The past, present and future of immunotherapy against tumor
Tumor is one of the most common lethal diseases in the world, with 14 million new cases diagnosed annually and is also the leading cause of deaths worldwide, causing 8.2 million deaths annually as World Health Organization (WHO) reported in the World Cancer Report 2014. Although the identification of a large amount of driver oncogenes and anti-oncogenes has resulted in improved treatment outcomes in some special subgroups of tumor patients, survival remains dismal as a whole and we still need to explore the novel therapeutic approaches. Considering that immune system plays a pivotal role in the tumorigenesis, immunotherapies in different kinds of tumors have been more and more crucial and promising.
Past
History of tumor immunotherapy
Our understanding of tumor is associated with the development and knowledge of the immune system. In the 1890s, Williams Coley, a surgeon in New York, demonstrated that streptococcal bacteria into the inoperable tumors of cancer patients could induce the immune response to combat tumors (1-3). Unfortunately, our scarce cognition of immune system has limited its development. During that time, immunotherapy was not considered a serious cancer therapy. Up to the middle of the last century, Coley’s work was firstly published and tumors were discovered to be recognized by the immune system. In the 1960s, some researchers suggested that lymphocytes continuously checked tissues through the recognition of tumor-associated antigens for transformed cells to destroy (4,5). Whilst others indicated that adjuvants could eradicate some tumors. In the 1970s and 1980s, immunologists searched for antibodies that would bind to tumors in the serum of tumor patients, and studies have demonstrated that lymphocytes activated with lectins or with interleukin-2 (IL-2) or interferons (IFNs) could target tumor cells in vitro (6-9). In the 1990s, a series of landmark events changed the prospects of tumor immunotherapy. Firstly, the first tumor-associated antigen was cloned (melanoma associated antigen 1) and immunogenic tumor antigens were discovered, suggesting that they may be recognized and cleared by the immune system (10-13). Secondly, tumor cells were shown to be highly genetically unstable. This could produce tumor-specific epitopes on the surface of the cells, which can distinguish them from normal cells (14). Thirdly, interferon-α2 (IFN-α2) was approved by the US Food and Drug Administration (FDA) for the adjuvant treatment of stage IIB/III melanoma in 1995. IL-2 was approved by the FDA in 1998 for the treatment of metastatic melanoma and renal cell carcinoma. These have led the tumor immunotherapy into a new era. In the 21th century, immunotherapy ushers in the new spring. Immunological checkpoint inhibitors are more and more significant and encouraging (Table 1) (15-24), such as the antibodies of cytotoxic T-lymphocyte antigen-4 (CTLA-4), programmed death-1 (PD-1), T-cell immunoglobulin- and mucin domain-3-containingmolecule 3 (TIM3), Lymphocyte-activation gene 3 (LAG3) and killer cell immunoglobulin-like receptor (KIR). Ipilimumab, the CTLA-4 blocking antibody (25), has provided the first phase III trial evidence of a survival benefit in advanced melanoma (26) and been approved by FDA for the immunotherapy of advanced metastatic melanoma.
