
Lung cancer biomarker testing: perspective from Europe
Background
The treatment of lung cancer is a prime example of rapidly developing options within current oncology. For optimal selection of individual patients for specific treatment, sole morphological (cytological or histological) diagnosis is no longer sufficient and up-to-date pathological reports should in many specific cancer types also include results of testing for several multiple molecular markers, namely different drugable mutations which can be targeted by modern drugs.
Lung cancer biomarker testing is performed in many countries in Europe. The manner in which predictive testing is performed, is usually governed and organized in a regional or national manner. National guidelines—sometimes based on international recommendations published by organizations like IASLC/CAP/AMP, ESMO, NCCN or ASCO—often play an important role (1).
A report about current situation in molecular testing in the central European region has been published recently (2). The authors used a questionnaire distributed in 2014-2015. This report provided information about non-small cell lung cancer management and testing of EGFR and ALK.
To obtain insight in the molecular landscape in more countries, a questionnaire of the Ryska paper (2) was updated (see Supplementary File) and distributed in Western and Central European countries to the pathologists participating at the Pulmonary Pathology Society meeting 26–28 June 2019 in Dubrovnik, Croatia - each country was represented by one responder.
Results and discussion
In total, responses from 21 pathologists from different Western and Central European countries were collected by the end of May 2019 (response rate 100%). Responses were obtained by representative of single institutions of a country. It is not excluded that local practices might be different than indicated, including variation in ‘on demand’, reflex’ or ‘diagnosis and predictive’ testing.
Analysis of the submitted answers indicate that a national cancer registry was available in 16 countries and a regional registration in an additional one, see Figure 1. The number of NSCLC patients per country ranged from 650–55,700 (mean 13,500).
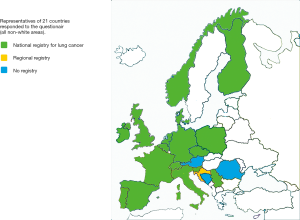
Diagnostic testing
The sample type used for molecular testing varied widely: mean and standard deviation were for ‘Cytology only’ 24%±14%, for ‘Histology only’ 45%±23% and for ‘Histology and Cytology’ 26%±20%. Most responders replied that all patients had pathological diagnoses of lung cancer, except for 6 responders, where the fraction of probable lung cancer patients without a pathological diagnosis varied from 2–15%.
Additional diagnostic (immuno)histochemistry (TTF1, p63/p40, mucin staining) is more or less routinely applied: mean 62% (range, 10–100%) to further specify the NSCLC diagnosis into individual histological subtypes.
The distribution for the histological subtypes was (mean and standard deviation): adenocarcinoma 47%±8%, squamous cell carcinoma 26%±7%, small cell lung carcinoma 15%±3%, NSCLC-not otherwise specified 6%±3% and others.
Testing guidelines
Awareness of international guidelines of IASLC/CAP/AMP (3), ASCO (4), and ESMO (5) was present in all countries. Many also have national guidelines: Austria (1) Belgium (6), Czech Republic (7), FRANCE (8), Germany (9), Netherlands (10), Norway (11), Slovenia (12), Spain (13).
Predictive testing
The inclusion criteria for predictive molecular testing were similar in all countries: adenocarcinoma, NSCLC favour adenocarcinoma and NSCLC NOS (not otherwise specified). In patients with squamous cell carcinoma testing was only performed on demand in those with advanced disease without a smoking history in line with ESMO (5), ASCO (4) and CAP/IASLC/AMP (3) guidelines. PD-L1 is tested for all subtypes of NSCLC.
Testing strategy
There are three strategies for predictive testing: ‘on demand’, ‘reflex testing’ and ‘diagnosis and prediction’.
With the ‘on demand’ (also called ‘bespoke’) testing approach the request to test is initiated by an oncologist/pulmonologist treating the patient after receiving the pathology report with lung cancer diagnosis. This approach has the advantage that only those patients with advanced NSCLC are tested, who also fit the clinical inclusion criteria for targeted treatment and are therefore in case of a positive test result likely to be treated. The disadvantages of this approach are (I) not all patients that may be eligible have predictive testing requested, (II) delay in predictive test results, as the test request by clinician is based on the availability of final histological diagnosis and (III) with no tissue management (see below), there is an increasing chance of an uninformative test outcome. Thus, in cases with small sample size with insufficient amount of remaining tumor cells, another biopsy may be necessary resulting in an additional delay. Of note, replacing the paraffin block in the microtome results in loss of tissue due to the uneven positioning in terms of µm, loosing 10–100% of the remaining tumor biopsy sample.
With the ‘reflex testing’ approach, the testing is initiated by the pathologist based on a diagnosis fulfilling the inclusion criteria. This has the advantages of (I) the application of optimal tissue management, and (II) a reduction of the waiting time for a predictive test result as predictive testing starts before communicating the diagnostic report to the clinician. The disadvantage is that also patients not eligible for treatment for various reasons (such as poor performance status, severe comorbidities, rejection of treatment by the patient) or patients with lower stages may be tested, some of whom may be cured by primary therapy.
