
Salvage surgery after definitive chemoradiotherapy for patients with non-small cell lung cancer
Introduction
According to the Longman Dictionary of Contemporary English, “to salvage” is defined as to save something from an accident or bad situation in which other things have already been damaged, destroyed, or lost. Therefore, the term “salvage surgery” is used for a surgery that is performed to save patients from treatment failure, in cases where other treatment options have already been used (1). In the treatment of lung cancer, salvage surgery most often refers to lung resection in cases of locally relapsed tumor after concurrent chemoradiotherapy (CRT) for locally advanced tumors (2). Salvage surgery usually does not include resection of distant oligometastases, such as surgeries for metastases of the brain or adrenal gland, but may include surgical resection after empiric conversion to trimodality therapy from CRT with curative intent (1). This is also referred to as “conversion surgery” (1,3,4). Thus, most of the indications are oncologic reasons, however in an exceptional example, lung resection for chronic bronchopleural fistula may be included in salvage surgery (1). In this review, we define salvage surgery as lung resection for the local control of a tumor, which was not planned initially, occurring after failure or insufficient treatment of the initial CRT. Figure 1 depicts possible clinical courses after CRT and the definition of salvage surgery used in this article.
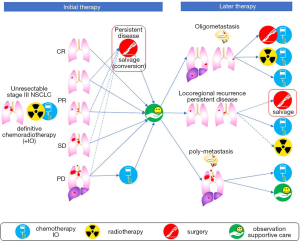
Approximately 30% of patients with non-small cell lung cancer (NSCLC) present with stage III disease (5). According to the TNM staging-8th edition, Stage III disease is subdivided into IIIA to IIIC, consisting of heterogeneous groups of tumors depending on the extent of the primary tumor as well as lymph nodes metastases (5). According to the latest version of the National Comprehensive Cancer Network (NCCN) guidelines, surgery is the preferred initial treatment for those with clinical T3 and technically resectable T4N0-1 tumors (6), both of which account for about 30% of clinical stage III disease (5). Treatment for patients with clinical stage IIIA-N2 disease (e.g., T1-2N2) is controversial. Some recommend definitive CRT for all these patients while others consider this is appropriate only for non-bulky, discrete, particularly single station, mediastinal node involvement as a resectable disease (7). In these cases, either induction chemo- or radio-therapy followed by surgery, or definitive CRT followed by surgical intervention, is recommended by the NCCN guidelines (6). However, some doctors prefer surgery with adjuvant chemotherapy (8). Although definitive CRT is recommended for category I disease according to the NCCN guidelines (6), the clinical outcome is not necessarily very good, in that the median overall survival (OS) was 27 or 25 months for those who received pemetrexed/cisplatin or etoposide/cisplatin with concurrent radiotherapy (RT), respectively, in the PROCLAIM study. In this trial, the percentage of patients who first relapsed within the radiation treatment field was 37% and 46%, respectively (9).
Recently, the phase III PACIFIC trial of durvalumab, human anti-PD-L1 antibody, compared with a placebo as maintenance therapy following definitive CRT has shown a 13% better 3-year OS rate (57% vs. 44% with a hazard ratio (HR) of 0.68 in unresectable stage III disease) (10,11). Following these reports, this regimen became the standard of care for those patients irrespective of PD-L1 expression (6). However, the European Medicines Agency suggested that durvalumab should be used for the patients who have tumors expressing PD-L1 on ≥1% of tumor cells, based on the results of post hoc analyses (12). Unfortunately, the incidence of locoregional failure has not been directly reported. However, the difference between the distant-metastasis free survival rate at 18 months of 64% and the progression-free survival rate of 50% read from the curve reported in 2018 (10) gives an approximation of the proportion of patients who are alive with only loco-regional recurrence, which is about 15%. Therefore, even in the immunotherapy era, a considerable number of locoregional recurrences still occur after non-surgical treatment only. Salvage surgery has been sporadically performed with the intention of improving local control of unresectable stage III disease and is still performed even after the introduction of durvalumab. However, the clinical significance is unclear, because it is almost impossible to conduct phase III randomized controlled trials for those patients.
In this review, we evaluated clinical outcomes of salvage surgery after definitive CRT for unresectable stage III NSCLC to gain insight into which patients are likely to benefit from salvage surgery.
Selection of articles reporting salvage surgery studies
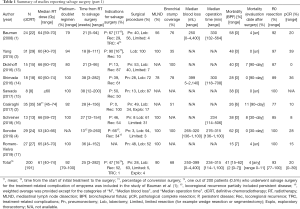
We searched PubMed for studies published from 2000 to 2019 regarding salvage surgery after definitive CRT for patients with NSCLC. Search terms included controlled terms (MeSH in PubMed and Emtree in Embase) as well as free text terms. Search terms expressing ‘non-small cell lung cancer’ were used in combination with ‘chemoradiotherapy’ and ‘recurrence’. Only full-length English-language articles were included in this review. Salvage surgery after stereotactic body radiation therapy, review articles and duplicate articles were excluded. After careful screening, nine retrospective studies were extracted for this review (1-4,13-17). All nine were single-center studies consisting of a small number of patients (median, 24 patients; range 8 to 35) (Table 1).
Full table
Summary of prior treatment and perioperative findings of salvage surgery (Table 1)
As an initial treatment, definitive CRT was performed in 191 of 200 patients (96%). Some patients were only treated either by radiotherapy or chemotherapy (1,3,4). The weighted average of median radiotherapy (RT) dose was 61 Gy, which varied between studies (range, 40 to 74 Gy). The platinum-doublet regimen was mainly used in 92% (weighted average) of patients.
