 95% CI: 0.21–068; P=0.002; Figure 1F). High CD8+ TIL density was correlated with longer OS (HR =0.43, 95% CI: 0.13–0.72; P=0.008) (Figure 1G). Univariate analysis found age (HR =0.416, P=0.031), TNM stage (HR =5.105, P<0.001), T stage (HR =2.182, P=0.014), lymph node metastasis (HR =1.926, P=0.045) were also associated with prolonged OS (Table 2). Multivariate analyses showed that positive PD-L1 expression (HR =0.352, 95% CI: 0.166–0.748; P=0.007) and high CD8+ TILs density (HR =0.261, 95% CI: 0.114–0.601; P=0.002) were independently associated with significantly longer OS (Table 2). We further divided the population into four groups according to PD-L1 expression and CD8+ TIL density. Patients with negative PD-L1 expression and low CD8+ TILs density had the shortest OS (HR =0.36, P=0.003), while the positive PD-L1 expression and high CD8+ TIL density group had the longest OS (HR =0.34, P=0.001) (Figure S2).

Full table

Features of included studies in the meta-analysis
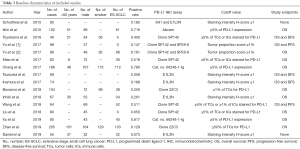
The detailed methodology of meta-analysis is summarized in Supplementary file 1 and Figure S1. Totally, 103 relevant publications were screened. The majority of the excluded publications were reviews, comments, duplications, or studies with incomplete data. A flowchart of publication selection was shown in Figure S3. The present study included 1,505 cases from 15 articles to investigate the prevalence of positive PD-L1 expression and its prognostic value in patients with SCLC (31-45). The main features of each study are summarized in Table 3 and Table S1.
Full table
Full table
Prevalence of positive PD-L1 expression and its prognostic value
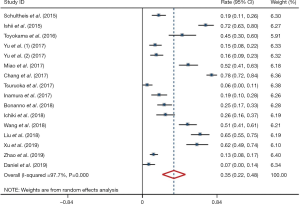
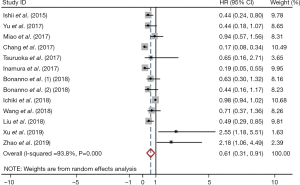
Meta-analysis showed that the prevalence of positive PD-L1 expression was 0.35 (95% CI: 0.22–0.48; Figure 2) and positive PD-L1 expression was correlated with significantly better OS (HR =0.61, 95% CI: 0.31–0.91; P<0.05; Figure 3). But both results exhibited high heterogeneity (I2=97.7%, P<0.001; I2=93.8%; P<0.001; respectively).
Publication bias
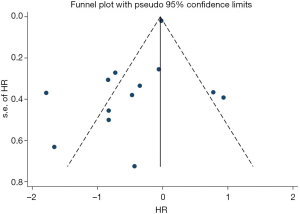
Sensitivity analysis was performed by deleting one study at one time to evaluate its effect on the pooled HRs. Deletion of the publication by Xu et al. or Zhao et al. slightly decreased the heterogeneity in the analysis of pooled HRs of PFS and OS (43,44). No other studies influenced the pooled results. Begg’s funnel plots and Egger’s tests were utilized to assess the publication bias. The Begg’s funnel plot was symmetric, and Egger’s tests suggested no evidence of publication bias (Figure S4).