The ‘diagnosis and prediction (D+P)’ approach requires a chain organisation based on a request from the clinician submitting the tissue (14) to the laboratory handling the specimen, through to the reporting process. The process starts with the clinician at the time of biopsy with the request for predictive testing if the histologic diagnosis fits the inclusion criteria. The request for predictive testing (D+P code) is printed on the paraffin blocks, indicating to the medical scientist/technician a specific cutting protocol of approximately 20 additional unstained sections, where the first and last will be used for routine haematoxylin and eosin stain for diagnostic purpose and the slides in between for both diagnostic (immunohistochemistry for tumor subtyping) and predictive testing, if the final diagnosis fits the inclusion criteria. This has the advantages that (I) only patients with advanced NSCLC will be tested who are likely to be treated in case of a positive predictive test are included and (II) optimal tissue management. The main disadvantage is that the protocol requires very close clinic-pathological collaboration and also slightly more handling time. In cases which do not fulfil criteria for predictive testing, not all initially cut sections are used. Sections ‘in between’ the haematoxylin stained sections are used in ~80% of the cases for immunohistochemical testing and in ~50% for predictive DNA testing.
Based on the responses in the questionnaire, the molecular testing strategy of ‘reflex testing’ is applied in 14 countries, ‘on demand’ testing in 6 countries [Ireland, Poland, Portugal, Romania, Serbia, Spain (some on demand, some reflex)], and diagnosis and prediction in 1 (locally in the Netherlands).
Site of testing
Most of the responders indicated that testing is performed locally, either in house or in a regional laboratory. The exceptions were Portugal with a national central laboratory and Romania with testing performed solely in an external commercial national or international institute.
Method of testing
Historically, molecular testing in lung cancer was initially performed for EGFR mutations with single gene (frequently real time PCR) testing. Gradually more genes were introduced to the testing process. KRAS mutation analysis was occasionally performed with the argument that if positive EGFR mutation testing was not necessary, because KRAS and EGFR mutations seemed to be mutually exclusive (15). Also, in at least one country (Hungary), use of first EGFR TKI was initially limited to patients with KRAS wild-type status (interestingly, not to EGFR mutant only). In time multiple parallel immunohistochemical testing for ALK, PD-L1 and recently ROS1 and NRTK IHC was frequently performed together with multiple gene testing on DNA.
Subsequently, next generation sequencing (NGS) evolved. In practice this meant the testing of mutations on a relatively small number of clinically relevant genes in one run (targeted NGS). Whole exome or whole genome sequencing is currently not performed in routine practice for predictive testing.
Responses to the question “If EGFR testing is performed in-house, what testing method(s) was applied were: 17x NGS and 5x real time PCR, see Figure 2. The reported fraction of inadequate testing ranged from 1–10% (mean 6%). The turnaround time for EGFR testing varied from 3–14 (mean 8) workdays.
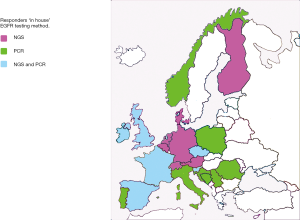
Availability of predictive testing
EGFR, ALK and PD-L1 testing was available in all countries. The antibodies used for PD-L1 were 22C3 (n=11), SP263 (n=7), SP142 (n=1) and E1L3N (n=1). In some countries (e.g., Austria), different laboratories are using different clones. Within the centers responding to the survey an approved (CE-IVD certified) test was used in 11 centres and in 7 a laboratory developed test was implemented.
ROS1 testing was available in most countries, except for United Kingdom, Romania and Serbia, see Figure 3. In Czech Republic, at the time of the responding, ROS1 was tested on demand. However, since November 2019 reimbursement was agreed for reflex testing. In Denmark ROS1 was fully implemented during the writing period.
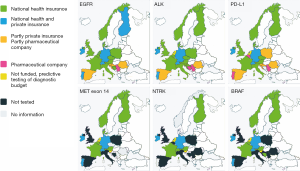
Funding for predictive testing
Following the European Medical Agency approval in many countries there are tightly regulated procedures for drug reimbursement. Frequently the drug can only be given when the predictive test is positive. The question is if across Europe reimbursement of the predictive test associated with the drug is obtained at the same time as the approval for the drug. A previous survey by the Central European Cooperative Oncology Group showed that both availability and reimbursement of testing of molecular alterations in NSCLC, which is essential for therapeutic decisions, varies widely between these countries (16). Not only is ‘reflex testing’ often substituted by analyses performed only ‘on demand’, but reimbursement of such assessments varies widely between unavailability and payments by the health care system or even pharmaceutical companies.
Reimbursement information of the current survey is shown in a graphical display for 6 different predictive tests (EGFR, ALK, ROS1, PD-L1, Met-Exon 14, and NTRK), see Figure 3.
For EGFR testing funding is available in most European countries, which evolved around 2010, but in some countries (Spain [partly private/public and partly pharmaceutical]], Croatia, Servia, Romania) a pharmaceutical company still fully or partly covers the cost of EGFR testing even in 2019. For ALK and PD-L1 the situation is more or less similar, but for the more recent predictive testing options the situation is dramatically worse.
