
Update on the pathologic diagnosis of malignant mesothelioma
Introduction
Malignant mesothelioma is a rare neoplasm of the serosal membranes. The World Health Organization provides guidance on the classification of malignant mesothelioma (1), but its diagnosis remains a challenge for many practicing surgical pathologists. To aid accurate diagnosis, the International Mesothelioma Interest Group (iMig) periodically publishes pathologic guidelines on the diagnosis of malignant mesothelioma, the most recent being the 2017 update (2-4). Since that time, numerous publications on biomarker utilization in malignant mesothelioma and on understanding of mesothelioma biology and genetics have led to a plethora of new and emerging concepts with implications in the diagnosis of malignant mesothelioma. The diagnostic guidelines will be updated based on this published literature from the last 3 years, and on experiences of an international group of leading pathologists in the field. These updates have been discussed by attendees of the Working Group for Multidisciplinary Classification of malignant mesothelioma (Lyon, France, July 2018) (5), International Mesothelioma Panel meeting (Washington, DC, March 2019) and Pulmonary Pathology Society Biennial Meeting (Dubrovnik, Croatia, June 2019).
Current strategies and updates in the utilization of immunohistochemical biomarkers
Establishment of mesothelial lineage
To establish mesothelial lineage by immunohistochemistry, current recommendations are to utilize a panel of at least two mesothelial markers [most commonly CK5/6, calretinin, WT-1, and/or D2-40 (podoplanin)] and two carcinoma markers (Ber-EP4, MOC-31, among others) (4). The exact panel of immunohistochemical markers used varies between the pleural and peritoneal cavities and depends upon the differential diagnosis. More recently, claudin-4, a component of epithelial tight junctions, has emerged as an excellent discriminatory marker in the differential between epithelial and mesothelial proliferations. Claudin-4 is now viewed by many expert mesothelioma pathologists, and supported by the literature, as a superior marker of epithelial differentiation (6-8). Positive immunoreactivity for claudin-4 is defined as strong membranous expression, with only granular cytoplasmic or very focal staining reported in mesothelioma (7,9). While the historically described mesothelial markers remain in use, more recently, HEG1 (heart development protein with EGF-like domains), has been described as a sensitive and specific marker of mesothelial differentiation with excellent discriminatory expression between mesothelial and epithelial proliferations (10-12). Currently, HEG1 is limited in use as it is not widely available outside of Japan.
Benign versus malignant mesothelial proliferations
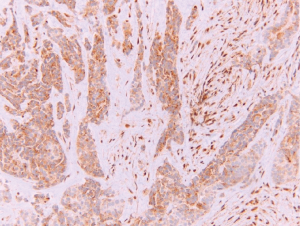
The diagnosis of a mesothelial proliferation as malignant is most easily accomplished by identification of invasion of the mesothelial cells into underlying tissue (lung, skeletal muscle, fibroadipose tissue, etc.), and invasion can be highlighted with immunohistochemistry directed against cytokeratin and/or calretinin (4). Identification of invasion can be difficult, especially on small biopsies, which may preclude evaluation of invasion into underlying tissue. Numerous markers have been proposed to differentiate benign from malignant mesothelial proliferations but have not achieved adequate sensitivity and specificity regarding this differential. More recently, nuclear loss of BRCA associated protein 1 (BAP1; Figure 1) has emerged as a specific marker of malignancy in mesothelial proliferations (13-16), although loss of BAP1 is not entirely specific for malignant mesothelioma and can be observed in melanoma, renal cell carcinoma, and other malignancies (17). Immunohistochemical loss of BAP1 does correlate with BAP1 mutation (13,18), however loss of BAP1 lacks sensitivity, only occurring in 50–65% of epithelioid malignant mesotheliomas, and around 15% of sarcomatoid malignant mesotheliomas (13,14,18-23). Cytoplasmic loss of MTAP (methylthioadenosine phosphorylase; Figure 2), which correlates to homozygous deletion of CDKN2A, has also recently emerged as a specific marker of malignancy in mesothelial proliferations, but like BAP1, lacks sensitivity (24-27). Lastly, nuclear loss of 5-hydroxymethylcytosine (5-hmC), a byproduct of gene demethylation, has been shown to be a specific marker of malignancy in mesothelial proliferations (28). By combining these various immunohistochemical markers, the vast majority of cases should be properly classified as either benign or malignant.

