Neoadjuvant chemotherapy combined with intraoperative radiotherapy is effective to prevent recurrence in high-risk non-small cell lung cancer (NSCLC) patients
Introduction
Different adjuvant treatment strategies have been developed to improve the survival of patients with non-small cell lung carcinoma (NSCLC) (1). Adjuvant chemotherapy (AC) has been established as the standard treatment for patients with completely resected stage II and III NSCLC (1). Nevertheless, postoperative chemotherapy increases the 5-year survival rate by only 4−5% (2,3). Neoadjuvant chemotherapy (NAC) can be considered as an alternative strategy for patients with NSCLC; however, the benefit in survival does not differ between the pre- and postoperative settings (4-6).
Hence, it is important to search, on the one hand, for markers predicting the risk of NSCLC recurrence and metastasis and to understand, on the other hand, molecular changes in the tumor and their association with patient survival.
Despite extensive efforts to identify factors related to NSCLC recurrence and distant metastasis, there is currently no consensus regarding the use of markers for predicting cancer prognosis (7,8). Tumor node metastasis (TNM) stage remains the most common prognostic parameter for lung cancer in clinical practice (9). However, prognosis significantly varies in patients with the same TNM stage.
NSCLC originates from changes in the airway epithelia: basal cell hyperplasia (BCH), squamous metaplasia (SM), dysplasia, and carcinoma in situ precede lung squamous cell carcinoma, whereas atypical adenomatous hyperplasia is the known precursor in the pathogenesis of lung adenocarcinoma (10,11). However, premalignant lesions, particularly BCH, SM, and dysplasia, are frequently observed in the bronchial epithelium of lung cancer patients (10). Our previous study showed that the co-presence of BCH and SM without dysplastic changes (BCH+SM+D−) in the small bronchi distant from the tumor is associated with a high (36.7%) incidence of NSCLC recurrence. In the case of BCH without SM and dysplasia (BCH+SM−D−), relapse was found only in 1.8% of the patients. No recurrence was detected in cases with SM combined with dysplasia (BCH−SM+D+) and with normal bronchial epithelium (BCH−SM−D−) (12). We hypothesized that epithelial-stromal interactions in the small bronchi distant from the tumor may reflect constitutive features of the inflammation in the patients and can be indirectly related to tumor-stromal interactions and cancer progression (12). Since tumor-stromal interactions determine the response to treatment (13), one cannot exclude that premalignant lesions in the bronchi may be also a marker of therapeutic efficacy.
In this regard, this study was aimed to assess the efficacy of various approaches to the treatment of recurrent NSCLC in patients with different premalignant lesions in the small bronchi distant from the tumor. We hypothesized that the efficiency of recurrence prevention in high-risk BCH+SM+D− patients with NSCLC can be associated with combined NAC and intraoperative radiation therapy (IORT). The article is presented in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/tlcr-19-719).
Methods
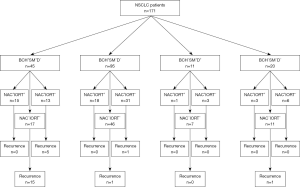
An overview of the design of the study is shown in Figure 1.
Patients
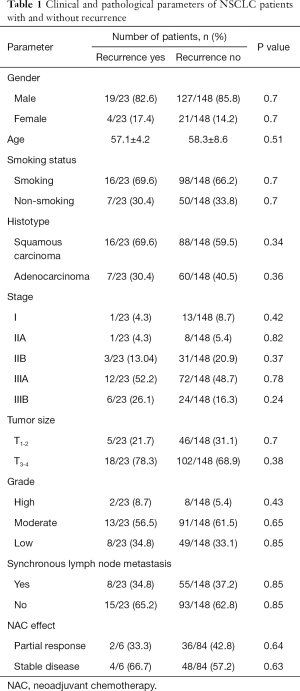
The study included 171 patients with NSCLC (T1-4N0-3M0) who were treated in the Cancer Research Institute, Tomsk NRMC between 2008 and 2015. The inclusion criteria were NSCLC (squamous cell carcinoma and adenocarcinoma), age >18 years, and an ECOG/WHO performance score <2. Patients were followed from the time of diagnosis for 5 years. The clinical and pathological parameters of patients with NSCLC depending on the presence or absence of recurrence are presented in Table 1. Patients were excluded if they were less than 18 years old, refused surgery, and had an ECOG/WHO performance score >2, small-cell lung cancer, associated severe diseases, cardiovascular and pulmonary decompensation.