Country specific funding information
Austria
In Austria, all predictive tests for EMA approved targeted therapies are reimbursed. There are different models of reimbursement, depending on the region, and on the provider of the testing (Medical Universities, state or private hospitals). This is a complicated system since there is no single national health care, or national insurance in Austria. Every employee has to be insured by the employer. There are several insurance companies and there is also insurance for the unemployed and retired people covered by the insurance system.
Belgium
In Belgium, a governmental commission has recently been set up (ComPerMed) where reimbursement of the medication is discussed together with the need and reimbursement of a companion diagnostic test. Nevertheless, although the time needed for the first part has much shortened in recent years, the second part takes a lot longer (up to several years), especially in regard to immunohistochemical tests performed exclusively by pathologists.
Bosnia and Herzegovina
In the Bosnia and Herzegovina there is no separate funding for applying predictive testing. Initially after drug approval, predictive tests are initially supported by pharmaceutical company and is available for all patients. After some time, if pharmaceutical company dismiss this support, expenses for predictive testing are payed from diagnostic budget.
Croatia
National health insurance made recommendations that are obligatory for predictive testing in Croatia, what is partly payed by National health insurance and partly supported by pharmaceutical companies (monoclonal antibodies for immunohisto/cytochemistry) and available for all patients.
Czech Republic
The predictive testing on NSCLC in Czech Republic is reimbursed by the National Health Insurance. However, reimbursement of the test depends on the availability of the treatments depending on such test—in other words, only tests with direct impact on patients’ clinical management are reimbursed. All laboratories register all tested samples (specific morphology type, methods used, results of the tests) on a voluntary basis in a national pathology registry of NSCLC.
Denmark
In Denmark the validation and application of predictive tests in public laboratories are covered from the diagnostic budget of the hospitals and there are no formal restrictions to availability. The predictive test will normally be discussed within the Danish group of lung pathologists (DaLuPa) before using it. The predictive tests are generally available from the initial period that the drug is available for all patients. Private laboratories do not perform these predictive tests.
Finland
In Finland the validation and application of predictive tests in public laboratories are covered from the diagnostic budget of the Health Care District and there are no formal restrictions to availability. After the approval of the drug by the European Medical Agency, it normally takes six months from the application for the Pharmaceuticals Pricing Board of the Ministry of Social Affairs and Health to approve the funding and the drug to be available for all patients.
France
In France the process of reimbursement is regulated by the French Ministry of Health for the different predictive tests in molecular biology. Until recent years, the French molecular platforms for oncology testing received a budget from the French NCI (Inca) according to their activity and the number of tests they did each year. Recently, another system of reimbursement has been set up, based on the size of the NGS panels used in oncology. This complex mode of functioning, called the “Référentiel des actes Innovants Hors Nomenclature” (RIHN) has been set up by the “Direction Générale de l'Offre de Soins” (DGOS).
Germany
In Germany, all predictive tests (immunohistochemical and molecular analyses) for NSCLC cases which are necessary for targeted therapies and are approved by the EMA, are reimbursed by health insurance companies. Tests and therapy are available for all patients. Approximately 73 million Germans have a national health insurance plan and 9 million Germans have a private health insurance plan. There is no significant delay in reimbursement.
Ireland
In Ireland the Health Service Executive (HSE) fund two public centres for molecular testing. Private insurance companies also pay for testing for patients with private health insurance.
Some hospitals, who wished to develop their own molecular testing service, are self-funding from their service (diagnostic) budget. In the first year of PDL1 testing in Ireland, funding was provided by the HSE but subsequently funding was rolled into individual hospital pathology departments’ budgets and is performed at five centres. Private insurance pays for PDL1 testing for patients with private health insurance.
Italy
Although in Italy the health-care system is a regionally organized National Health Service (SSN), the Livelli Essenziali di Assistenza (LEA) has at national level exclusive authority in determining the minimum obligatory tests to be performed in all regions. Each region may individually define the additional tests (and tariffs).
Netherlands
In the Netherlands the administrative process of reimbursement is tightly regulated in 2 price categories. This precludes immediate adaptation of pricing and funding of predictive testing when the testing panel increases. Nederlandse Zorg Autoriteit (NZA) decides on the testing categories. The Dutch Scientific Society proposes the table that assigns the various techniques to a category. Hospitals subsequently negotiate with the insurance companies and payment will be obtained over the next budget year. After drug approval (included a separate budget) the delay in funding for the test by national health insurance companies is around 3 years. Test innovation and validation is not covered by health insurance. As the total health care budget is restricted, the increasing expenses for predictive testing effectively, in most hospitals comes down to a budget cut. Nevertheless, predictive tests are generally available from the initial period that the drug, but access to testing and the completeness of predictive testing varies across hospitals.
Norway
In Norway all the predictive testing is government funded. The outpatient clinic testing is paid for directly by the Helfo (The Norwegian Health Economics Administration). For NGS there is full reimbursement, but for the immunostains (alk, ros1 and PD-L1) and FISH the reimbursement is lower than the realistic price. The difference is covered by the pathology department's own "diagnostic” budget. The inpatient testing is paid for by the budget of the hospital department submitting the biopsy/cytology specimen. The hospitals that perform predictive testing in Lung Cancer in Norway are Public.
