
18F-FDG PET/CT total lesion glycolysis is associated with circulating tumor cell counts in patients with stage I to IIIA non-small cell lung cancer
Introduction
Five-year survival of non-small cell lung cancer (NSCLC) patients remains low at 18% (1). 18F-fluoro-2-deoxy-D-glucose (18F-FDG) PET/CT, representing active tumor biology based on glucose metabolism, is the standard imaging modality for NSCLC staging (2). 18F-FDG uptake parameters are predictive of poor outcome in NSCLC and other cancer types (3,4). Increased 18F-FDG uptake on PET is associated with higher tumor glucose metabolism, which correlates with metastatic spread (5). Standardized uptake values (SUVavg/max) are parameters typically reported in staging PET imaging for NSCLC, calculated as the ratio of radioactivity per unit volume of a region of interest (ROI) to the activity per unit whole body volume (6). However, combined measures of metabolic and volumetric tumor information, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG) (calculated as MTV × SUVavg), may be a more appropriate predictor for micrometastatic spread than SUVs (7,8).
Circulating tumor cells (CTCs)—defined as CKpos/EpCAMpos/CD45neg with a DAPIpos nucleus—are found in the blood of cancer patients and correlate negatively with survival in NSCLC (9). CTCs have been implicated in development of metastases (10). Due to their simple detection, CTC liquid biomarker research is a most active field in translational oncology. The linkage between CTCs and tumor glucose metabolism is poorly understood (11). Since both CTCs and PET activity are associated with metastatic spread, we hypothesized that metabolic PET parameters correlate with CTCs in NSCLC.
This is a pilot study that demonstrates that TLG is associated with CTCs in non-metastatic NSCLC patients, in contrast to SUVmax/avg. Results suggest that TLG may be a better marker than SUVs for micrometastatic spread of NSCLC.
Methods
Subjects
Institutional Review Board approval was obtained (IRB2010166/IRB2004401-VA). Prospectively, treatment-naïve NSCLC patients that underwent staging for surgery for stage I-IIIA (AJCC 8th ed.) between July 2016 to December 2017 and healthy never-smokers were enrolled and signed informed consents.
18F-FDG PET/CT
Imaging was performed with Biograph 32-Slice PET/CT scanners (Siemens, Germany), approximately one hour after 18F-FDG intravenous injection using commercially available Medrad® Intego PET Infusion System. Dose of 18F-FDG injected was based on body weight and equaled 0.04 mCi/pound body weight. The acquisition time per bed ranged from 1–4 minutes, based on BMI (with longest times applied for patients with severe morbid obesity). Patients fasted overnight and underwent blood glucose testing prior to scanning [median 95 mg/dL (range, 72–130)]. Attenuation correction of images was performed with low-dose CT without intravenous contrast. MIM Software (Cleveland, OH) was used to determine SUVmax, SUVavg, MTV was calculated by 3D region isocontour-based VOIs (12). TLG was calculated as SUVavg × MTV.
CTC detection
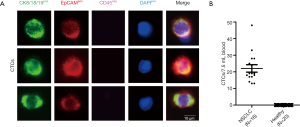
Seven point five milliliter of blood were drawn in CellSave® tubes (Menarini, Huntingdon Valley, PA), and CTCs were enriched by size with CellSieve™ microfilter (Creatv MicroTech, Rockville, MD), as described (13,14). In alignment with the FDA-approved definition, CTCs were identified by immunofluorescence as CKpos/EpCAMpos/CD45neg with a DAPIpos nucleus.
Statistical analysis
Research personnel were blinded to study-specific outcomes by separation from the recruiting staff. Statistical significance refers to a P<0.05. Analyses were performed using Prism 5.00 (GraphPad, La Jolla, CA).
Results
Characteristics of subjects and CTC detection
In total, 36 subjects were prospectively recruited: 16 NSCLC patients and 20 healthy never-smokers (Table 1). All NSCLC patients carried CTCs, however none of the 20 healthy controls had CTCs detectable (Figure 1).
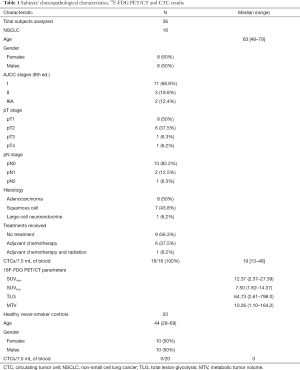
Full table
18F-FDG-PET/CT uptake parameter associations with CTCs
To associate metabolic parameters with CTCs, linear regression analyses were performed (Figure 2). We found a significant positive association between MTV and CTCs [slope= 0.13, 95% CI, (0.03, 0.23), F(1,15)=7.60, P=0.016, r2=0.35], and TLG and CTCs [slope =0.03, 95% CI, (0.01, 0.05), F(1,15)=9.81, P=0.007, r2=0.41]. No significant correlations between SUVmax and CTCs [slope= ‒0.21, 95% CI, (‒0.93, 0.51), F(1,15)=0.38, P=0.546, r2=0.03] or SUVavg and CTCs [slope =‒0.42, 95% CI, (‒1.79, 0.95), F(1,15)=0.43, P=0.522, r2=0.03] were found. There was no significant association between pathologic tumor size (pT) and CTCs, or pT and metabolic parameters.