Full table
Diagnosis
The histological diagnosis of lung cancer was made according to the IASLC/ATS/ERS lung adenocarcinoma classification (14) and the WHO criteria (15) and was confirmed by immunohistochemistry using a panel of antibodies: TTf-1 (clone 8G7G3/1, Dako), Napsin A (Napsin A, Rabbit Polyclonal, Cell Marque), p63 (Rabbit Polyclonal, Leica) or TTf-1 (clone 8G7G3/1, Dako), CK7 (clone OV-TL 12/30, Dako), CK20 (clone Ks 20.08, Dako), and CEA (clone AMT28, Dako). Tumor stage was determined according to the TNM classification (16).
Morphological analysis
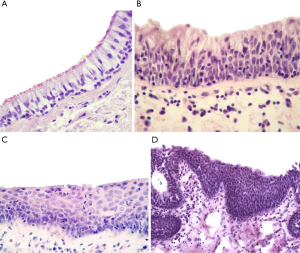
The samples of lung tissue, mainly small bronchi (d=0.5–2 mm), obtained at a distance of ~3 cm from the tumor edge during surgery were used to assess the type of morphological lesions in bronchial epithelium. The samples were collected before IORT and thus not irradiated. Nevertheless, when the samples have been already obtained the affected area was exposed to irradiation. The samples were fixed in 10% neutral formalin for 24 h, rinsed with a mixture of isopropanol, and embedded in paraffin. The sections (5–6 µm thick) were stained with hematoxylin and eosin. Morphological analysis was performed using an Axio Scope A1 light microscope (Carl Zeiss). BCH, SM, and dysplasia (Figure 2) were identified in the bronchial epithelium using standard recommendations (17,18).
Treatment
In the preoperative period, 52.6% (90/171) of the patients received NAC according to the following regimen: paclitaxel 175 mg/m2 IV on day 1 or carboplatin AUC 6 IV on day 1. A total of 2–3 cycles with a 3-week interval were performed.
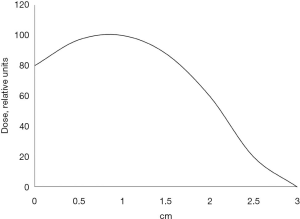
During radical surgery, 41.1% (37/90) of the patients received single-dose IORT. Of these, 37.8% (14/37) of the patients were treated with cisplatin prior to radiation therapy to achieve radiosensitization. Cisplatin was given intravenously 2 and 1 day prior to surgery, as well as two hours before IORT on the day of surgery in a dose of 6 mg/m2. IORT was performed using a small-sized pulsed betatron MIB-6E (19) with a 6 MeV electron beam and collimators (radiation field size: 4 cm × 7 cm). A maximum single dose of IORT was 15 Gy at the level of the 80% isodose whose depth was 1.5−2 cm. Figure 3 shows the distribution of absorbed dose on the beam axis in the tissue-equivalent material. In pneumonectomy, the irradiated area included tracheobronchial angle, paratracheal, paravenous and bifurcation regions. In lobectomy, irradiation was additionally given to the basal part of the remaining lobe with bronchopulmonary lymph nodes. During the postoperative period, 47.4% (81/171) of the patients received platinum-based AC: 37.0% (30/81) of the patients were treated with vinorelbine/carboplatin; 32.1% (26/81) of the patients—paclitaxel/carboplatin; 23.5% (19/81) of the patients—gemcitabine/carboplatin; 4.9% (4/81) and 2.5% (2/81) of the patients—irinotecan/carboplatin and etoposide/cisplatin, respectively.
Follow-up
After termination of treatment, patients were followed up every three months during the first year, every six months for the following three years and every year over the following two years. Follow-up included general physical examination, chest radiography (frontal and lateral projections), chest computed tomography (CT) scan, bronchoscopy with a morphological examination, abdominal ultrasound, brain CT scan, and bone scan.
Statistical analysis
Only patients with available data regarding treatment and 5-year follow-up were included in the statistical analysis. Intergroup differences were assessed using Student’s t-test, Yates-corrected Chi-square test, and Fisher’s exact test. The Kaplan-Meier method was used to generate survival curves and calculate the 5-year overall and recurrence-free survival. The survival data on all patients were censored on the date of the last follow-up visit or death from other causes. The log-rank test was used to compare the survival differences. The Cox proportional hazards model was used to identify the risk factors associated with survival. The results were considered statistically significant at P<0.05. All analyses were performed using the STATISTICA 7.0 software (StatSoft).