Poland
In Poland national health payer (Narodowy Fundusz Zdrowia, NFZ) reimburses molecular or genetic procedures. The cost of routine pathological procedures including immunohistochemical tests is incorporated into surgical or endoscopic procedures and is also covered by NFZ. Immunohistochemical biomarkers (ALK/IHC or PD-L1) are not extra paid. The costs of positive PD-L1 tests are coupled with the treatment for reimbursement covered by NFZ. Currently, pharmaceutical company bears the costs of negative tests.
Portugal
In Portugal, National Health Service supports molecular pathology tests for every Portuguese citizen. PD-L1 22C3 DAKO is supported by industry. Private Hospitals perform predictive testing with patient insurance.
Romania
In Romania predictive tests are usually supported by pharmaceuticals company and is available for all patients after visiting the oncologist for a ‘pharma voucher’, if the patients requires testing before this moment the costs are borne by the patient.
Serbia
In the Republic of Serbia the registration of molecular tests for oncology diagnostic procedures lays within “Medicines and Medical Devices Agency of Serbia (ALIMS)”. After the approval by the agency, test can be used for molecular diagnostics in oncology.
All the tests ALIMS approved tests are funded by the industry. Thus, not reimbursed by the National Health Insurance Fund. Outside this regulation, private testing and private application of the drugs is available.
Slovenia
Predictive biomarker testing in lung cancer patients follows national recommendations and is available for all patients (12). Reimbursement for major predictive tests is funded by pharmaceutical companies and is supervised by national health insurance company. Other tested predictive biomarkers are not reimbursed.
Spain
In Spain the situation differs between various autonomous communities and hospitals. In the past, most testing was funded by the pharmaceutical industry but since 2018 there have been different efforts to finance the testing by national health system. Currently, there is a mixture where funding is covered by national healthcare authority as part of NGS panel in some and in others IHC is provided by pharmaceutical industry.
United Kingdom
In the UK, funding for molecular testing comes from the National Health Service (NHS) after it has been approved by the National Institute for Health and Care Excellence (NICE) following an in-depth analysis, including cost-benefit considerations. Currently, approval by NICE and reimbursement is limited to EGFR, ALK, ROS1 and PD-L1 expression. Additional testing for other molecular tests may be performed if relevant for treatments funded by the patient via private insurance or other routes such as the Cancer Drugs Fund (CDF). New predictive tests are typically funded on an ad hoc basis in conjunction with the associated pharmaceutical company.
It should be noted that in England and Wales there is a change to move all molecular testing to 7 genomic laboratory hubs (GLHs) that will be the sole recipients of funding from the NHS for performing any cancer molecular tests. This is due to occur in April 2020, and for most solid organ tumours molecular profiling will be performed with NGS panels, but for specific cases, including some haematological and paediatric tumours whole genome sequencing (WGS) will also be available and funded.
Switzerland
In Switzerland predictive biomarker testing is available for all patients with advanced stage NSCLC and covered by health insurance companies. Beside testing for drugs approved by the Swiss authority Swissmedic, broad molecular profiling is usually performed to allow enrolment in clinical trials and access to off label use of available drugs. Testing guidelines are provided by the Swiss Society of Pathology (17,18) and are closely linked to the NCCN guidelines (18). Noteworthy, there are no central regulations regarding predictive testing, which allows for dynamic and flexible expansion of existing testing algorithms by new targets.
Future perspective
That funding is important shows a study of Vrdoljak and colleagues, where a correlation of health expenditures per capita and mortality-to-incidence ratio is shown (19). Large differences in cancer epidemiology, in the majority of oncology care parameters and in treatment outcomes, exist between Central and Eastern European countries and neighbouring Western countries. Mortality-to-incidence ratios are much less favourable in Central and Eastern European countries, and cancer mortality age-standardized rates are among the worst in the world (2). In general, a higher national income and health care budget is associated with a higher cancer incidence rate and lower mortality rate (12).
In line with a report about lung cancer in Africa and the Middle East in countries with a recent history of war (or still at war), the development in modern health care, expressed in terms of predictive testing for lung cancer, is at an essentially slower rate than in other countries or has no recognizable progress (20).
The extension of the questionnaire about molecular testing in the central European region (2) shows in this study that for recent biomarkers the availability and reimbursement of diagnoses of molecular alterations in NSCLC, varies widely between different, also western European, countries. Reimbursement of such assessments varies widely between unavailability and payments by the health care system or even pharmaceutical companies.
The support for testing from alternative sources, such as the pharmaceutical industry, is no doubt partly compensating for the lack of public health system support, but it is not a viable or long-term solution, as it is hampered by a series of ethical issues and conflicts of interests (2).
Currently, 74% of the predictive tests are performed with a laboratory developed test and 26% with in-vitro diagnostics kits (21). An additional future financial hurdle is that the European Medical Agency has provided a regulation that laboratories should use in-vitro diagnostics kits, which are in general much more costly than the laboratory developed tests. This will potential have a huge financial impact on testing for all countries. This regulation is peculiar as after a learning phase laboratory developed tests do not perform worse than commercial assays (22,23) and, if followed, will especially be beneficial for the companies generating these kits at the expense of the national health budget. Needless to say, that in countries with an already limited health care budget, postponing the start of biomarker testing such a regulation backfires and has a counterproductive effect.