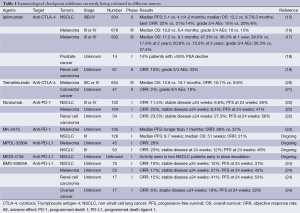
Full table
Immune response to tumor
Immune response to tumor is a complex process (27). Both innate and adaptive immunity play the part in this process. Adaptive immune response is the major and vital process in the immune response to tumor. Generally, this process can be divided into two steps: induction or activation phase and effector phase. In the induction phase, specialized antigen present cells (APCs), also called dendritic cells (DCs) play the critical role. It can handle the antigens from the tumor and present them to naive T cells. Before this process, DCs must receive an immunogenic maturation stimulus, such as microbial peptides or pro-inflammatory cytokines, so that it can be activated upon capture and antigen presentation. Without such a stimulus, an opposite reaction will induce tolerance by T-cell deletion and/or the production of regulatory T cells (Tregs) (28-30). Immunogenic maturation signals not only can be derived from necrotic tumor cells, but also can be therapeutically administered. DC maturation is induced by activated pattern recognition receptors such as Toll-like receptor (TLR). Therefore, TLR ligands or agonist antibodies may be used to stimulate the DCs. Such stimulated DCs will process the captured antigen and present it on MHC class II molecules, at which point they are transported to the draining lymph node, interact with T cells and induce an immune response. After MHC and processed antigens binding together, T-cell receptor (TCR) will interact with them and a co-stimulatory signal in the form of either plasma membrane ligands on the DC that interacts with stimulatory or inhibitory receptors on the T-cell, or in the form of secreted cytokines, would take place for the purpose of the effective immune response to tumor cells (31). If a co-stimulatory signal is received, T cells will become activated. The immune response will enter the effector phase. T-cell mediated immune response is modulated by stimulatory and inhibitory signals. Immune co-stimulatory molecules include CD28, CD137, glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), and OX-40 (32-35). This co-stimulatory signal can be in the form of interaction with plasma membrane ligands of TNF family or the B7 family on the DCs with activating receptors on the T cells. Both the TNF and the B7 family of ligands interact with activating receptors, whereas only B7 family ligands interact with inhibitory receptors. When the activated T-cell travels to the tumor, it will recognize the antigens and eliminate the tumor. However, the tumor possesses some defense mechanisms. During the immune response, the immune system prevents attacking “self” cells under the help of immunological checkpoints. Unfortunately, tumor cells can use these checkpoints as a defense mechanism to deliver the inhibitor signals. The dysfunction of these immune checkpoints can lead to tumor tolerance and eventually allow for tumor “escape” from the immune system.
Current therapy targets
Immunotherapy has made marvelous progress in recent years. IL-2 and IFNs have been approved by FDA in some kinds of tumors. Other cytokines remain uncertainty on antitumor effect. A therapeutic cancer vaccine intends to treat an existing cancer by strengthening the body’s natural defense against cancer. Early attempts to use such technologies were of limited success. As research continues, a number of promising vaccine candidates based on different types of antigenic stimulus have now been evaluated in clinical studies. DCs and T cells based therapy experienced a flexuose development process and it still need our more research. Recently, inhibitors targeting the immune checkpoints have shown more and more exciting and promising antitumor effect, especially targeting the inhibitory receptor CTLA-4, PD-1 and programmed death ligand-1 (PD-L1).
Present
Cytokines
Interleukin-2 (IL-2)
IL-2 is a pleiotropic cytokine produced after antigen activation, which was first discovered in supernatants of activated human T cells in 1976 (36). It can promote CD8+ T-cell and NK cell cytotoxicity activity, and modulate T-cell differentiation programs in response to antigen, promoting naïve CD4+ T-cell differentiation into T helper-1 (Th1) and T helper-2 (Th2) cells while inhibiting T helper-17 (Th17) differentiation (37-39). Moreover, IL-2 is essential for the development and maintenance of Tregs and for activation-induced cell death, thereby mediating tolerance and limiting inappropriate immune reactions (40). IL-2 immunotherapy has been used for many years. It can result in complete remission of 5-10% of patients with metastatic melanoma and renal cell carcinoma, with lack of recurrence for as long as 25 years and potential cures of 70% of these individuals (41). A study including 270 melanoma patients reported CR in 17 patients (6%) and PR in 26 (10%) (42). In 2000, the follow-up study reported CR median duration of at least 59 months, although this had not been reached in their patient population. Based on this, IL-2 was approved by FDA for the treatment of metastatic melanoma in 1998. A limitation of IL-2 is its toxicity, including severe capillary leak syndrome that can accompany such treatment.
In order to improve the outcome with IL-2, many studies have suggested that combination of IL-2-based immunotherapy and chemotherapy or targeted therapy may be the breakthrough. The initial results of combined IL-2 and chemotherapy were promising (43). However, two phase III trials were unable to produce statistically significant response improvements and overall survival (OS) benefit (44,45). In another phase II study (46), 70 consecutive patients with advanced non-small cell lung cancer (NSCLC) were divided into gefitinib (G) and gefitinib + IL-2 (GIL-2) group. In the GIL-2 group and G-group, the author observed: an overall response rate of 16.1% and 5.1%; a disease control rate of 41.9% and 41%; a median time to progression of 3.5 and 4.1 months; a median OS of 20.1 and 6.9 months (P=0.002). This study showed that IL-2 could increase the efficacy of gefitinib. Whether other epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) combined IL-2 have this effect need our further research.