Results
The following types of premalignant changes were detected in the small bronchi distant for the tumor in NSCLC patients: BCH, SM, and grade I–III dysplasia. These changes were found not only separately but also in various combinations with each other within the same tissue sample. BCH was the most frequently observed bronchial lesion. In most cases [55.6% (95/171)], BCH was detected in the absence of other epithelial changes (BCH+SM−D−), while the co-presence of BCH and SM without dysplasia (BCH+SM+D−) was less frequent [26.3% (45/171); P=0.002]. Dysplasia was found in 6.4% (11/171) of the patients typically together with SM (BCH−SM+D+). As shown previously, all of these bronchial changes developed at sites of chronic inflammation (20). Normal bronchial epithelium (BCH−SM−D−) without any signs of chronic inflammation in the bronchi was detected in 11.7% (20/171) of the cases.
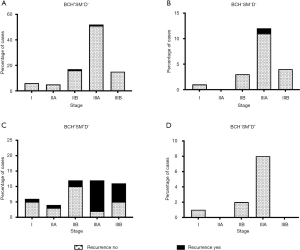
In the 5-year period, 13.4% (23/171) of the patients with NSCLC had a recurrence. Of those, 87.0% (20/23) of the patients belonged to the BCH+SM+D− group, and 13.0% (3/23) of the patients had other variants of bronchial lesions (BCH+SM−D− and BCH−SM−D−). No recurrence was detected in the BCH−SM+D+ patients (0/11). Based on these data, BCH+SM+D− patients were classified as having a high risk of recurrence, whereas BCH+SM−D−, BCH−SM+D+, and BCH−SM−D− cases were considered as low risk.
The recurrence was regional (metachronous metastases in the thoracic lymph nodes) in 82.6% (19/23) of the cases and local (a relapse in the bronchial stump) in 17.4% (4/23) of the patients. It should be noted that NSCLC recurrence is typically mentioned in the literature without specifying metachronous lymph node metastases or bronchial stump relapse.
Association of NSCLC recurrence with tumor stage
In the low-risk groups (BCH+SM−D− and BCH−SM−D−), recurrence was observed mainly in stage IIIA patients: 1.9% (1/52) and 8.3% (1/12) of the cases, respectively (Figure 4A,B). In the high-risk group (BCH+SM+D−), recurrence occurred at any stage but was predominant in stage IIIA [83.3% (10/12)] and IIIB [54.5% (6/11)] of the patients (P=0.007; Figure 4C). As indicated above, no recurrence was detected in BCH−SM+D+ patients (Figure 4D).
Efficacy of NAC and IORT in the prevention of NSCLC recurrence
NSCLC patients were divided into the following study groups depending on the type of therapy: only NAC (NAC+IORT−), NAC in combination with intraoperative radiation therapy (NAC+IORT+), and untreated patients (NAC−IORT−). The recurrence rate was assessed in each group in the 2- and 5-year follow-up period (Figure 5A,B).
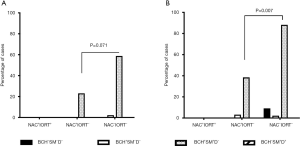
In the 2-year period, recurrence was observed in 8.2% (14/171) of the NSCLC patients. Recurrence was common in the high-risk (BCH+SM+D−) group compared to patients with other bronchial lesions and their absence (28.9% (13/45) vs. 0.8% (1/26); P<0.001). In this group, recurrence tended to occur more often in NAC−IORT− patients compared to NAC+IORT− cases [58.8% (10/17) vs. 23.1% (3/13); P=0.071; Figure 5A]. No recurrence was detected in NAC+IORT+ patients (0/15). In the low-risk (BCH+SM−D−) group, a relapse was only in one patient out of 95 (1.05%) cases, and that was the case without treatment [2.2% (1/46); Figure 5A]. In another low-risk (BCH−SM−D−) group, no recurrence was observed regardless of the type of treatment (Figure 5A).