In the biomarker testing landscape of an increasing number of genes needed to be tested, see Figure 3. At least for a large number of genes, implementation of simultaneous testing of multiple genes at the same time using the next-generation sequencing approach in clinical diagnostic molecular pathology laboratories is the way forward (24). It is likely that for NSCLC the financial break-even point between performing in parallel individual tests for EGFR, BRAF, Her2, ALK, ROS1, NTRK, MET exon14 and PD-L1 and replacement with DNA testing for mutations, small deletions/insertions, RNA testing for gene fusion and PD-L1 IHC has been reached. This change in NGS testing approach definitely requires more collaboration between regional laboratories.
Participating in external quality assessment programs on predictive testing has been performed in the past in some countries France (25), Germany (26), Italy (27,28), Netherlands (23) or by nation overarching European organisations: ESP (29,30), UKNEQAS (31), EMQN (32), NordiQC (22,33) and should be maintained in the future to establish predictive testing of optimal quality. The published data clearly demonstrate that regular participation in the established EQA programs results in improvement of the performance of individual laboratories (29,30).
Ideally, a structured access to testing and reimbursement should be the aim in order to provide patients with appropriate therapeutic options. As biomarker enabled therapies deliver a 50% better probability of outcome success (21), improved and unbiased reimbursement remains a major challenge for the future.
Supplementary
NSCLC Molecular testing May 2019
Q1: Does your country have a population-based cancer registry recording data on NSCLC incidence of NSCLC?
Please enter your choice:
□ a. Yes, national registry If yes, please provide registry name(s):
□ b. Yes, regional registry If yes, please provide registry name(s):
□ c. No
□ d. Don’t know
Q2: What proportion of NSCLC patients in your country have Stage IIIB/IV disease at diagnosis?
.% in year ….
Answer based on
□ Data records ; source….
□ Best estimate
Q3: In the last year for which data are available, how many cases of NSCLC (any stage) were diagnosed in your country?
Answer based on
□ Data records ; source….
□ Best estimate
Q4: In 2018, how many cases of NSCLC (any stage) were seen at your centre?
…. number
Q5: Of these cases in Q4, what proportion was diagnosed using tissue material originating from the following sources (answer should total 100%):
Cytology only …%
Histology only …%
Concurrent histology and cytology …%
No microscopic diagnosis …%
Q6: For each stage of disease shown below, please indicate the proportion of NSCLC patients at your centre for whom a surgical sample from the primary tumour is available?
Stage I …%
Stage II …%
Stage III …%
Stage IV …%
Q7: How many hospitals in your country, where lung cancer is treated, have an in-house pathology department that routinely performs morphological diagnostics (cytology and/or histopathology) of lung cancer?
Number: ….
Q8: In what proportion of NSCLC cases tested by your laboratory in 2018 was immunohistochemistry used to confirm/finalise the histological diagnosis (TTF1, p63/p40, mucin stain, .etc.)?
Percentage: …%
Q9: What proportion of the lung cancer cases diagnosed in your country are classified as the following histological subtypes?
Adenocarcinoma (incl favour adeno) …%
Squamous cell carcinoma (incl favour SqCC) …%
NSCLC NOS (biopsy only by definition) …%
Small cell carcinoma …%
Large cell carcinoma (resection specimen only by definition) …%
Other …%
Q10: Are NSCLC patients in your country currently tested for mutation status?
□ a. Yes, all of them
□ b. Yes, some of them
□ c. No
□ d. Don’t know
Q11: How is EGFR testing in your country currently funded? (Please specify all that apply)
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q12: Which histological subtypes of NSCLC are tested for mutation status in your country? (Please specify all that apply)
□ Adenocarcinoma (incl favour adeno)
□ Squamous cell carcinoma (incl favour SqCC)
□ NSCLC NOS (biopsy only by definition)
□ Small cell carcinoma
□ Large cell carcinoma (resection specimen only by definition)
□ Other
Q13: Does your country follow guidelines to select which histological subtypes of NSCLC undergo EGFR testing?
□ a. Yes, national testing guidelines
□ b. Yes, European testing guidelines
□ c. Yes, local guidelines
□ d. Yes, other guidelines
□ e. No, we have no guidelines
□ f. No, we cannot follow them (due to financial limitations, tissue availability, lack of cooperation, etc.)
If you follow guidelines to guide EGFR testing, please provide a reference*)
……….
*) PMID/website link/attach PDF please, if locoregional
Q14: Does your centre follow guidelines to select which histological subtypes of NSCLC undergo EGFR testing?
□ a. Yes, national testing guidelines
□ b. Yes, European testing guidelines
□ c. Yes, local guidelines
□ d. Yes, other guidelines
□ e. No, we have no guidelines
□ f. No, we cannot follow them (due to financial limitations, tissue availability, lack of cooperation, etc.)
If you follow guidelines to guide EGFR testing, please provide a reference*)
….
*) PMID/website link/attach PDF please, if locoregional
Q15: Which stages of NSCLC are tested for mutation status at initial diagnosis at your centre?