Discussion
Accumulating evidence indicated that blockade of PD-1/PD-L1 interaction yielded a narrow antitumor effect in patients with ES-SCLC when compared with NSCLC. As the most important factors of antitumor immune response, PD-L1 expression and CD8+ TIL often determines whether anti-PD-1/PD-L1 antibodies works or not in various solid tumors (46,47). Understanding of PD-L1 expression and CD8+ TIL in SCLC could contribute to the research and development of more effective immune checkpoints blockade therapy. Furthermore, clarifying the prognostic value of PD-L1 expression and CD8+ TILs density in patients with SCLC would be helpful to precisely choose the sub-populations who could most benefit from anti-PD-1/PD-L1 antibodies therapy. In order to achieve these aims, the present study investigated the clinicopathological parameters of PD-L1 expression and CD8+ TIL density, and their correlations with clinical outcome in patients with surgically resected SCLC. The current results showed that the prevalence of PD-L1 expression in surgically resected SCLC is lower than that published for NSCLC. There was no any association between PD-L1 expression or CD8+ TIL density and clinicopathological parameters in SCLC. Positive PD-L1 expression and high CD8+ TIL density was independently correlated with better prognosis in patients with SCLC, and PD-L1 expression plus CD8+ TIL density could more precisely differentiate sub-populations with discrepant OS after surgical resection. Moreover, a meta-analysis of 15 published articles with 1,505 cases confirmed the lower prevalence and prognostic value of PD-L1 expression in patients with SCLC.
The prevalence of positive PD-L1 expression was 39.3% and 35.0% in the pooled analysis, which was lower than that reported in NSCLC (25,48-51). Although the detection of PD-L1 expression was influenced by a multitude of factors including laboratory conditions, testing platform and process, PD-L1 antibody assay and so on, most of published studies consistently reported the relatively low rate of PD-L1 expression in SCLC. For example, Yu et al. reported that the overall prevalence of PD-L1 expression in tumors was 16.5% with a tumor proportion score (TPS) cutoff ≥1% by using two approved anti-PD-1/PD-L1 antibodies (SP142 and clone 28-8) in 249 SCLC patients (34). Similarly, Zhao et al. reported that only 12.9% of 205 patients with surgically resected SCLC had positive PD-L1 expression by using clone 22C3 with a cutoff value of 1% (44). Interestingly, these two studies included patients from different ethnicities, indicating that low rate of PD-L1 expression is common in patients with SCLC. However, Chang et al. observed that the frequency of PD-L1 overexpression in tumors was 78.0% in 186 patients with SCLC (36), which was comparable to the expression rate in NSCLC. Of note, most of the included cases in Chang’s study was diagnosed with stage IV SCLC (60.2%). As they mentioned in the study, high PD-L1 expression was significantly associated with stage IV disease (P=0.048) (36), which could be partially explain the high prevalence of PD-L1 overexpression in their cohort. Taken together, our results together with other findings suggested that overall frequency of PD-L1 expression in SCLC is low and not influenced by the ethnicity. Whether disease stage of SCLC had impact on the prevalence of PD-L1 expression need further study.
To better select the targeted population who had the tendency to express PD-L1 and CD8+ TIL, we did the analysis of association between clinicopathological parameters and PD-L1 expression or CD8+ TIL density. We observed no any association between PD-L1 expression or CD8+ TIL density and clinicopathological features in current cohort, mainly due to the small sample size. Although the previous studies suggested that PD-L1 expression or CD8+ TIL density was significantly correlated with patient age, the absence of nodal metastasis, the presence of vascular invasion, disease stage, primary tumor size, normal levels of serum neuron-specific enolase (NSE) and lactate dehydrogenase (LDH) (33,35,36,42-44), all of these studies are retrospective design together with small sample size. It still warrants the future studies with large sample size to investigate the correlations between clinicopathological features and PD-L1 expression or CD8+ TIL density.
In our study, we observed that positive PD-L1 expression and high CD8+ TIL density was independently correlated with prolonged OS in surgically resected SCLC. Specifically, the meta-analysis of 15 publications with 1,505 cases further validated the prognostic significance of positive PD-L1 expression in SCLC. Similar to our findings, a number of studies reported the correlation between PD-L1 expression and better prognosis in SCLC (32,36,38,42). High CD8+ TIL density was also previously reported to be associated with improved OS (41,45). However, several studies indicated that PD-L1 expression was not the independent prognostic factor for OS. Even some of them revealed that positive PD-L1 expression was correlated with worse OS (43,44). The potential reasons for this inconsistent result should consider the different populations, testing process and technical difference for PD-L1 expression, as well as the heterogeneity of PD-L1 expression in tumor or immune cells. Nevertheless, when we utilized PD-L1 expression plus CD8+ TIL density to predict the clinical outcome, we observed that it could more precisely stratify the total population into two groups with different prognoses after surgical resection, indicating the incorporation of these factors into multivariable prognostic models worth further exploration.