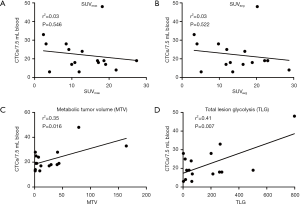
A multivariate linear regression analysis predicting CTCs from MTV, controlling for AJCC stage, was performed. This overall model was not significant [F(2,13)=2.20, P=0.061]—due to a non-significant slope for stage. The intercept was 18.06 [95% CI, (8.23, 27.89)]. MTV was a significant predictor for CTCs [slope= 0.13, 95% CI, (0.02, 0.23), P=0.008]. AJCC stage was not a significant predictor for CTCs [slope= ‒0.25, 95% CI, (‒5.74, 6.24), P=0.930]. Residuals for MTV were normally distributed and homoscedastic. The r2 was 0.35 in this multivariate regression model. Similarly, we did a multivariate linear regression predicting CTCs from TLG, controlling for stage. The regression model was significant (F(2,13)=5.01, P=0.024). The intercept was 14.50 [95% CI, (4.57, 24.44)]. TLG was a significant predictor for CTCs (slope= 0.03, 95% CI, (0.01, 0.05), P=0.008). Stage was not a significant predictor for CTCs [slope= 1.91, 95% CI, (‒3.72, 7.53), P=0.477]. Residuals for TLG were normally distributed and homoscedastic. The r2 was 0.44 in this multivariate linear regression.
This pilot study results suggest that MTV and TLG, in contrast to SUVmax/avg, are potential predictors for micrometastatic hematogenous spread measured by CTCs in NSCLC patients (Figure 3).
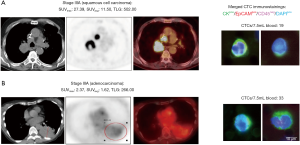
Discussion
SUVmax is the standard metabolic parameter applied for clinical NSCLC staging (15). However the TLG, a measure that takes the metabolic tumor volume burden into account, may provide a better reflection of cancer biology (7). Our findings suggest that in addition to MTV, TLG is a more accurate marker than SUVs for micrometastatic NSCLC spread represented by CTCs. Correlation of SUVmax with poor outcome in NSCLC has been suggested, but conflicting data exist (3,5,16). Independently, 18F-FDG PET/CT TLG and CTCs in the blood predict increased risk of recurrence in NSCLC, but the association of 18F-FDG uptake parameters with CTCs is poorly understood (11,17-19). We performed metabolic 18F-FDG PET/CT analysis in primary lung tumors, but not in loco-regional lymph node metastases (if present). Although we did not include NSCLC patients with distant metastases, the study is limited by population heterogeneity. The majority of patients had lung tumors with no loco-regional nodal metastases, but three patients had ipsilateral intrapulmonary/hilar and/or mediastinal lymph node metastases.
In metastatic NSCLC patients undergoing chemotherapy, a correlation between a decrease in CTCs and SUVmax has been demonstrated (19). In another cohort of metastatic NSCLC patients that often have multiple tumor sites with different metabolic uptake, no correlation between CTCs and metabolic parameters, including TLG, was noted (20). In contrast, our study focused on non-metastatic NSCLC patients that might be less diverse than metastatic patients, which may explain why an association between TLG and CTCs was observed. In a comparable group of NSCLC patients undergoing surgery, presence of CTCs one month after resection correlated with SUVmax, but TLG was not analyzed in this study (17). In a heterogeneous group of NSCLC patients of all stages, CTCs detected by a non-EpCAM-based method neither correlated with SUVmax, nor TLG (18). Interestingly, presence of CTC clusters (≥2 CTCs in aggregate) was associated with TLG, but it needs to be taken into consideration that clusters are more prevalent in metastatic NSCLC (21). EpCAM-independent detection technologies yield higher CTC counts due to down-regulation of EpCAM during epithelial-mesenchymal transition of cancer cells (18). However, we chose to identify CTCs as CKpos/EpCAMpos—in alignment with the FDA-approved definition (22).
This pilot study links TLG to CTCs in non-metastatic NSCLC patients. Although we exclusively studied patients with local tumors or loco-regional nodal disease (without distant metastases), the extent of tumor burden varied amongst patients which may affect glucose metabolism and CTC shedding. Interpretation of our results are limited by small sample size and should be confirmed in larger cohorts.
Conclusions
18F-FDG PET/CT TLG, in contrast to SUVs, is associated with presence of CTCs in stage I-IIIA NSCLC patients. TLG might be an appropriate marker than SUVs for micrometastatic spread of NSCLC measured by CTCs in the blood.