□ a. All stages
□ b. Stage IIIB and stage IV only
□ c. Do not know
Q16: Which strategy is used for EGFR testing?
□ a. Reflex testing: every patient is tested automatically (based on histological type)
□ b. On-demand: requested by the clinician who took the biopsy
□ c. On-demand: requested by clinician who is treating the patient (e.g., oncologist)
□ d. Other strategy (please specify): ……….
Q17: In what proportion of the NSCLC cases which are eligible for EGFR testing in your country is testing actually performed?
Percentage: …. %
Q18: In what proportion of the NSCLC cases eligible for testing at your centre is EGFR testing actually performed?
Percentage: …. %
Q19: Please list any exclusion criteria inclusion/exclusion criteria for EGFR testing in your country? E.g., gender, smoking history, performance status, KRAS status, etc.
…………………………………… ………………………………. …………………………… ………………….
Q20: Where is EGFR testing for the NSCLC patients treated at your centre performed?
□ a. In-house testing laboratory
□ b. External national laboratory
□ c. External laboratory (outside of your country)
□ d. External national commercial laboratory
□ e. External international commercial laboratory (outside of your country)
Q21: If EGFR testing is performed in-house, what test method(s) do you use?
Please specify all that apply:
□ a. Direct sequencing
□ b. Immunohistochemistry with mutation-specific antibody
□ c. Real-time PCR (e.g., ARMS, TheraScreen®, Cobas®)
□ d. Pyrosequencing
□ e. NGS
□ f. WES
□ g. WGS
□ h. Other method please specify:
□ i. I do not know what method is used
Q22: If EGFR testing is performed in-house, what proportion of tissue specimens are inadequate for testing?
Percentage: …. %
Q23: Please indicate the reason(s) why specimens are inadequate (answer should total 100%)
a. Tissue specimen too small: … %
b. Inadequate fixation/storage of tissue specimen: … %
c. Not enough tumour cells in the tissue sample: … %
d. Other reason(s): … %
Q24: What is the average turnaround time of EGFR testing for your patients? (I.e. time from when specimen is sent to the laboratory for analysis until final test results are received.)
Indicate average number of days: ……
Q25: Is ALK testing available for NSCLC patients in your country?
□ a. Yes;
□ b. No;
□ c. Do not know
Q26: Are NSCLC patients in your country currently tested for rearrangements?
□ a. Yes, all of them
□ b. Yes, some of them selected by histology (indicate histological subtypes that are tested):
i. Adenocarcinoma (incl. NSCLC, favour adenocarcinoma)
ii. NSCLC-NOS
iii. Squamous cell carcinoma (incl. NSCLC favour squamous cell carcinoma)
iv. Large cell neuroendocrine carcinoma
v. Other
□ c. No
□ d. Don’t know
Q27: How is ALK testing currently funded in your country?
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q28: Please list any other inclusion/exclusion criteria for ALK testing E.g., gender, smoking history, performance status, EGFR/KRAS status, etc.
…………………………………… ………………………………. …………………………… ………
Q29: Does your centre follow guidelines to select which histological types of NSCLC undergo ALK testing?
□ a. Yes, national testing guidelines
□ b. Yes, European testing guidelines
□ c. Yes, local guidelines
□ d. Yes, other guidelines
□ e. No, we have no guidelines
□ f. No, we cannot follow them (due to financial limitations, tissue availability, lack of cooperation, etc.)
If you follow guidelines to guide EGFR testing, please provide a reference ………./PMID/attach PDF
Q30: What strategy is used for ALK testing?
□ a. Reflex testing: every patient is tested automatically (based on histological type)
□ b. On-demand: requested by the clinician who took the biopsy
□ c. On-demand: requested by clinician who is treating the patient (e.g., oncologist)
□ d. Other strategy (please specify): ……….
Q31: Which stages of NSCLC are tested for rearrangements at the time of the initial diagnosis at your centre?
□ a. All stages
□ b. Stage IIIB and stage IV only
□ c. Do not know
Q32: What method(s) are currently used for ALK testing in your country? (Please specify all that apply, and indicate details of specific antibodies or kits used)
□ a. Immunohistochemistry ; please specify antibody….
□ b. Immunohistochemical screening, followed by FISH
□ c. FISH
□ d. RT-PCR
□ e. DNA sequencing
□ f. Other method, please specify
Q33: Are lung cancer patients in your country tested for KRAS in NSCLC?
□ a. Yes
□ b. No
Q34: In your country, are multidisciplinary lung cancer teams established in routine clinical practice?
□ a. Yes
□ b. No
□ c. Yes, but only in specialised lung cancer treatment centres
□ d. Do not know
Q35: In your country, is it mandatory for all lung cancer cases to be discussed by a multidisciplinary tumour board before any primary treatment is initiated?
□ a. Yes, according to local guidelines
□ b. Yes, according to local practice
□ c. No
□ d. Selected cases only
□ e. Do not know
Q36: In reality, what proportion of lung cases are actually discussed at the multidisciplinary tumour board in your country?
Best estimate Percentage: …...%
Q37: In reality, what proportion of lung cancer cases are actually discussed at the multidisciplinary tumour board at your centre?
Best estimate Percentage: …...%
Q38: Is PD-L1 tested for NSCLC in your centre?