Several limitations of this study should be acknowledged. Firstly, the number of eligible patients and identified publications in meta-analysis were relatively small and all of them were retrospective studies, which suggested that the findings should be interpreted with caution and large-scale studies are still warranted. Secondly, PD-L1 antibody assay used in this study is clone E1L3N. Whether it could result in the low detection rate of PD-L1 expression in SCLC remained undetermined. Thirdly, publication bias is inevitable since we identified several meeting abstracts without detailed publications and not included these them for the meta-analysis. Fourthly, the data quality of each included article in meta-analysis was heterogeneous due to a series of confounding factors (PD-L1 antibody assay, laboratory conditions, testing process, cutoffs of positive PD-L1 expression, etc.) that made direct comparisons difficult.
In conclusion, this study reported that PD-L1 expression and CD8+ TIL density had a lower expression level and particular clinicopathological feature in patients with SCLC when compared with NSCLC. Both positive PD-L1 expression and high CD8+ TIL density was independently correlated with longer OS, and combination of PD-L1 expression and CD8+ TIL density could further stratify the total population into two groups with discrepant prognosis, suggesting that a meaningful graded prognostic evaluation for patients with surgically resected SCLC should incorporate PD-L1 expression and CD8+ TILs.
Supplementary
Supplementary file 1 Methodology of meta-analysis
Publication search
We conducted a literature review of publication search via the online databases including PubMed/Medline, Cochrane Library, EMBASE, Web of Science, and Google Scholar through May 2019, using “lung cancer” and “PD-L1”, and their corresponding words. Titles and abstracts were firstly reviewed to determine publications. We collected the data on the association of PD-L1 expression with prognosis, and clinicopathological characteristics in patients with small-cell lung cancer (SCLC). This analysis was performed in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement.
Publication selection, data extraction and quality assessment
Studies met the following criteria were identified: (I) evaluated positive PD-L1 expression in patients with SCLC; (II) PD-L1 expression was tested on tumor samples, instead of the peripheral blood or cell lines or any other types of tissue; (III) published data could assess the rate of positive PD-L1 expression and/or high risk on overall survival (OS). Publications were excluded if they were: (I) reviews, case-only studies, editorial, comment, or familial studies; (II) inadequate data for analysis of rate and/or high risk with 95% confidence intervals (CIs); and (III) repeat of previous studies or replicated samples. Two reviewers independently evaluated the study eligibility.
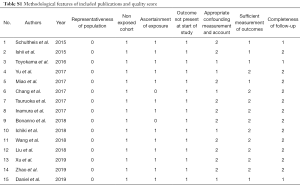
The following information from the eligible studies: name of first author, publication year, study population, number of age >60 years old, number of male, number of smoker, number of extensive-stage SCLC (ES-SCLC), rate of positive PD-L1 expression with 95% CIs, cut-off value of positive PD-L1 expression, anti-PD-L1 antibody assay, and hazard ratio (HR) for OS with related 95% CIs, were extracted. We only chose the results of multivariate analysis when univariate and multivariate analysis were simultaneously reported. Two reviewers independently extracted the data. Disagreements were solved by discussion. As we previously mentioned (25,52,53), two reviewers independently assessed the study quality via using the listed factors.
Acknowledgments
Funding: This study was supported in part by grants from the National Natural Science Foundation of China (No. 81672286 and 81772467), Shanghai Municipal Science and Technology Commission Basic Research Innovation Plan (No. 16JC1405900), Shanghai Municipal Science and Technology Commission Medical Guidance Project (No. 16411964400).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the ethics committee of Shanghai Pulmonary Hospital (FK-17-0113) and conducted in line with the provisions of the Declaration of Helsinki.