Sarcomatoid malignant mesothelioma differential diagnosis
Although large confirmatory studies are needed, there is evidence that GATA3 may be a relatively specific mesothelial marker in the differential between sarcomatoid mesothelioma and sarcomatoid carcinoma of the lung (29). Conversely, MUC4 may be a relatively specific marker of carcinoma in the differential of lung carcinoma versus mesothelioma, and may also be utilized in the differentiation between the sarcomatoid forms of lung carcinoma and mesothelioma (30,31). Beyond the diagnostic challenge differentiating sarcomatoid malignant mesothelioma from sarcomatoid carcinoma, is differentiating sarcomatoid malignant mesothelioma from other sarcomas. Keratin positivity should be observed, at least focally, in a sarcomatoid malignant mesothelioma, which should rule out most, but not all sarcomas. Genetic testing for sarcoma and other mesenchymal neoplasm specific translocations may also be utilized, especially if immunohistochemistry is inconclusive (32-37).
Updates in epithelioid malignant mesothelioma: subtyping according to prognostic factors and nuclear grade
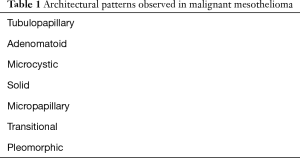
It is well documented in the literature that epithelioid malignant mesothelioma carries a better prognosis than biphasic and sarcomatoid cases. Numerous reports have published on specific histologic parameters that have been shown to stratify patients into prognostic groups within epithelioid malignant mesothelioma. Accepted architectural patterns in epithelioid malignant mesothelioma are shown in Table 1. Architectural patterns, as well as cytologic and stromal features, are now recommended to be reported on diagnostic specimens as these features may help with prognostication or improve diagnostic accuracy (5).
Full table
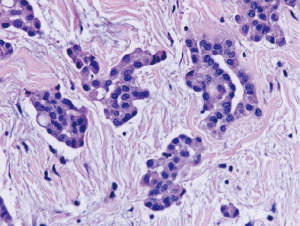
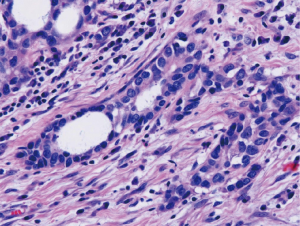
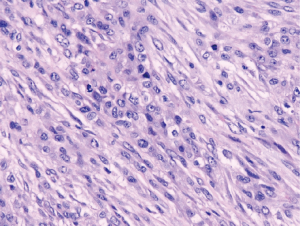
Nuclear grading schemes have been proposed which are able to stratify epithelioid malignant mesothelioma into distinct prognostic groups (Table 2) (38,39). While the most recent iMig diagnostic guidelines did not formally endorse grading of epithelioid malignant mesothelioma, it is now favored by international consensus (5). While the previously published grading systems utilized a three tier approach based on nuclear atypia (Figures 3,4,5) and mitotic count, a two tier system of high (nuclear grade 2 with necrosis and nuclear grade 3) and low grade (nuclear grade 1 or nuclear grade 2 without necrosis) is favored and proposed (5).

Full table
Updates in biphasic malignant mesothelioma: issues with reproducibility, classification, and lingering issues
Biphasic malignant mesothelioma is arbitrarily defined by the most recent WHO as a malignant mesothelial tumor with at least 10% each of sarcomatoid and epithelioid components (1). Although robust data is lacking, two studies showed that prognostic cutoffs can be set at different percent sarcomatoid component (40,41). With this in mind, some experts believe in dropping the 10% requirement altogether. It is imperative, even on small biopsies, to recognize and record the percent of epithelioid and sarcomatoid components to properly diagnosis a tumor as biphasic (5). The challenges surrounding the diagnosis of biphasic malignant mesothelioma may stem from low interobserver reproducibility from lack of a standard definition (41). Other studies have shown better interobserver reproducibility in the identification of sarcomatoid components (42), and improved identification of mesothelial subtype following training (43). Lastly, appropriate classification of spindled mesothelial cells as benign or malignant hinders histologic subtyping of malignant mesothelioma. Recent studies have demonstrated discordant staining between epithelioid and sarcomatoid components for malignancy specific marker BAP1 (18,41). The exact role, if any, immunohistochemistry may play in the workup of biphasic mesothelioma is yet to be determined.