□ a. Yes
□ b. No
Q39: Which PD-L1 antibody is used for NSCLC in your centre?
□ a. 22.8
□ b. 22C3
□ c. SP263
□ d. SP142
□ e. Other, please specify: ………. …….
Q40: How is PD-L1 testing currently funded in your country?
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q41: Is this an approved assay or a laboratory developed test (LDT)?
□ a. EMA or FDA Approved
□ b. LDT
□ c. do not know
Q42: Is this PD-L1 assay clinically validated or technically validated ?
□ a. technically validated
□ b. clinically
□ c. do not know
Q43: Is MET exon 14 skipping tested for NSCLC in country?
□ a. Yes please provide method: … …. ….
□ b. No
Q44: If Yes on Q43: How is MET exon 14 skipping testing currently funded in your country?
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q45: Is BRAF mutation tested for NSCLC in your country?
□ a. Yes please provide method: … …. ….
□ b. No
Q46: If Yes on Q45: How is BRAF testing currently funded in your country?
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q47: Is NTRK fusion tested for NSCLC in your country?
□ a. Yes please provide method: ...............
□ b. No
Q48: If Yes on Q47: How is NTRK testing currently funded in your country?
Please specify all that apply:
□ a. National healthcare authority
□ b. National health insurance company
□ c. Private insurance
□ d. Pharmaceutical industry
□ e. Other source(s); please provide details
□ f. No funding
Q49: Are lung cancer patients in your country tested for any other molecular biomarkers (e.g., NRG1)? Please provide details below.
- NRG1
- ROS1?
- RET?
………… …………. …………. ……………….
Acknowledgments
The support of Dr. Paul C. Diegenbach with the figures is greatly appreciated.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Sanja Dacic) for the series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” published in Translational Lung Cancer Research. The article was sent for external peer review organized by the Guest Editor and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr.2020.04.07). The series “Selected Highlights of the 2019 Pulmonary Pathology Society Biennial Meeting” was commissioned by the editorial office without any sponsorship or funding. ET reports personal fees from Abbvie, personal fees from Takeda, grants and personal fees from Pfizer, personal fees from Thermofisher, outside the submitted work; BW reports personal fees from Astra-Zeneca, personal fees from Roche, personal fees from MSD, personal fees from Merck, personal fees from Pfizer, outside the submitted work; AH reports personal fees from Astrazeneca, personal fees from Roche, during the conduct of the study; IS reports personal fees and non-financial support from Roche pharma, personal fees from Roche diagnostics, personal fees from Abbvie, personal fees and non-financial support from MSD, personal fees and non-financial support from Pfizer, personal fees from Takeda, personal fees and non-financial support from BMS, non-financial support from Astra-Zeneca, non-financial support from Boeringher, outside the submitted work; LB reports personal fees and non-financial support from AstraZeneca, personal fees from Roche, personal fees from Boehringer-Ingelheim, personal fees and non-financial support from MSD, personal fees from Merck, personal fees from Takeda, outside the submitted work; MS reports personal fees from Boehringer Ingelheim, personal fees from Hammermed, personal fees from MSD Polska, personal fees from Astra Zeneca Pharma Poland, non-financial support from Pfizer Polska, outside the submitted work; AR reports grants and personal fees from AstraZeneca, grants, personal fees and non-financial support from MSD, grants from Roche, personal fees and non-financial support from BMS, during the conduct of the study. Other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Popper HH, Gruber-Mösenbacher U, Hutarew G, et al. Recommendations of the Austrian Working Group on Pulmonary Pathology and Oncology for predictive molecular and immunohistochemical testing in non-small cell lung cancer. Memo 2016;9:191-200. [Crossref]
- Ryska A, Berzinec P, Brcic L, et al. NSCLC molecular testing in Central and Eastern European countries. BMC Cancer 2018;18:269. [Crossref] [PubMed]
- Lindeman NI, Cagle PT, Aisner DL, et al. Updated Molecular Testing Guideline for the Selection of Lung Cancer Patients for Treatment With Targeted Tyrosine Kinase Inhibitors: Guideline From the College of American Pathologists, the International Association for the Study of Lung Cancer, and the Association for Molecular Pathology. Arch Pathol Lab Med 2018;142:321-46. [Crossref] [PubMed]
- Kalemkerian GP, Narula N, Kennedy EB, et al. Molecular Testing Guideline for the Selection of Patients With Lung Cancer for Treatment With Targeted Tyrosine Kinase Inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/ Association for Molecular Pathology Clinical Practice Guideline Update. J Clin Oncol 2018;36:911-9. [Crossref] [PubMed]
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv192-iv237. [Crossref]
- Pauwels P, Remmelink M, Hoton D, et al. Lung Cancer : Belgian Guidelines. Belg J Med Oncol 2018;12:233-8.
