
An advanced non-small cell lung cancer patient with epidermal growth factor receptor sensitizing mutation responded to toripalimab in combination with chemotherapy after resistance to osimertinib: a case report
Introduction
For patients with untreated EGFR-mutated advanced non-small cell lung cancer (NSCLC), according to data from FLAURA study, the third-generation tyrosine kinase inhibitor (TKI) targeting epidermal growth factor receptor (EGFR), osimertinib, has demonstrated a high response rate of 80%, median progression-free survival (PFS) of 18.6 months, median overall survival (OS) of 38.6 months for patients with (1,2). Additionally, the National Comprehensive Cancer Network (NCCN) guideline has recommended osimertinib as the preferred first-line therapy for EGFR-mutated NSCLC patients based on these facts (3). With the widespread clinical use of osimertinib, how to manage patients who get acquired resistance to osimertinib is a growing clinical challenge.
Toripalimab (JS001) is a humanized immunoglobulin G4 monoclonal antibody targeting programmed cell death-1 (PD-1). Toripalimab has received its first global conditional approval in China on December 17th, 2018, for the treatment of unresectable or metastatic melanoma after the failure of previous systemic therapy (4). There are several ongoing clinical trials in various malignancies, including NSCLC.
Here, we would present a case of an advanced NSCLC patient with EGFR exon 19 deletion (19DEL) who has responded to combined therapy of pemetrexed, carboplatin with toripalimab after acquiring resistance to osimertinib. We hope this case report will supply a new insight into the treatment of advanced NSCLC resistance to osimertinib in clinical practice. We present the following case following the CARE-Guideline (5).
Case presentation
A 48-year-old male was admitted to our hospital on June 20th, 2017, complaining of cough and dyspnea for 3 weeks. The patient was previously in good health without any medical history. He had no exposure to tobacco and no family history of cancer.
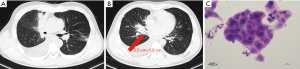
On physical examination, he had enlarged the right cervical lymph node. A baseline chest computed tomography (CT) scan revealed a right middle lobe mass of 3.5 cm × 1.8 cm with obstructive inflammation and right pleural effusion (Figure 1A,B). Bone scan, brain magnetic resonance imaging (MRI), and abdomen B-ultrasound showed no active findings. We performed a biopsy of the right cervical lymph node and pathology results indicated lung adenocarcinoma (Figure 1C). Genetic testing found EGFR 19DEL mutation. The patient was then diagnosed with right middle lobe lung adenocarcinoma after taking together all the information: T4N3M1b-stage IVa with EGFR 19DEL. He was recommended the 3rd-generation of EGFR-TKI as first-line therapy and he took 80mg of osimertinib orally for once per day since June 22nd, 2017. The best response was the partial response (PR) (Figure 2) and toxicity related to treatment was slight and manageable, with grade I° rash and I° diarrhea.
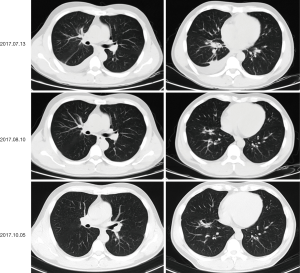
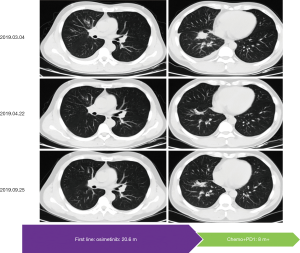
After 20.6 months of PFS, chest CT on March 4th, 2019, showed a recurrence lesion in the right middle lobe accompanied by right pleural effusion. Bone scan, brain MRI and abdomen B-ultrasound showed no metastatic lesions. Pathology analysis of cell block from hydrothorax showed adenocarcinoma, and EGFR mutation testing revealed 19DEL without any concomitant mutation. At that time, a phase II clinical study (NCT03513666) was recruiting patients, which was conducted by our hospital to evaluate the efficacy of toripalimab combined with chemotherapy in EGFR mutated advanced NSCLC patients failed to prior EGFR-TKI therapies but not acquired EGFR T790M. After the strict screening, the patient took part in the study and received the treatment of pemetrexed/carboplatin plus toripalimab since March 22nd, 2019. Fortunately, this patient did respond to this combination therapy with confirmed PR (Figure 3). The patient has a continuous survival benefit from this kind of treatment at the time of this article submission, with over 8 months of PFS benefit and over 28 months of OS benefit (Figure 4).