Acknowledgments
We are very grateful to all subjects and their families for their voluntary participation, and to Blanche L. Lasta, RN, and Ken Baker, RN, for assistance in trial conductance.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.10). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Institutional Review Board of University of Missouri (IRB2010166/IRB2004401-VA) and informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cronin KA, Lake AJ, Scott S, et al. Annual Report to the Nation on the Status of Cancer, part I: National cancer statistics. Cancer 2018;124:2785-800. [Crossref] [PubMed]
- van Tinteren H, Hoekstra OS, Smit EF, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet 2002;359:1388-93. [Crossref] [PubMed]
- Paesmans M, Berghmans T, Dusart M, et al. Primary tumor standardized uptake value measured on fluorodeoxyglucose positron emission tomography is of prognostic value for survival in non-small cell lung cancer: update of a systematic review and meta-analysis by the European Lung Cancer Working Party for the International Association for the Study of Lung Cancer Staging Project. J Thorac Oncol 2010;5:612-9. [Crossref] [PubMed]
- Choi ES, Ha SG, Kim HS, et al. Total lesion glycolysis by 18F-FDG PET/CT is a reliable predictor of prognosis in soft-tissue sarcoma. Eur J Nucl Med Mol Imaging 2013;40:1836-42. [Crossref] [PubMed]
- Vesselle H, Salskov A, Turcotte E, et al. Relationship between non-small cell lung cancer FDG uptake at PET, tumor histology, and Ki-67 proliferation index. J Thorac Oncol 2008;3:971-8. [Crossref] [PubMed]
- Na F, Wang J, Li C, et al. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol 2014;9:834-42. [Crossref] [PubMed]
- Larson SM, Erdi Y, Akhurst T, et al. Tumor Treatment Response Based on Visual and Quantitative Changes in Global Tumor Glycolysis Using PET-FDG Imaging. The Visual Response Score and the Change in Total Lesion Glycolysis. Clin Positron Imaging 1999;2:159-71. [Crossref] [PubMed]
- Davison J, Mercier G, Russo G, et al. PET-based primary tumor volumetric parameters and survival of patients with non-small cell lung carcinoma. AJR Am J Roentgenol 2013;200:635-40. [Crossref] [PubMed]
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63. [Crossref] [PubMed]
- Pantel K, Alix-Panabieres C. Functional Studies on Viable Circulating Tumor Cells. Clin Chem 2016;62:328-34. [Crossref] [PubMed]
- Yu JQ, Cristofanilli M. Circulating tumor cells and PET. J Nucl Med 2011;52:1501-4. [Crossref] [PubMed]
- Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging 2015;42:328-54. [Crossref] [PubMed]
- Manjunath Y, Upparahalli SV, Avella DM, et al. PD-L1 Expression with Epithelial Mesenchymal Transition of Circulating Tumor Cells Is Associated with Poor Survival in Curatively Resected Non-Small Cell Lung Cancer. Cancers (Basel) 2019. [Crossref] [PubMed]
- Manjunath Y, Upparahalli SV, Suvilesh KN, et al. Circulating tumor cell clusters are a potential biomarker for detection of non-small cell lung cancer. Lung Cancer 2019;134:147-50. [Crossref] [PubMed]
- Coleman RE. PET in lung cancer. J Nucl Med 1999;40:814-20. [PubMed]
- Vanhove K, Mesotten L, Heylen M, et al. Prognostic value of total lesion glycolysis and metabolic active tumor volume in non-small cell lung cancer. Cancer Treat Res Commun 2018;15:7-12. [Crossref] [PubMed]
- Bayarri-Lara CI, de Miguel Perez D, Cueto Ladron de Guevara A, et al. Association of circulating tumour cells with early relapse and 18F-fluorodeoxyglucose positron emission tomography uptake in resected non-small-cell lung cancers. Eur J Cardiothorac Surg 2017;52:55-62. [Crossref] [PubMed]
- Nair VS, Keu KV, Luttgen MS, et al. An observational study of circulating tumor cells and (18)F-FDG PET uptake in patients with treatment-naive non-small cell lung cancer. PLoS One 2013;8:e67733. [Crossref] [PubMed]
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401. [Crossref] [PubMed]
- Morbelli S, Alama A, Ferrarazzo G, et al. Circulating Tumor DNA Reflects Tumor Metabolism Rather Than Tumor Burden in Chemotherapy-Naive Patients with Advanced Non-Small Cell Lung Cancer: (18)F-FDG PET/CT Study. J Nucl Med 2017;58:1764-9. [Crossref] [PubMed]
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15. [Crossref] [PubMed]
- Adams DL, Martin SS, Alpaugh RK, et al. Circulating giant macrophages as a potential biomarker of solid tumors. Proc Natl Acad Sci U S A 2014;111:3514-9. [Crossref] [PubMed]