References
- Tsoukalas N, Aravantinou-Fatorou E, Baxevanos P, et al. Advanced small cell lung cancer (SCLC): new challenges and new expectations. Ann Transl Med 2018;6:145. [Crossref] [PubMed]
- Sen T, Gay CM, Byers LA. Targeting DNA damage repair in small cell lung cancer and the biomarker landscape. Transl Lung Cancer Res 2018;7:50-68. [Crossref] [PubMed]
- Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer 2015;121:664-72. [Crossref] [PubMed]
- Asai N, Ohkuni Y, Kaneko N, et al. Relapsed small cell lung cancer: treatment options and latest developments. Ther Adv Med Oncol 2014;6:69-82. [Crossref] [PubMed]
- Tokudome N, Yamamoto N. Development of predictive liquid biomarkers for response to treatment in small cell lung cancer. Transl Cancer Res 2017;6:S348-52. [Crossref]
- Altan M, Chiang AC. Management of Small Cell Lung Cancer: Progress and Updates. Cancer J 2015;21:425-33. [Crossref] [PubMed]
- Nicholson AG, Chansky K, Crowley J, et al. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:300-11.
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64. [Crossref] [PubMed]
- Schoenhals JE, Seyedin SN, Anderson C, et al. Uncovering the immune tumor microenvironment in non-small cell lung cancer to understand response rates to checkpoint blockade and radiation. Transl Lung Cancer Res 2017;6:148-58. [Crossref] [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 2015;27:450-61. [Crossref] [PubMed]
- Galluzzi L, Chan TA, Kroemer G, et al. The hallmarks of successful anticancer immunotherapy. Sci Transl Med 2018. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Fehrenbacher L, Spira A, Ballinger M, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016;387:1837-46. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017;389:255-65. [Crossref] [PubMed]
- Ready N, Farago AF, de Braud F, et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J Thorac Oncol 2019;14:237-44. [Crossref] [PubMed]
- Antonia SJ, Lopez-Martin JA, Bendell J, et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): a multicentre, open-label, phase 1/2 trial. Lancet Oncol 2016;17:883-95. [Crossref] [PubMed]
- Hellmann MD, Callahan MK, Awad MM, et al. Tumor Mutational Burden and Efficacy of Nivolumab Monotherapy and in Combination with Ipilimumab in Small-Cell Lung Cancer. Cancer Cell 2018;33:853-861.e4. [Crossref] [PubMed]
- Horn L, Mansfield AS, Szczesna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med 2018;379:2220-9. [Crossref] [PubMed]
- Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197-218. [Crossref] [PubMed]
- Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359:1350-5. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Bai Y, Chen X, Hou L, et al. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol Med 2018;15:434-42. [Crossref] [PubMed]
- Yang H, Shi J, Lin D, et al. Prognostic value of PD-L1 expression in combination with CD8(+) TILs density in patients with surgically resected non-small cell lung cancer. Cancer Med 2018;7:32-45. [Crossref] [PubMed]
- Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012;4:127ra37. [Crossref] [PubMed]
- Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014;515:563-7. [Crossref] [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [Crossref] [PubMed]
- Tokito T, Azuma K, Kawahara A, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer 2016;55:7-14. [Crossref] [PubMed]
- Schultheis AM, Scheel AH, Ozretic L, et al. PD-L1 expression in small cell neuroendocrine carcinomas. Eur J Cancer 2015;51:421-6. [Crossref] [PubMed]
- Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2015;10:426-30. [Crossref] [PubMed]