Interferons (IFN)
IFNs are naturally secreted glycoproteins produced by almost every cell type, which can prevent the host from microbial attack and tumor cells invasion (47,48). Its family is classified into three different groups. Type I IFNs consist of IFN-α (comprised of 13 subtypes), IFN-β, IFN-κ, IFN-δ, IFN-ε, IFN-τ, IFN-ω, and IFN-ξ. Type II IFN contains one member, IFN-γ. Type III IFNs consist of 3 members: IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28A) (49,50). All three types of IFNs can induce apoptosis of tumor cells. The mechanism is unclear, but JAK/STAT signaling pathway remains fundamental in initiating the apoptotic signals of IFNs is clear after extensive studies (51,52).
Multiple clinical trials about IFN-based immunotherapy were designed to test its antitumor effects in a variety of tumors after 1980s, including chronic myelogenous leukemia (CML), melanoma, hairy cell leukemia and other types of solid tumors. IFN-α2b was first shown to be beneficial in stage II/III melanoma. A number of studies had demonstrated that adjuvant IFN can significantly increase disease-free survival (DFS) and in some studies, OS (53-57). FDA has approved IFN-a2b as the adjuvant therapy for melanoma patients in 1995. Although IFN immunotherapy is effective in a small subset of tumor patients, it is also associated with high toxicity. For the purpose of high survival benefits, IFN in combination with other treatment modalities is now exploring. One study reported that IFN-α combined with ribavirin, an inhibitor of RNA metabolism, could prevent recurrence and occurrence of hepatocellular carcinoma (HCC) in difficult-to-treat patients with high titers of hepatitis C virus (58). Moreover, patients with HCC show increased responsiveness to IFN therapy when combined with 5-FU.
Vaccines
Traditionally, vaccines to infectious diseases contained an inactivated form of the pathogen, so that they would stimulate an immune response, but not risk the development of disease from the pathogen. Tumor immunologists mimicked this approaches and developed the cancer vaccines, which is to produce an immune response that eliminates cancer cells and produces long-lasting immunity. Theoretically, these vaccines could result in the tumor death and elimination. However, most of the clinical trials proved these methods ineffective. As the representative example, an irradiated, polyvalent, whole-cell melanoma vaccine known as Canvaxin, which seemed promising in phase II studies of melanoma patients at risk for relapse, revealed no benefit for patients in phase III study (59,60).
Nowadays, most cancer vaccine approaches use a specific target antigen that is only expressed in the cancer. One of antigen-specific vaccines, melanoma-associated antigen-A3 (MAGE-A3), a family of tumor specific antigens that is expressed on variety of tumor cells, is promising during recent studies. A phase II trial studying the efficacy and safety of the vaccine was performed in 182 patients with MAGE-A3 positive, resected stage IB/II NSCLC (61). The results showed a positive trend in DFS and OS. In addition, the vaccine was very well tolerated resulting in a good compliance. These encouraging results lead to a phase III trial, which is to explore the efficacy of MAGE-A3 vs. placebo in adjuvant setting for patients with MAGE-A3 positive stage IB, II or IIIA NSCLC. Survival benefit is anticipated.
Another vaccine approach we will focus on is DC vaccines. As we said above, DCs are the key immune cells that can process the antigens and present them to naive T in the adaptive immune response to tumor. The first clinical trial with ex vivo DCs was performed in 1996 (62). With the progress in the understanding of the biology of DCs, we have developed many DC-based novel vaccine strategies. As Palucka reported (63), DCs can be exploited for vaccination against cancer through various means including: (I) non-targeted peptide/protein and nucleic acids-based vaccines captured by DCs in vivo; (II) vaccines composed of antigens directly coupled to anti-DC-antibodies; or (III) vaccines composed of ex vivo generated DCs that are loaded with antigens. All these methods are assessed in ongoing clinical trials.