Pleomorphic and transitional mesothelioma
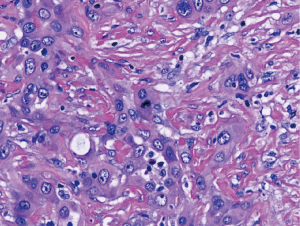
Malignant mesothelioma can show marked cytologic atypia with anaplasia and giant cells. The 2015 WHO defines such changes when present in epithelioid malignant mesothelioma, as pleomorphic mesothelioma (1). Noting that anaplasia and marked nuclear pleomorphism is not restricted to epithelioid subtype, recent guidelines suggest including pleomorphism as a cytologic feature of both epithelioid and sarcomatoid malignant mesothelioma (5). The transitional pattern of malignant mesothelioma (Figure 6) has been defined in the 2015 WHO as a feature of epithelioid malignant mesothelioma showing “sheet-like growth pattern in which the cells are cohesive but have elongated morphology” (1). However, recent data shows that tumors with transitional features have survival curves more closely resembling those of sarcomatoid malignant mesothelioma than epithelioid, and are genetically more similar to sarcomatoid mesothelioma than epithelioid; these studies favor transitional features as a subset of sarcomatoid subtype (41,44).
Mesothelioma in situ
With advancements in understanding mesothelial biology and with increased utilization of immunohistochemical biomarkers, mesothelial malignancy specific markers, namely BAP1 and MTAP, have been demonstrated to be lost in mesothelium which does not show invasion or other features of malignancy. These few reported cases, termed malignant mesothelioma in situ, support the notion that a malignant mesothelioma in situ lesion likely exists prior to invasive disease (45-47).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See:
References
- WHO Classification of Tumours of the Lung, Pleura, Thymus, and Heart. Lyon: IARC, 2015.
- Husain AN, Colby TV, Ordonez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133:1317-31. [PubMed]
- Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2013;137:647-67. [Crossref] [PubMed]
- Husain AN, Colby TV, Ordonez NG, et al. Guidelines for Pathologic Diagnosis of Malignant Mesothelioma 2017 Update of the Consensus Statement From the International Mesothelioma Interest Group. Arch Pathol Lab Med 2018;142:89-108. [Crossref] [PubMed]
- Nicholson AG, Sauter JL, Nowak AK, et al. EURACAN/IASLC proposals for updating the histologic classification of pleural mesothelioma: towards a more multidisciplinary approach. J Thorac Oncol 2020;15:29-49. [Crossref] [PubMed]
- Chaouche-Mazouni S, Scherpereel A, Zaamoum R, et al. Claudin 3, 4, and 15 expression in solid tumors of lung adenocarcinoma versus malignant pleural mesothelioma. Ann Diagn Pathol 2015;19:193-7. [Crossref] [PubMed]
- Ohta Y, Sasaki Y, Saito M, et al. Claudin-4 as a marker for distinguishing malignant mesothelioma from lung carcinoma and serous adenocarcinoma. Int J Surg Pathol 2013;21:493-501. [Crossref] [PubMed]
- Ordóñez NG. Value of claudin-4 immunostaining in the diagnosis of mesothelioma. Am J Clin Pathol 2013;139:611-9. [Crossref] [PubMed]
- Kushitani K, Amatya VJ, Okada Y, et al. Utility and pitfalls of immunohistochemistry in the differential diagnosis between epithelioid mesothelioma and poorly differentiated lung squamous cell carcinoma. Histopathology 2017;70:375-84. [Crossref] [PubMed]
- Tsuji S, Washimi K, Kageyama T, et al. HEG1 is a novel mucin-like membrane protein that serves as a diagnostic and therapeutic target for malignant mesothelioma. Sci Rep 2017;7:45768. [Crossref] [PubMed]
- Matsuura R, Kaji H, Tomioka A, et al. Identification of mesothelioma-specific sialylated epitope recognized with monoclonal antibody SKM9-2 in a mucin-like membrane protein HEG1. Sci Rep 2018;8:14251. [Crossref] [PubMed]