Discussion
Understanding and naming the resistance mechanisms to osimertinib is critical, as its use has increased widely. The acquired resistance mechanisms to osimertinib have been widely reported in a second-line setting, which is usually manifested as the loss of the T790M mutation (6). Resistance mechanisms encompass EGFR-dependent way, which are more likely to arise in cases with EGFR T790M mutation retained, including EGFR G796/C797, L792, G724, and L718/G719 tertiary mutations, and EGFR-independent way, through activation of alternative bypass pathways, aberrant downstream signaling or histologic transformation, such as alterations in MET, BRAF, KRAS, PIK3CA, RET and small-cell lung cancer (SCLC) transformation (7-10). Ramalingam and colleagues reported the putative resistance mechanisms to first-line osimertinib therapy in nine out of 19 patients, including EGFR C797S mutation, amplification of MET, EGFR, and KRAS; alteration in MEK1, KRAS, PIK3CA, JAK2, and HER2, however, acquired EGFR T790M mutation was not detected in this patient cohort (11). These data showed that the emergence of T790M under osimertinib treatment was not expected to be a resistance mechanism. To recapitulate, the types of resistance mechanisms to osimertinib are similar in these two settings, but there are some differences in frequencies. The most common resistance mechanism to osimertinib in second-line setting is EGFR C797S tertiary mutation, accounting for 10–26% (10), while in first-line setting, the frequency of the C797S mutation was 7%, making it the second most frequent mechanism, behind MET amplification with 25% of proportion (11).
The intra-patient heterogeneity and multiple resistance mechanisms are a major challenge in developing an efficient treatment strategy to counteract tumor progression. Re-treating with first or second-generation EGFR-TKIs or developing fourth-generation EGFR-TKIs were studied in patients with EGFR-dependent resistance (12-14). Combining osimertinib with drugs targeting other downstream pathways, such as c-MET inhibitor crizotinib (15), BRAF inhibitor trametinib (16), AXL inhibitor cabozantinib (17), or RET inhibitors BLU-667 (8), constitutes a promising strategy for overcoming osimertinib resistance, but clinical evidence regarding the effectiveness of these approaches lacks so far. Therefore, to explore treatment options following disease progression on osimertinib is urgently awaited.
Immune checkpoint blockades (ICB), targeting PD-1 and its ligand (PD-L1), have shown promising anti-tumor activity across a broad range of cancers. The result from the KeyNote-001 study showed that the 5-year OS rate was 29.6% for advanced NSCLC patients with a PD-L1 tumor proportion score of 50% or greater (18). Driven genes alteration is a documented detrimental factor for ICB monotherapy (19). A phase II trial (NCT02879994) of pembrolizumab in TKI naive patients with EGFR mutation-positive, advanced NSCLC, and PD-L1-positive were ceased due to lack of efficacy. Only 1 patient had an objective response (9%); however, repeat gene analysis showed an incorrect report of an EGFR mutation (20). In the TATTON study, a combination of osimertinib and durvalumab showed encouraging efficacy results, both in TKIpre-treated and TKI-naive patients, but the study was terminated owing to the high rate of pulmonary toxicity (20).
Nevertheless, in a report of IMpower 150, patients with EGFR or ALK genetic alterations could receive help from the combination therapy of atezolizumab, bevacizumab, carboplatin, and paclitaxel (21). These data suggested that ICB combined chemotherapy or anti-angiogenesis drugs might be a therapeutic choice for patients with driven genes. The phase II clinical trial (NCT03513666), which the patient was recruited into, was aimed to explore the possibility of toripalimab combined chemotherapy and overcome the resistance to EGFR-TKI. The results have been reported in the 2019 WCLC meeting that patients without EGFR T790M who were treated with toripalimab combined chemotherapy achieved 50% of objective response rate and 7.0 months of median PFS (22). This treatment modality has an unexpected effect on this patient. However, it is regrettable that the patient did not undergo next-generation sequencing (NGS) tests to search for resistance mechanisms. Interestingly, based on the promising results of the phase II study, a randomized, double-blind, placebo-controlled, phase III study is underway to evaluate the efficacy and safety of toripalimab or placebo combined with chemotherapy in advanced NSCLC patients after failure on EGFR-TKI treatment (NCT03924050).