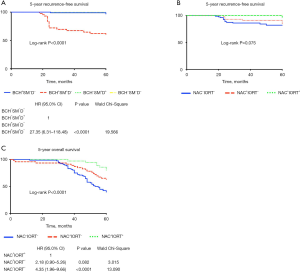
In the 5-year period, recurrence was detected in 23/171 (13.4%) of the patients with NSCLC. The highest relapse frequency was also in high-risk BCH+SM+D− patients [44.5% (20/45)]. Of these, recurrence was observed in 88.2% (15/17) of the untreated cases and in 38.5% (5/13) of the NAC+IORT− cases (P=0.007) (Figure 5B). In untreated patients, recurrence was local in 20.0% (3/15) of the cases and regional in 80.0% (12/15) of the cases. In the NAC+IORT− group, percentages were the same: 20.0% (1/5) of the patients with local recurrence and 80% (4/5) of the cases with regional recurrence. In the NAC+IORT+ group, no recurrence was detected (0/15; Figure 5B).
The 5-year recurrence-free survival was shorter in BCH+SM+D− patients (HR 27.35; 95% CI: 6.31–118.48; P<0.0001) than in cases with other bronchial lesions or normal epithelium (Figure 6A) and tended to be decreased in NAC−IORT− patients (log-rank P=0.075; Figure 6B). The 5-year overall survival was also worse in NAC−IORT− (HR 4.35; 95% CI: 1.96–9.66; P<0.0001) and tended to be decreased in NAC+IORT− (HR 2.18; 95% CI: 0.90–5.26; P=0.082) patients as compared with cases received the combination treatment (Figure 6C).
Efficacy of AC in the prevention of NSCLC recurrence
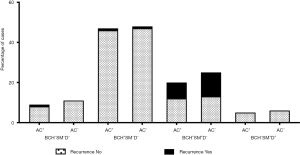
AC was administered in 43.5% (10/23) of the patients with recurrent NSCLC. In the low-risk BCH−SM−D− group, 45.0% (9/20) of the patients received AC. As noted above, relapse occurred only in one patient, and only when AC was used (Figure 7). In another low-risk BCH+SM−D− group, AC was administered in 49.5% (47/95) of the cases. Relapse was detected in one AC-treated patient and one AC-naïve patient (P>0.05; Figure 7). In high-risk BCH+SM+D− patients, AC was used in 44.4% (20/45) of the patients. Relapse occurred in 36.4% (8/20) of the cases received AC and in 48% (12/25) of the untreated cases (P>0.05; Figure 7). No differences in recurrence were found between patients treated with different AC regimens (Table 2).
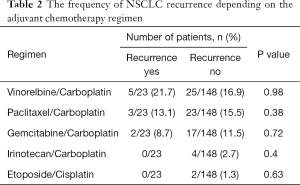
Full table
Discussion
Despite improvements in treatment, the 5-year survival rate for NSCLC remains poor mainly because cancer is detected in later stages (21). Even if lung cancer is diagnosed at stages I and II, postoperative recurrence develops in almost a quarter of patients, most of which die of this recurrent disease (22,23). NSCLC relapse may occur due to various reasons such as non-radical surgery (leaving residual disease), manifestation of multifocality, etc. TNM stage is considered as main factor for predicting NSCLC prognosis, mainly the risk of recurrence and distant metastasis (9,24). Several years ago, a new pattern of tumor invasion—“spread through air spaces” (STAS) has been proposed as a significant risk factor of distant and locoregional recurrence in lung adenocarcinoma (25). Subsequent studies showed that STAS is an independent predictor for recurrence-free survival in lung squamous cell carcinoma (26,27). We previously found that the co-presence of BCH and SM (BCH+SM+D−) in the small bronchi distant from the tumor is associated with a high risk of NSCLC recurrence (12). The mechanism of this observation is suggested to be related to the genetic determination of immune and stromal reactions in the development of chronic bronchitis and various types of epithelial-stromal interactions associated with them. Since metastatic tumor spread including metachronous lymph node metastasis is largely controlled by the immune tumor microenvironment, one can expect that immune-stromal reactions in bronchi can be associated with microenvironmental features in the tumor.
In this study, recurrence was also observed mainly in BCH+SM+D−patients and represented mostly by metachronous metastases in the thoracic lymph nodes. Interestingly, the common association of recurrence with a more advanced tumor stage was found only in high-risk BCH+SM+D− patients. Thus, the co-presence of BCH and SM in the small bronchi is associated with regional recurrence of NSCLC. Local recurrence was a rare event and observed in the proximal or main bronchi. Based on this fact, one can understand why recurrence was absent in cases with dysplasia in the small bronchi. Such dysplasia could be a cause of recurrence only in cases when carcinogenesis would be in the post-operative period de novo.