T-cell based therapy
T-cell is the indispensable part in the adaptive immune response to tumors. DCs would interact with T cells after processing the tumor antigens, and activated T cells would trigger a series of reactions to kill tumor cells. The initial T-cell based therapy was reported by Rosenberg in 1988 (64). Up to now, this method has been developed different strategies. For instance, tumor infiltrating lymphocyte (TIL) based strategy has been demonstrated that it has less risk for autoimmunity. National Cancer Institute group summarized its 10-year experience with phase II clinical trials. The results showed that the clinical response rate is 51% and continuing complete regression over 5 years is 13%. Moreover, recent study indicated that most TILs are directed to unknown antigens instead of known tumor antigens, probably mutated self-proteins that are not expressed in other tissues (65). Another impressive strategy is chimeric antigen receptor T (CAR-T) cells based therapy. The structure of chimeric antigen receptor (CAR) is an extracellular single chain antibody and an intracellular TCR signaling domain. CAR-T is the T cells expressed the CAR, which obtained from the peripheral blood. As an escape mechanism, MHC expression would downregulate in many tumor cells so that T cells cannot recognize them. With the help of CAR, the antigen is still identified by the antibody and via the intracellular TCR signaling domain the T-cell becomes activated. The clinical trials in hematologic tumors have shown promising results (66-68). It is worth mentioning that γδ T-cell-based immunotherapy is becoming more and more impressive. γδ T cells recognize pathogens and transformed cells in a HLA-unrestricted manner. It can share characteristics of both the innate and adaptive immune system, displaying both innate cytotoxic functions and antigen-presenting capability, particularly in the presence of antibody-opsonized target cells. In a systematic review of clinical trials about γδ T-cell for cancer immunotherapy, the author found that γδ T-cell-based immunotherapy is superior to current second-line therapies for advanced renal cell carcinoma and prostate cancer, but not for NSCLC. Furthermore, some studies have suggested that γδ T cells and some antitumor antibodies such as rituximab can be successfully combined for the treatment of tumors since it can overcome the immunosuppression (69-71).
Immune checkpoints pathway
Cytotoxic T-lymphocyte antigen-4 (CTLA-4)
When an antigen was presented to TCR, the MHC and B7 molecules on APCs would bind to CD28 on T cells leading to the activation of CD4 and CD8 cells. CTLA-4 can also bind to B7 but conduct the inhibitory signals. Mice with CTLA-4 knockout have been shown to have lethal lymphoproliferation, which indicated that CTLA4 plays an important role in inhibiting T-cell activation (72). Ipilimumab is a humanized monoclonal antibody that binds to CTLA-4, thereby preventing it from binding to B7 and reducing the inhibition of T-cell activation by CTLA-4. Its inhibition also reduces Tregs, ultimately leading to an accelerated immune response to tumor associated antigens. Ipilimumab has provided the survival benefit to advanced melanoma in a phase III trial and been approved by FDA for the immunotherapy of advanced metastatic melanoma. In a randomized phase II trial in patients with advanced NSCLC, patients received ipilimumab had the longer immune-related progression-free survival (PFS), especially patients with squamous histology had longer OS (15). Ipilimumab is also being studied in combination with EGFR and ALK tyrosine kinase inhibitors in NSCLC (NCT01998126).
Programmed death-1 (PD-1)
PD-1 receptor is expressed in the CD4, CD8, Tregs and NK cells. Its ligands include PD-L1 (B7-H1) and PD-L2 (B7-DC). In many tumors, PD-1 is up regulated in TILs, while a large amount of tumors have increased PD-L1 expression. When PD-1 binds to PD-L1 between tumor cells and lymphocytes, it would result in T-cell anergy and APCs not processing tumor antigens. Nivolumab is a fully human IgG4 monoclonal PD-1 antibody without detectable antibody-dependent cellular cytotoxicity (ADCC). In a phase I study of patients with advanced stage solid tumor, including NSCLC, prostate cancer, renal cell carcinoma, colorectal cancer and melanoma (22). In the NSCLC cohort, the objective response rate (ORR) was 17% with a median duration of response of 74 weeks (range, 6.1-133.9 weeks). In the melanoma cohort, ORR was 31%. In comparison to ipilimumab, nivolumab was generally well tolerated with less frequent immune-related adverse effects (AEs). Therefore, three phase III trials are being conducted to evaluate the role of nivolumab in patients with advanced melanoma (NCT01721746, NCT01721772, NCT01844505). Furthermore, combining PD-1 antibody with a cancer vaccine that also works in the activation phase may provide some benefit. In a study using mice, combining PD-1 antibody with the stimulated DC maturation through activation of TLR pathways showed a synergistic effect (73).