- Chapel DB, Churg A, Santoni-Rugiu E, et al. Molecular pathways and diagnosis in malignant mesothelioma: A review of the 14th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2019;127:69-75. [Crossref] [PubMed]
- Nasu M, Emi M, Pastorino S, et al. High Incidence of Somatic BAP1 alterations in sporadic malignant mesothelioma. J Thorac Oncol 2015;10:565-76. [Crossref] [PubMed]
- Yoshimura M, Kinoshita Y, Hamasaki M, et al. Diagnostic application of BAP1 immunohistochemistry to differentiate pleural mesothelioma from metastatic pleural tumours. Histopathology 2017;71:1011-4. [Crossref] [PubMed]
- Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol 2015;39:977-82. [Crossref] [PubMed]
- McGregor SM, Dunning R, Hyjek E, et al. BAP1 facilitates diagnostic objectivity, classification, and prognostication in malignant pleural mesothelioma. Hum Pathol 2015;46:1670-8. [Crossref] [PubMed]
- Carbone M, Yang H, Pass HI, et al. BAP1 and cancer. Nat Rev Cancer 2013;13:153-9. [Crossref] [PubMed]
- Righi L, Duregon E, Vatrano S, et al. BRCA1-Associated Protein 1 (BAP1) Immunohistochemical Expression as a Diagnostic Tool in Malignant Pleural Mesothelioma Classification: A Large Retrospective Study. J Thorac Oncol 2016;11:2006-17. [Crossref] [PubMed]
- Wu D, Hiroshima K, Yusa T, et al. Usefulness of p16/CDKN2A fluorescence in situ hybridization and BAP1 immunohistochemistry for the diagnosis of biphasic mesothelioma. Ann Diagn Pathol 2017;26:31-7. [Crossref] [PubMed]
- Hwang HC, Sheffield BS, Rodriguez S, et al. Utility of BAP1 Immunohistochemistry and p16 (CDKN2A) FISH in the Diagnosis of Malignant Mesothelioma in Effusion Cytology Specimens. Am J Surg Pathol 2016;40:120-6. [Crossref] [PubMed]
- Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol 2015;28:1043-57. [Crossref] [PubMed]
- Berg KB, Dacic S, Miller C, et al. Utility of Methylthioadenosine Phosphorylase Compared With BAP1 Immunohistochemistry, and CDKN2A and NF2 Fluorescence In Situ Hybridization in Separating Reactive Mesothelial Proliferations From Epithelioid Malignant Mesotheliomas. Arch Pathol Lab Med 2018;142:1549-53. [Crossref] [PubMed]
- Hwang HC, Pyott S, Rodriguez S, et al. BAP1 Immunohistochemistry and p16 FISH in the Diagnosis of Sarcomatous and Desmoplastic Mesotheliomas. Am J Surg Pathol 2016;40:714-8. [Crossref] [PubMed]
- Kinoshita Y, Hida T, Hamasaki M, et al. A combination of MTAP and BAP1 immunohistochemistry in pleural effusion cytology for the diagnosis of mesothelioma. Cancer Cytopathol 2018;126:54-63. [Crossref] [PubMed]
- Hamasaki M, Kinoshita Y, Yoshimura M, et al. Cytoplasmic MTAP expression loss detected by immunohistochemistry correlates with 9p21 homozygous deletion detected by FISH in pleural effusion cytology of mesothelioma. Histopathology 2019;75:153-5. [Crossref] [PubMed]
- Hida T, Hamasaki M, Matsumoto S, et al. Immunohistochemical detection of MTAP and BAP1 protein loss for mesothelioma diagnosis: Comparison with 9p21 FISH and BAP1 immunohistochemistry. Lung Cancer 2017;104:98-105. [Crossref] [PubMed]
- Chapel DB, Schulte JJ, Berg K, et al. MTAP immunohistochemistry is an accurate and reproducible surrogate for CDKN2A fluorescence in situ hybridization in diagnosis of malignant pleural mesothelioma. Mod Pathol 2020;33:245-54. [Crossref] [PubMed]
- Chapel DB, Husain AN, Krausz T. Immunohistochemical evaluation of nuclear 5-hydroxymethylcytosine (5-hmC) accurately distinguishes malignant pleural mesothelioma from benign mesothelial proliferations. Mod Pathol 2019;32:376-86. [Crossref] [PubMed]