The most effective relapse prevention therapy was the combination of NAC and IORT that resulted in the absence of recurrence in high-risk BCH+SM+D− patients during the 5-year follow-up period. The overall and recurrence-free survival was also highest in patients who received the combination treatment. The high efficacy of NAC and IORT in the prevention of recurrence may be related to the suppression of metachronous metastases in the mediastinal lymph nodes. Taken together, these data indicate that the type of premalignant lesions in the small bronchi distant from the tumor (~3 cm from the tumor edge) is an indirect sign that allows stratifying patients with NSCLC according to the recurrence risk and anti-relapse effect of preoperative therapy. It draws attention to the special role of IORT without which recurrence prevention was less effective.
IORT is a feasible and promising technique for the treatment of NSCLC patients. This treatment has several important advantages over conventional radiation therapy that include, for example, the high precision delivery of large doses of radiation in an exposed and precisely defined tumor bed saving adjacent critical normal structures and reducing radiotoxicity (28). Nevertheless, the current evidence regarding the use of IORT in lung cancer patients is limited and represented by small prospective single-institution pilot trials. In stage I or II NSCLC, IORT resulted in excellent rates of local control (70–100%) in medically inoperable patients (28). In stage III NSCLC, NAC with paclitaxel/carboplatin and IORT with radiosensitization by cisplatin significantly increased the 5-year survival compared to surgery alone (47.9% vs. 29.2%, P<0.05) (29). The advantage of our results is the possibility to select patients with a high risk of recurrence that are sensitive to NAC and IORT and low-risk patients in which the combined treatment is inadvisable. This can be used in clinical practice for the prognosis of locoregional recurrence in patients with NSCLC and the planning preventive therapy. The specificity of this approach can be increased by additional studies that will allow identifying patients in which recurrence does not develop during 5-year follow-up despite the co-presence of BCH and SM in the small bronchi.
Some limitations of this study should be addressed. First, the study group is small and the obtained findings need to be validated in independent and large cohorts. Second, it remains unclear if IORT alone can be effective for recurrence prevention. And third, further studies are required to understand why recurrence does not develop in one-half of the high-risk patients with the co-presence of BCH and SM.
In conclusion, the co-presence of BCH and SM in the small bronchi of NSCLC patients is a significant risk factor of recurrence. NAC combined with IORT has a high efficacy in the prevention of recurrence in high-risk NSCLC patients.
Acknowledgments
Funding: This study was funded by the Russian Foundation for Basic Research (No 17-29-06002; Sampling).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-19-719
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-19-719
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-19-719). OVP reports grants from Russian Foundation for Basic Research, during the conduct of the study; in addition, OVP has a patent RU Patent 2538642 issued, a patent RU Patent 2489718 issued, a patent RU Patent 2498305 issued, and a patent RU Patent 2293323 issued. SVM has a patent RU Patent 2348357 issued, a patent RU Patent 2431450 issued, and a patent RU Patent 2361580 issued. SAT has a patent RU Patent 2348357 issued, a patent RU Patent 2538642 issued, a patent RU Patent 2431450 issued, and a patent RU Patent 2361580 issued. EVD reports grants from Russian Foundation for Basic Research, during the conduct of the study; in addition, EVD has a patent RU Patent 2538642 issued. VMP has a patent RU Patent 2538642 issued, a patent RU Patent 2489718 issued, a patent RU Patent 2498305 issued, and a patent RU Patent 2293323 issued. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The procedures followed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The study protocol “Intraoperative radiation therapy and radiosensitization in combined treatment of non-small cell lung cancer” has been reviewed by the Coordination Committee of the Tomsk Cancer Research Institute and approved by the Ethical Committee of the Tomsk Cancer Research Institute (the approval number is 12/20.11.2008). The present study was approved by the institutional review board of the Cancer Research Institute, Tomsk NRMC 10 December 2012 (the number of approval is 16). All patients signed an informed consent for voluntary participation.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pirker R, Filipits M. Adjuvant Therapy in Patients With Completely Resected Non-small-cell Lung Cancer: Current Status and Perspectives. Clin Lung Cancer 2019;20:1-6. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Westeel V, Quoix E, Puyraveau M, et al. A randomised trial comparing preoperative to perioperative chemotherapy in early-stage non-small-cell lung cancer (IFCT 0002 trial). Eur J Cancer 2013;49:2654-64. [Crossref] [PubMed]
- Brandt WS, Yan W, Zhou J, et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non-small cell lung cancer: A propensity-matched analysis. J Thorac Cardiovasc Surg 2019;157:743-753.e3. [Crossref] [PubMed]
- Scagliotti GV, Vokes EE. 51 - Adjuvant and Neoadjuvant Chemotherapy for Early-Stage Nonsmall Cell Lung Cancer. In: Pass HI, Ball D, Scagliotti GV. editors. IASLC Thoracic Oncology (Second Edition). Content Repository Only! Philadelphia, 2018:512-524.e4.