- Nádory plic. Available online: íce-web.pdfhttp://www.patologie.info/soubory/all/2019-5_Guideline-pl
- Available online: https://www.e-cancer.fr
- Available online: https://www.leitlinienprogramm-onkologie.de/leitlinien/lungenkarzinom/
- Available online: https://www.oncoline.nl/niet-kleincellig-longcarcinoom
- Christensen NL, Jekunen A, Heinonen S, et al. Lung cancer guidelines in Sweden, Denmark, Norway and Finland: a comparison. Acta Oncologica 2017;56:943-8. [Crossref] [PubMed]
- Boc N, Vrankar M, Kern I, et al. 2019. Priporočila za obravnavo bolnikov s pljučnim rakom.
- Garrido P, Conde E, de Castro J, et al. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: a National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin Transl Oncol 2020;22:989-1003. [Crossref] [PubMed]
- Bubendorf L, Lantuejoul S, de Langen AJ, et al. Nonsmall cell lung carcinoma: diagnostic difficulties in small biopsies and cytological specimens. Eur Respir Rev 2017;26:170007. [Crossref] [PubMed]
- Scoccianti C, Vesin A, Martel G, et al. Prognostic value of TP53, KRAS and EGFR mutations in nonsmall cell lung cancer: the EUELC cohort. Eur Respir J 2012;40:177-84. [PubMed]
- Ryska A, Buiga R, Fakirova A, et al. Non‐Small Cell Lung Cancer in Countries of Central and Southeastern Europe: Diagnostic Procedures and Treatment Reimbursement Surveyed by the Central European Cooperative Oncology Group. Oncologist 2018;23:e152-8. [Crossref] [PubMed]
- Available online: https://www.sgpath.ch/docs/QRL/QRL_SGPath_Lunge_2017.pdf
- Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Vrdoljak E, Bodoky G, Jassem J, et al. Cancer Control in Central and Eastern Europe: Current Situation and Recommendations for Improvement. Oncologist 2016;21:1183-90. [Crossref] [PubMed]
- Jazieh AR, Algwaiz G, Errihani H, et al. Lung Cancer in the Middle East and North Africa Region. J Thorac Oncol 2019;14:1884-91. [Crossref] [PubMed]
- Available online: https://www.diaceutics.com/2019/10/22/diaceutics-pharma-precision-medicine-readiness-report-2019-its-time-to-rethink-the-testing-treatment-relationship/
- Nordiqc, Imunohistochemical, Control, Quality. 2018. PD-L1 Assessment Run C3. Available online: http://www.nordiqc.org/downloads/assessments/108_102.pdf
- Thunnissen E, Bovée JVMG, Bruinsma H, et al. EGFR and KRAS quality assurance schemes in pathology: generating normative data for molecular predictive marker analysis in targeted therapy. J Clin Pathol 2011;64:884-92. [Crossref] [PubMed]
- Deans ZC, Costa JL, Cree I, et al. Integration of next-generation sequencing in clinical diagnostic molecular pathology laboratories for analysis of solid tumours; an expert opinion on behalf of IQN Path ASBL. Virchows Arch 2017;470:5-20. [Crossref] [PubMed]
- Dequeker EMC, Keppens C, Egele C, et al. Three Rounds of External Quality Assessment in France to Evaluate the Performance of 28 Platforms for Multiparametric Molecular Testing in Metastatic Colorectal and Non-Small Cell Lung Cancer. J Mol Diagn 2016;18:205-14. [Crossref] [PubMed]
- Petersen I, Dietel M, Geilenkeuser WJ, et al. EGFR immunohistochemistry as biomarker for antibody-based therapy of squamous NSCLC - Experience from the first ring trial of the German Quality Assurance Initiative for Pathology (QuIP®). Pathol Res Pract 2017;213:1530-5. [Crossref] [PubMed]
- Normanno N, Pinto C, Taddei G, et al. Results of the First Italian External Quality Assurance Scheme for Somatic EGFR Mutation Testing in Non-Small-Cell Lung Cancer. J Thorac Oncol 2013;8:773-8. [Crossref] [PubMed]
- Marchetti A, Barberis M, Papotti M, et al. ALK rearrangement testing by FISH analysis in non-small-cell lung cancer patients: results of the first italian external quality assurance scheme. J Thorac Oncol 2014;9:1470-6. [Crossref] [PubMed]
- Tembuyser L, Tack V, Zwaenepoel K, et al. The relevance of external quality assessment for molecular testing for ALK positive non-small cell lung cancer: results from two pilot rounds show room for optimization. PLoS One 2014;9:e112159. [Crossref] [PubMed]
- Keppens C, Tack V, ’t Hart N, et al. A stitch in time saves nine: External quality assessment rounds demonstrate improved quality of biomarker analysis in lung cancer. Oncotarget 2018;9:20524-38. [Crossref] [PubMed]
- Ibrahim M, Parry S, Wilkinson D, et al. ALK Immunohistochemistry in NSCLC: Discordant Staining Can Impact Patient Treatment Regimen. J Thorac Oncol 2016;11:2241-7. [Crossref] [PubMed]
- Patton S, Normanno N, Blackhall F, et al. Assessing standardization of molecular testing for non-small-cell lung cancer: results of a worldwide external quality assessment (EQA) scheme for EGFR mutation testing. Br J Cancer 2014;111:413-20. [Crossref] [PubMed]
- 2017. NordiQC PD-L1 assessment run C1. Available online: http://www.nordiqc.org/downloads/assessments/96_102.pdf