- Berg KB, Churg A. GATA3 Immunohistochemistry for Distinguishing Sarcomatoid and Desmoplastic Mesothelioma From Sarcomatoid Carcinoma of the Lung. Am J Surg Pathol 2017;41:1221-5. [Crossref] [PubMed]
- Mawas AS, Amatya VJ, Kushitani K, et al. MUC4 immunohistochemistry is useful in distinguishing epithelioid mesothelioma from adenocarcinoma and squamous cell carcinoma of the lung. Sci Rep 2018;8:134. [Crossref] [PubMed]
- Amatya VJ, Kushitani K, Mawas AS, et al. MUC4, a novel immunohistochemical marker identified by gene expression profiling, differentiates pleural sarcomatoid mesothelioma from lung sarcomatoid carcinoma. Mod Pathol 2017;30:672-81. [Crossref] [PubMed]
- Travis WD. Sarcomatoid neoplasms of the lung and pleura. Arch Pathol Lab Med 2010;134:1645-58. [PubMed]
- Doyle LA, Vivero M, Fletcher CD, et al. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol 2014;27:390-5. [Crossref] [PubMed]
- Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol 2007;31:509-20. [Crossref] [PubMed]
- Antonescu CR, Suurmeijer AJ, Zhang L, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol 2015;39:957-67. [Crossref] [PubMed]
- Anderson T, Zhang L, Hameed M, et al. Thoracic epithelioid malignant vascular tumors: a clinicopathologic study of 52 cases with emphasis on pathologic grading and molecular studies of WWTR1-CAMTA1 fusions. Am J Surg Pathol 2015;39:132-9. [Crossref] [PubMed]
- van de Rijn M, Barr FG, Xiong QB, et al. Poorly differentiated synovial sarcoma: an analysis of clinical, pathologic, and molecular genetic features. Am J Surg Pathol 1999;23:106-12. [Crossref] [PubMed]
- Kadota K, Suzuki K, Colovos C, et al. A nuclear grading system is a strong predictor of survival in epitheloid diffuse malignant pleural mesothelioma. Mod Pathol 2012;25:260-71. [Crossref] [PubMed]
- Rosen LE, Karrison T, Ananthanarayanan V, et al. Nuclear grade and necrosis predict prognosis in malignant epithelioid pleural mesothelioma: a multi-institutional study. Mod Pathol 2018;31:598-606. [Crossref] [PubMed]
- Vigneswaran WT, Kircheva DY, Ananthanarayanan V, et al. Amount of Epithelioid Differentiation Is a Predictor of Survival in Malignant Pleural Mesothelioma. Ann Thorac Surg 2017;103:962-6. [Crossref] [PubMed]
- Galateau Salle F, Le Stang N, Nicholson AG, et al. New Insights on Diagnostic Reproducibility of Biphasic Mesotheliomas: A Multi-Institutional Evaluation by the International Mesothelioma Panel From the MESOPATH Reference Center. J Thorac Oncol 2018;13:1189-203. [Crossref] [PubMed]
- Dacic S, Le Stang N, Husain A, et al. Interobserver variation in the assessment of the sarcomatoid and transitional components in biphasic mesotheliomas. Mod Pathol 2020;33:255-62. [Crossref] [PubMed]
- Brcic L, Vlacic G, Quehenberger F, et al. Reproducibility of Malignant Pleural Mesothelioma Histopathologic Subtyping. Arch Pathol Lab Med 2018;142:747-52. [Crossref] [PubMed]
- Salle FG, Le Stang N, Tirode F, et al. Comprehensive molecular and pathological evaluation of transitional mesothelioma assisted by deep learning approach: a multi institutional study of the International Mesothelioma Panel from MESOPATH Reference Center. J Thorac Oncol 2020;15:1037-53. [Crossref] [PubMed]
- Churg A, Hwang H, Tan L, et al. Malignant mesothelioma in situ. Histopathology 2018;72:1033-8. [Crossref] [PubMed]
- Churg A, Galateau-Salle F, Roden AC, et al. Malignant mesothelioma in situ: morphologic features and clinical outcome. Mod Pathol 2020;33:297-302. [Crossref] [PubMed]
- Minami K, Jimbo N, Tanaka Y, et al. Malignant mesothelioma in situ diagnosed by methylthioadenosine phosphorylase loss and homozygous deletion of CDKN2A: a case report. Virchows Arch 2020;476:469-73. [Crossref] [PubMed]