Programmed death ligand-1 (PD-L1)
As one of PD-1 ligand, PD-L1 is a member of the B7superfamily, which is expressed in T and B cells, macrophages and DCs. It is also up regulate in many kinds of tumors. Recent study has demonstrated that PD-L1 expression was associated with vascular invasion and higher-grade differentiation but not associated with EGFR/KRAS mutations or ALK rearrangement (74). The monoclonal antibodies of PD-L1 undergoing clinical development include the monoclonal antibodies MPDL3280A, MEDI4736, BMS-936559 and MSB0010718C. The Fc domain of MPDL3280A has been engineered to avoid ADCC. In a phase I study of MPDL3280A in pre-treated patients with advanced NSCLC, the ORR was 24% and the 24-week PFS was 46%. The higher ORR is in patients with PD-L1 positive and former/current smokers than PD-L1 negative tumors (100% vs. 15%) and never smoker (25% vs. 16%), respectively. MEDI-4736 is an engineered human IgG1 antibody. In its phase I trial, patients with solid tumors had the clinical activity and durable disease stabilization with no dose limiting toxicities or grade 3-4 treatment-related AEs. Another phase I trial combining this antibody with the anti-CTLA4 antibody, tremelimumab, is under plan (NCT01975831).
Future perspectives
With the advances in the understanding of tumor heterogeneity, we have laid more and more emphasis on the targeted therapy and personalized medicine. The first and most important step is to select the targeted patients with different tumors. In other words, we should identify the predictive markers which can predict the antitumor effect and survival benefit before the implementation of immunotherapies. In the past studies, researchers have reported that some predictive markers were all associated with a better response to IL-2 therapy in melanoma patients, such as a good performance status (e.g., Eastern Cooperative Oncology Group 0 or 1), normal lactate dehydrogenase levels, metastasis to less than three organs and cutaneous (43,75). In a prospective study, authors have investigated whether there was a correlation between responsiveness of patients with metastatic melanoma to immunotherapy with IL-2 and IFN-α and the HLA type of the patients. Two HLA alleles that have been shown to function as restriction elements in vitro were observed more frequently in responding patients, namely Cw7 (P=0.014) and A1 (P=0.19). No association was found for A2 and B44. These observations provide evidence that the responsiveness of metastatic melanoma to immunotherapy with IL-2 is associated with certain HLA types (76).
In the immunotherapy with checkpoint inhibitors such as CTLA-4, we have identified some predictive markers that may translate into clinical application such as absolute lymphocyte count (ALC), inducible costimulator (ICOS), HLA-DR and CD45RO (77). The ALC is routinely measured to exclude lymphopenia associated with some therapies and the rate of rise in ALC was identified to correlate with clinical benefit (78). The correlation between CTLA-4 blockade and ICOS was first described in an analysis of 6 bladder cancer patients receiving ipilimumab in the pre-operative setting. Studies have shown a positive correlation between ipilimumab treatment and frequency of CD4+ cells expressing high levels of ICOS (79). One study of 12 patients treated with tremelimumab has demonstrated a correlation between HLA-DR and CD45RO with clinical response (80). Furthermore, PD-1 may be predictive marker in the nivolumab therapy. In a preliminary study, results showed that 36% of the patients that were positive for PD-L1 ligand expression had an objective response to anti-PD-1 therapy compared with none of the 17 patients with PD-1 negative tumors. Preliminary data suggest a relationship between PD-L1 expression on tumor cells and objective response (22). The above results indicated that immunotherapy is similar the molecular targeted therapy. Both of them have the special benefit patients with tumors. Before therapy implementation, we should identify these patients with help of methods of molecular biology.
Another significant direction is the combination therapy. The concept of combination therapy has been a common approach in tumor therapy. As an example, a platinum-based doublet for NSCLC therapy is the successful case in combination cytotoxic chemotherapeutic agents with other drugs. We have summarized the some ongoing clinical trials about the combinations between different immunotherapies especially the checkpoints inhibitors in Table 2. To date, some studies have confirmed that combinations between different immunotherapies are more effective than single strategy. As we have mentioned before, EGFR-TKIs (gefitinib) combined with IL-2 have showed the more survival benefit and less AEs than gefitinib for NSCLC treatment in a phase II study (46). IFN-α combined with ribavirin could prevent recurrence and occurrence of HCC in difficult-to-treat patients with high titers of hepatitis C virus (58). In the preclinical studies, combination TIM-3 or LAG-3 blockade with PD-1 blockade is more effective than blocking either receptor alone (81,82). Another study suggested that combination blockade of the PD-1/PD-L1 and CTLA-4 allows tumor-specific T cells that would otherwise be inactivated to continue to expand and carry out effector functions, thereby shifting the tumor microenvironment from suppressive to inflammatory (83). However, the combination of immunotherapeutic agents with one another and with chemotherapy regimens requires careful investigation in their consideration because some combinations of targeted therapy and chemotherapy have been found to be of benefit, other combinations may be against.