In summary, we described a case that a 48-year-old male with EGFR-mutated advanced NSCLC, who showed a favorable response to the first-line osimertinib with PR for 20.6 months, overcame the acquired resistance to osimertinib by a combination of pemetrexed /carboplatin and toripalimab and achieved PR for over 8 months. This case suggested that immune checkpoint inhibitors combined chemotherapy may be a new way to overcome acquired resistance to osimertinib. However, further validation was still in need.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (grant number: 81874036), whose sponsor was Chunxia Su-- the correspondence of this manuscript.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.02.09). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Faber AC, Burns TF. An expanding role for osimertinib for the treatment of ErbB family driven NSCLC. Transl Cancer Res 2018;7:S787-91. [Crossref]
- NCCN Clinical Practice Guidelines in Oncology. Non-small Cell Lung Cancer (Version 1. 2019). Available online: https://www.nccn.org/professionals/physician_gls/
- Keam SJ. Toripalimab: First Global Approval. Drugs 2019;79:573-8. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Lettig L, Sahnane N, Pepe F, et al. EGFR T790M detection rate in lung adenocarcinomas at baseline using droplet digital PCR and validation by ultra-deep next generation sequencing. Transl Lung Cancer Res 2019;8:584-92. [Crossref] [PubMed]
- Yang Z, Yang N, Ou Q, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097-107. [Crossref] [PubMed]
- Piotrowska Z, Isozaki H, Lennerz JK, et al. Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov 2018;8:1529-39. [Crossref] [PubMed]
- Ma L, Chen R, Wang F, et al. EGFR L718Q mutation occurs without T790M mutation in a lung adenocarcinoma patient with acquired resistance to osimertinib. Ann Transl Med 2019;7:207. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of resistance mechanisms and clinical implications in patients with EGFR T790M–positive lung cancer and acquired resistance to osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Wang S, Song Y, Liu D. EAI045: the fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett 2017;385:51-4. [Crossref] [PubMed]
- Liu Y, Li Y, Ou Q, et al. Acquired EGFR L718V mutation mediates resistance to osimertinib in non-small cell lung cancer but retains sensitivity to afatinib. Lung Cancer 2018;118:1-5. [Crossref] [PubMed]
- Pakkala S, Ramalingam SS. Personalized therapy for lung cancer: striking a moving target. JCI Insight 2018;3:120858. [Crossref] [PubMed]
- Zhu VW, Schrock AB, Ali SM, et al. Differential response to a combination of full-dose osimertinib and crizotinib in a patient with EGFR-mutant non-small cell lung cancer and emergent MET amplification. Lung Cancer (Auckl) 2019;10:21-6. [Crossref] [PubMed]
- La Monica S, Minari R, Cretella D, et al. Acquired BRAF G469A Mutation as a Resistance Mechanism to First-Line Osimertinib Treatment in NSCLC Cell Lines Harboring an EGFR Exon 19 Deletion. Target Oncol 2019;14:619-26. [Crossref] [PubMed]
- Tian Y, Zhang Z, Miao L, et al. Anexelekto (AXL) increases resistance to EGFR-TKI and activation of AKT and ERK1/2 in non-small cell lung cancer cells. Oncol Res 2016;24:295-303. [Crossref] [PubMed]
- Gettinger S, Horn L, Jackman D, et al. Five-Year Follow-Up of Nivolumab in Previously Treated Advanced Non-Small-Cell Lung Cancer: Results From the CA209-003 Study. J Clin Oncol 2018;36:1675-84. [Crossref] [PubMed]
- Gainor JF, Shaw AT, Sequist LV, et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin Cancer Res 2016;22:4585-93. [Crossref] [PubMed]
- Lisberg A, Cummings A, Goldman JW, et al. A Phase II Study of Pembrolizumab in EGFR-Mutant, PD-L1+, Tyrosine Kinase Inhibitor Naive Patients With Advanced NSCLC. J Thorac Oncol 2018;13:1138-45. [Crossref] [PubMed]
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N Engl J Med 2018;378:2288-301. [Crossref] [PubMed]
- Zhang J, Zhou C, Zhao Y, et al. A PII Study of Toripalimab, a PD-1 mAb, in Combination with Chemotherapy in EGFR plus Advanced NSCLC Patients Failed to Prior EGFR TKI Therapies. J Thorac Oncol 2019;14S:S292. [Crossref]