- Toyokawa G, Takada K, Haratake N, et al. Favorable Disease-free Survival Associated with Programmed Death Ligand 1 Expression in Patients with Surgically Resected Small-cell Lung Cancer. Anticancer Res 2016;36:4329-36. [PubMed]
- Yu H, Batenchuk C, Badzio A, et al. PD-L1 Expression by Two Complementary Diagnostic Assays and mRNA In Situ Hybridization in Small Cell Lung Cancer. J Thorac Oncol 2017;12:110-20. [Crossref] [PubMed]
- Miao L, Lu Y, Xu Y, et al. PD-L1 and c-MET expression and survival in patients with small cell lung cancer. Oncotarget 2017;8:53978-88. [PubMed]
- Chang YL, Yang CY, Huang YL, et al. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget 2017;8:18021-30. [PubMed]
- Tsuruoka K, Horinouchi H, Goto Y, et al. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer 2017;108:115-20. [Crossref] [PubMed]
- Inamura K, Yokouchi Y, Kobayashi M, et al. Relationship of tumor PD-L1 (CD274) expression with lower mortality in lung high-grade neuroendocrine tumor. Cancer Med 2017;6:2347-56. [Crossref] [PubMed]
- Bonanno L, Pavan A, Dieci MV, et al. The role of immune microenvironment in small-cell lung cancer: Distribution of PD-L1 expression and prognostic role of FOXP3-positive tumour infiltrating lymphocytes. Eur J Cancer 2018;101:191-200. [Crossref] [PubMed]
- Ichiki Y, Matsumiya H, Mori M, et al. Predictive factors of postoperative survival among patients with pulmonary neuroendocrine tumor. J Thorac Dis 2018;10:6912-20. [Crossref] [PubMed]
- Wang H, Li Z, Dong B, et al. Prognostic significance of PD-L1 expression and CD8+ T cell infiltration in pulmonary neuroendocrine tumors. Diagn Pathol 2018;13:30. [Crossref] [PubMed]
- Liu J, Lu Z, Wang W, et al. Programmed death-ligand 1 positivity can predict improved survival and a lower risk of brain metastasis in patients with resectable small cell lung cancer. Oncol Lett 2018;16:2373-81. [PubMed]
- Xu Y, Cui G, Jiang Z, et al. Survival analysis with regard to PD-L1 and CD155 expression in human small cell lung cancer and a comparison with associated receptors. Oncol Lett 2019;17:2960-8. [PubMed]
- Zhao X, Kallakury B, Chahine JJ, et al. Surgical Resection of SCLC: Prognostic Factors and the Tumor Microenvironment. J Thorac Oncol 2019;14:914-23. [Crossref] [PubMed]
- Carvajal-Hausdorf D, Altan M, Velcheti V, et al. Expression and clinical significance of PD-L1, B7-H3, B7-H4 and TILs in human small cell lung Cancer (SCLC). J Immunother Cancer 2019;7:65. [Crossref] [PubMed]
- Kim TK, Herbst RS, Chen L. Defining and Understanding Adaptive Resistance in Cancer Immunotherapy. Trends Immunol 2018;39:624-31. [Crossref] [PubMed]
- Apetoh L, Smyth MJ, Drake CG, et al. Consensus nomenclature for CD8(+) T cell phenotypes in cancer. Oncoimmunology 2015;4:e998538. [Crossref] [PubMed]
- Hirsch FR, McElhinny A, Stanforth D, et al. PD-L1 Immunohistochemistry Assays for Lung Cancer: Results from Phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2017;12:208-22. [Crossref] [PubMed]
- Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181-8. [Crossref] [PubMed]
- Schmidt LH, Kummel A, Gorlich D, et al. PD-1 and PD-L1 Expression in NSCLC Indicate a Favorable Prognosis in Defined Subgroups. PLoS One 2015;10:e0136023. [Crossref] [PubMed]
- Sun JM, Zhou W, Choi YL, et al. Prognostic Significance of PD-L1 in Patients with Non-Small Cell Lung Cancer: A Large Cohort Study of Surgically Resected Cases. J Thorac Oncol 2016;11:1003-11. [Crossref] [PubMed]
- Jiang T, Xu X, Qiao M, et al. Comprehensive evaluation of NT5E/CD73 expression and its prognostic significance in distinct types of cancers. BMC Cancer 2018;18:267. [Crossref] [PubMed]
- Jiang T, Qiao M, Zhao C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother 2018;67:713-27. [Crossref] [PubMed]