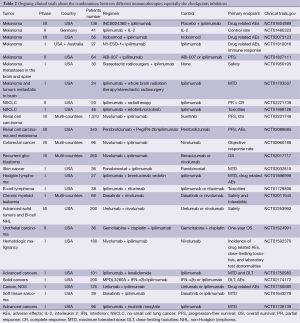
Full table
Conclusions
Immunotherapy is becoming more and more encouraging and promising in tumor therapy. Different strategies based on the various mechanism of immune response to tumor have been developed and showed antitumor effect in some degree. In particular, immunological checkpoint inhibitor is one of the critical immunotherapeutic agents. However, before immunotherapies implementation, we should identify the targeted patients with the help of predictive markers. Furthermore, combination between different strategies would become the major therapy methods to tumors in the future. Ultimately, the timing of immunotherapy administration, duration of treatment and the tolerance of initial treatment may be the future challenges.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin Orthop Relat Res 1991;3-11. [PubMed]
- Topalian SL, Weiner GJ, Pardoll DM. Cancer immunotherapy comes of age. J Clin Oncol 2011;29:4828-36. [PubMed]
- Nauts HC, Fowler GA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man; a critical analysis of 30 inoperable cases treated by Coley's mixed toxins, in which diagnosis was confirmed by microscopic examination selected for special study. Acta Med Scand Suppl 1953;276:1-103. [PubMed]
- Klein G, Sjogren HO, Klein E, et al. Demonstration of resistance against methylcholanthrene-induced sarcomas in the primary autochthonous host. Cancer Res 1960;20:1561-72. [PubMed]
- Burnet FM. Immunological aspects of malignant disease. Lancet 1967;1:1171-4. [PubMed]
- Strausser JL, Mazumder A, Grimm EA, et al. Lysis of human solid tumors by autologous cells sensitized in vitro to alloantigens. J Immunol 1981;127:266-71. [PubMed]
- Grimm EA, Mazumder A, Zhang HZ, et al. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155:1823-41. [PubMed]
- Mazumder A, Grimm EA, Zhang HZ, et al. Lysis of fresh human solid tumors by autologous lymphocytes activated in vitro with lectins. Cancer Res 1982;42:913-8. [PubMed]
- Kirkwood JM, Ernstoff MS. Interferons in the treatment of human cancer. J Clin Oncol 1984;2:336-52. [PubMed]
- Urban JL, Schreiber H. Tumor antigens. Annu Rev Immunol 1992;10:617-44. [PubMed]
- Boon T, Cerottini JC, Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994;12:337-65. [PubMed]
- Boon T, van der Bruggen P. Human tumor antigens recognized by T lymphocytes. J Exp Med 1996;183:725-9. [PubMed]
- Coulie PG, Van den Eynde BJ, van der Bruggen P, et al. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat Rev Cancer 2014;14:135-46. [PubMed]
- Fenton RG, Longo DL. Genetic instability and tumor cell variation: implications for immunotherapy. J Natl Cancer Inst 1995;87:241-3. [PubMed]
- Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012;30:2046-54. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Small EJ, Tchekmedyian NS, Rini BI, et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin Cancer Res 2007;13:1810-5. [PubMed]
- Yang JC, Hughes M, Kammula U, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825-30. [PubMed]
- Ribas A, Kefford R, Marshall MA, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J Clin Oncol 2013;31:616-22. [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [PubMed]
- Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clin Cancer Res 2011;17:6958-62. [PubMed]
- McDermott D, Haanen J, Chen TT, et al. Efficacy and safety of ipilimumab in metastatic melanoma patients surviving more than 2 years following treatment in a phase III trial (MDX010-20). Ann Oncol 2013;24:2694-8. [PubMed]
- Kirkwood JM, Butterfield LH, Tarhini AA, et al. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012;62:309-35. [PubMed]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol 2003;21:685-711. [PubMed]
- Darrasse-Jèze G, Deroubaix S, Mouquet H, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. J Exp Med 2009;206:1853-62. [PubMed]