- Uramoto H, Tanaka F. Recurrence after surgery in patients with NSCLC. Transl Lung Cancer Res 2014;3:242-9. [PubMed]
- Vargas AJ, Harris CC. Biomarker development in the precision medicine era: lung cancer as a case study. Nat Rev Cancer 2016;16:525-37. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Denisov EV, Schegoleva AA, Gervas PA, et al. Premalignant lesions of squamous cell carcinoma of the lung: The molecular make-up and factors affecting their progression. Lung Cancer 2019;135:21-8. [Crossref] [PubMed]
- Kadara H, Scheet P, Wistuba II, et al. Early Events in the Molecular Pathogenesis of Lung Cancer. Cancer Prev Res (Phila) 2016;9:518-27. [Crossref] [PubMed]
- Pankova OV, Denisov EV, Ponomaryova AA, et al. Recurrence of squamous cell lung carcinoma is associated with the co-presence of reactive lesions in tumor-adjacent bronchial epithelium. Tumour Biol 2016;37:3599-607. [Crossref] [PubMed]
- Perelmuter VM, Tashireva LA, Savelieva OE, et al. Mechanisms behind prometastatic changes induced by neoadjuvant chemotherapy in the breast cancer microenvironment. Breast Cancer (Dove Med Press) 2019;11:209-19. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart. 4th ed. Lyon: IARC Press, 2015.
- Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th ed. Wiley-Blackwell, Hoboken, NJ, 2010.
- Dacic S. Pulmonary preneoplasia. Arch Pathol Lab Med 2008;132:1073-8. [PubMed]
- Kerr MK. The Classification of Pre-invasive Lesions. In: Cagle PT, Beasley MB, Dacic S, et al. editors. Molecular Pathology of Lung Cancer. New York: Springer, 2012:35-52.
- Choynzonov EL, Musabaeva LI, Lisin VA, et al. Novel technique of intraoperative electron radiation therapy with external radiation therapy for combined modality treatment in cancer patients. Oncosurgery 2010;2:26-36.
- Pankova OV, Perelmuter VM, Savenkova OV, et al. Characteristics of bronchial mucosal inflammatory response in sites of basal cell hyperplasia and squamous metaplasia combined with squamous cell lung cancer. Sib J Oncol 2012;5:28-33.
- Duma N, Santana-Davila R, Molina JR. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Boyd JA, Hubbs JL, Kim DW, et al. Timing of local and distant failure in resected lung cancer: implications for reported rates of local failure. J Thorac Oncol 2010;5:211-4. [Crossref] [PubMed]
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81. [Crossref] [PubMed]
- Liu A, Hou F, Qin Y, et al. Predictive value of a prognostic model based on pathologic features in lung invasive adenocarcinoma. Lung Cancer 2019;131:14-22. [Crossref] [PubMed]
- Kadota K, Nitadori J, Sima CS, et al. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J Thorac Oncol 2015;10:806-14. [Crossref] [PubMed]
- Kadota K, Kushida Y, Katsuki N, et al. Tumor Spread Through Air Spaces Is an Independent Predictor of Recurrence-free Survival in Patients With Resected Lung Squamous Cell Carcinoma. Am J Surg Pathol 2017;41:1077-86. [Crossref] [PubMed]
- Lu S, Tan KS, Kadota K, et al. Spread through Air Spaces (STAS) Is an Independent Predictor of Recurrence and Lung Cancer-Specific Death in Squamous Cell Carcinoma. J Thorac Oncol 2017;12:223-34. [Crossref] [PubMed]
- Calvo FA, Aristu J, Usychkin S, et al. Intraoperative Radiotherapy in Lung Cancer: Methodology (Electrons or Brachytherapy), Clinical Experiences and Long-Term Institutional Results. In: Jeremic B. editor. Advances in Radiation Oncology in Lung Cancer. Berlin Heidelberg: Springer, 2011:461-76.
- Dobrodeev AI, Zav'ialov AA, Tyzikov SA, et al. The results of combined treatment for stage III lung cancer including the use of intraoperative radiotherapy. Vopr Onkol 2013;59:606-10. [PubMed]