- Kalinski P, Muthuswamy R, Urban J. Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev Vaccines 2013;12:285-95. [PubMed]
- Gray JC, Johnson PW, Glennie MJ. Therapeutic potential of immunostimulatory monoclonal antibodies. Clin Sci (Lond) 2006;111:93-106. [PubMed]
- Farres MN, Sabry MK, Ahmed EE, et al. OX40 ligand: a potential costimulatory molecule in atopic asthma. J Asthma 2014;51:573-7. [PubMed]
- Woerly G, Roger N, Loiseau S, et al. Expression of CD28 and CD86 by human eosinophils and role in the secretion of type 1 cytokines (interleukin 2 and interferon gamma): inhibition by immunoglobulin a complexes. J Exp Med 1999;190:487-95. [PubMed]
- Nocentini G, Ronchetti S, Cuzzocrea S, et al. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol 2007;37:1165-9. [PubMed]
- Shao Z, Schwarz H. CD137 ligand, a member of the tumor necrosis factor family, regulates immune responses via reverse signal transduction. J Leukoc Biol 2011;89:21-9. [PubMed]
- Morgan DA, Ruscetti FW, Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science 1976;193:1007-8. [PubMed]
- Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol 2010;10:225-35. [PubMed]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 2010;140:845-58. [PubMed]
- Szabo SJ, Sullivan BM, Peng SL, et al. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol 2003;21:713-58. [PubMed]
- Sakaguchi S, Yamaguchi T, Nomura T, et al. Regulatory T cells and immune tolerance. Cell 2008;133:775-87. [PubMed]
- Rosenberg SA. Raising the bar: the curative potential of human cancer immunotherapy. Sci Transl Med 2012;4:127ps8.
- Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 1999;17:2105-16. [PubMed]
- Keilholz U, Conradt C, Legha SS, et al. Results of interleukin-2-based treatment in advanced melanoma: a case record-based analysis of 631 patients. J Clin Oncol 1998;16:2921-9. [PubMed]
- Keilholz U, Punt CJ, Gore M, et al. Dacarbazine, cisplatin, and interferon-alfa-2b with or without interleukin-2 in metastatic melanoma: a randomized phase III trial (18951) of the European Organisation for Research and Treatment of Cancer Melanoma Group. J Clin Oncol 2005;23:6747-55. [PubMed]
- Ridolfi R, Chiarion-Sileni V, Guida M, et al. Cisplatin, dacarbazine with or without subcutaneous interleukin-2, and interferon alpha-2b in advanced melanoma outpatients: results from an Italian multicenter phase III randomized clinical trial. J Clin Oncol 2002;20:1600-7. [PubMed]
- Bersanelli M, Buti S, Camisa R, et al. Gefitinib plus interleukin-2 in advanced non-small cell lung cancer patients previously treated with chemotherapy. Cancers (Basel) 2014;6:2035-48. [PubMed]
- Rees RC. MHC restricted and non-restricted killer lymphocytes. Blood Rev 1990;4:204-10. [PubMed]
- Kotredes KP, Gamero AM. Interferons as inducers of apoptosis in malignant cells. J Interferon Cytokine Res 2013;33:162-70. [PubMed]
- Kontsek P, Karayianni-Vasconcelos G, Kontseková E. The human interferon system: characterization and classification after discovery of novel members. Acta Virol 2003;47:201-15. [PubMed]
- Vilcek J. Novel interferons. Nat Immunol 2003;4:8-9. [PubMed]
- Gamero AM, Larner AC. Vanadate facilitates interferon alpha-mediated apoptosis that is dependent on the Jak/Stat pathway. J Biol Chem 2001;276:13547-53. [PubMed]
- Scarzello AJ, Romero-Weaver AL, Maher SG, et al. A Mutation in the SH2 domain of STAT2 prolongs tyrosine phosphorylation of STAT1 and promotes type I IFN-induced apoptosis. Mol Biol Cell 2007;18:2455-62. [PubMed]
- Kirkwood JM, Strawderman MH, Ernstoff MS, et al. Interferon alfa-2b adjuvant therapy of high-risk resected cutaneous melanoma: the Eastern Cooperative Oncology Group Trial EST 1684. J Clin Oncol 1996;14:7-17. [PubMed]
- Eggermont AM, Suciu S, MacKie R, et al. Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 2005;366:1189-96. [PubMed]
- Eggermont AM, Suciu S, Santinami M, et al. Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 2008;372:117-26. [PubMed]
- Garbe C, Radny P, Linse R, et al. Adjuvant low-dose interferon {alpha}2a with or without dacarbazine compared with surgery alone: a prospective-randomized phase III DeCOG trial in melanoma patients with regional lymph node metastasis. Ann Oncol 2008;19:1195-201. [PubMed]
- Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst 2010;102:493-501. [PubMed]
- Kudo M. Adjuvant therapy after curative treatment for hepatocellular carcinoma. Oncology 2011;81:50-5. [PubMed]
- Morton DL, Foshag LJ, Hoon DS, et al. Prolongation of survival in metastatic melanoma after active specific immunotherapy with a new polyvalent melanoma vaccine. Ann Surg 1992;216:463-82. [PubMed]
- Morton DL, Hsueh EC, Essner R, et al. Prolonged survival of patients receiving active immunotherapy with Canvaxin therapeutic polyvalent vaccine after complete resection of melanoma metastatic to regional lymph nodes. Ann Surg 2002;236:438-48; discussion 448-9. [PubMed]
- Vansteenkiste J, Zielinski M, Linder A, et al. Adjuvant MAGE-A3 immunotherapy in resected non-small-cell lung cancer: phase II randomized study results. J Clin Oncol 2013;31:2396-403. [PubMed]
- Hsu FJ, Benike C, Fagnoni F, et al. Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 1996;2:52-8. [PubMed]
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. [PubMed]
- Yang JC, Rosenberg SA. Current approaches to the adoptive immunotherapy of cancer. Adv Exp Med Biol 1988;233:459-67. [PubMed]
- Junker N, Kvistborg P, Køllgaard T, et al. Tumor associated antigen specific T-cell populations identified in ex vivo expanded TIL cultures. Cell Immunol 2012;273:1-9. [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25.
- Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725-33. [PubMed]
- Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013;368:1509-18. [PubMed]
- Braza MS, Klein B, Fiol G, et al. γδ T-cell killing of primary follicular lymphoma cells is dramatically potentiated by GA101, a type II glycoengineered anti-CD20 monoclonal antibody. Haematologica 2011;96:400-7. [PubMed]
- Capietto AH, Martinet L, Fournié JJ. Stimulated γδ T cells increase the in vivo efficacy of trastuzumab in HER-2+ breast cancer. J Immunol 2011;187:1031-8. [PubMed]
- Tokuyama H, Hagi T, Mattarollo SR, et al. V gamma 9 V delta 2 T cell cytotoxicity against tumor cells is enhanced by monoclonal antibody drugs--rituximab and trastuzumab. Int J Cancer 2008;122:2526-34. [PubMed]
- Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270:985-8. [PubMed]
- Mangsbo SM, Sandin LC, Anger K, et al. Enhanced tumor eradication by combining CTLA-4 or PD-1 blockade with CpG therapy. J Immunother 2010;33:225-35. [PubMed]
- Yang CY, Lin MW, Chang YL, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014;50:1361-9. [PubMed]
- Phan GQ, Attia P, Steinberg SM, et al. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol 2001;19:3477-82. [PubMed]
- Scheibenbogen C, Keilholz U, Mytilineos J, et al. HLA class I alleles and responsiveness of melanoma to immunotherapy with interferon-alpha (IFN-alpha) and interleukin-2 (IL-2). Melanoma Res 1994;4:191-4. [PubMed]
- Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol 2010;37:473-84. [PubMed]
- Willett CG, Duda DG, di Tomaso E, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol 2009;27:3020-6. [PubMed]
- Liakou CI, Kamat A, Tang DN, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A 2008;105:14987-92. [PubMed]
- Comin-Anduix B, Lee Y, Jalil J, et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J Transl Med 2008;6:22. [PubMed]
- Sakuishi K, Apetoh L, Sullivan JM, et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 2010;207:2187-94. [PubMed]
- Woo SR, Turnis ME, Goldberg MV, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res 2012;72:917-27. [PubMed]
- Curran MA, Montalvo W, Yagita H, et al. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010;107:4275-80. [PubMed]