The time from definitive CRT to salvage surgery also varied. When combining data from the nine studies, the weighted average of median time from definitive CRT to salvage surgery was 25 weeks with a wide range of 3 to 282 weeks. The timing of salvage surgery is determined by various factors; conversion surgery is usually performed earlier than true salvage surgery for recurrent disease, and patients with rapid local recurrence may undergo surgery earlier. Salvage surgery for persistent disease was performed in 47% of patients and for locoregional recurrence in 52%. A total of 16% of patients underwent conversion surgery after definitive CRT for persistent disease that were converted from “initially unresectable” to “potentially resectable” (1,3,4). By contrast, the study of Dickhoff et al. excluded patients who underwent surgery less than 12 weeks after the last day of RT in order to minimize the effect of the interval between the end of RT and surgery (13).
Lobectomies (63%) and mediastinal lymph node dissections (90%) were the most common procedures of salvage surgery. However, it is noted that pneumonectomy was performed in 28%, considerably higher than that in lung resection in general, which was 1% in a Japanese registry study (18) and 7% in the United States (19). R0 resection was achieved in 93% of patients (range, 77% to 100%). In addition, complicated resections including broncho- or pulmonary artery plasty are frequently reported (4). Bronchial stump coverage was performed in a total of 68% of patients.
Surgery after radiation is accompanied by technical difficulty due to radiation-induced fibrosis that obliterates the planes between vital structures such as the superior vena cava, pulmonary artery or trachea, and surrounding structures. Salvage surgery is even more difficult due to the long interval between radiation and surgery. As a result, blood loss is greater and the operation time is longer. Three studies showed median blood loss volumes of 250 to 399 mL (range, 0 to 4,400 mL) (1,4,14) and four studies showed median operation times of 234 to 315 minutes (range, 114 to 1,100 minutes) (1,4,14,16). Morbidity occurred in 41% (weighted median) of patients and bronchial stump fistula occurred in 2% of patients.
Treatment-related mortality rates were calculated at different time points of 30 days (4,16), 90 days (2,13,14), and unspecified (1,3,15,17). The weighted average mortality rate of all nine studies was 4% (range, 0 to 11%) in total: 0% in 3 Japanese studies (4,14,15), and 6% (range, 0–11%) in six non-Japanese studies (1-3,13,16,17). Considering the 30-day mortality rate for overall lung cancer surgery was reported as 0.4% in Japan (18) and 3% in the United States (19), the mortality rate of salvage surgery is acceptable, probably because the surgery was performed for carefully selected patients and was most likely performed by experienced surgeons in academic medical centers.
Long-term outcomes of salvage surgery after definitive CRT (Table 2)
Full table
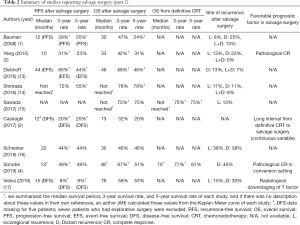
The long-term outcomes after salvage surgery were not uniformly evaluated. The median recurrence-free survival (RFS), and 3- and 5-year RFS rates after salvage surgery in each study ranged from 10 to 22 months, and 31–72% and 23–55%, respectively (3,4,14,16). The median OS, and 3- and 5-year OS rates after salvage surgery ranged from 13 to 76 months, and 32–78% and 20–78%, respectively (1-4,13-17).
What are the outcomes of non-surgical treatment for locoregional recurrence? The median OS of patients who received only chemotherapy or only RT (including proton) for locoregional recurrence after definitive CRT was reportedly 9 months (20) or 11–15 months (21-23), respectively, which is shorter than survival after salvage surgery. Although there should be selection biases, salvage surgery may at least offer a non-inferior outcome and can be an option in these situations.
Appropriate patient selection for salvage surgery (Table 2)
So far, we have seen varying clinical outcomes for patients who underwent salvage surgery. Some are almost cured, while the other may suffer from serious complications/short survival periods. Therefore, there is a great need to identify factors that predict favorable prognoses that can be evaluated before surgery.
The time from RT to salvage surgery may have prognostic implications. One study showed a positive relationship between OS and a long interval between RT and salvage surgery. Casiraghi et al. also found that this long RT-to-surgery interval, as a continuous variable, was predictive of longer OS with a HR of 0.90 (2). This is interpreted to indicate that the longer interval between RT and surgery is a surrogate marker of the less aggressive nature of the tumor and thus longer progression-free survival in a true salvage setting. In contrast, Sonobe et al. did not find any significant effect of RT-to-surgery interval on patient survival (4). However, in this study, 66% of salvage surgery was performed in a conversion setting where this interval should have been short. These patients should have had a good response enough to conversion from unresectable tumor to resectable tumor, which should be a surrogate of longer survival as well.
Vielva et al. revealed that downstagings of T and N factors were found in 19 (70%) and 17 (63%) patients, respectively, using preoperative imaging studies by computed tomography or fluorodeoxyglucose-positron emission tomography (17). In their study, patients with T-downstaging showed significantly better OS than did those without.
In induction therapy followed by surgery, tumor regression with 10% or less viable residual tumor cells as determined by histologic examination was proposed as a surrogate marker for OS in a prospective (24), as well as a retrospective, study (25). In the present analysis, pathological complete response (pCR) in resected specimens was found in a median of 20% of patients (range 0 to 39%). Two studies showed that pCR is also a surrogate marker of longer OS (3,4). For example, Yang et al. found that patients with pCR showed significantly longer median OS than did those without (60 vs. 20 months, respectively; P=0.03) (3). This information is usually obtained after surgery, following examination of the entire tumor, and is not useful for patient selection for salvage surgery.
Conclusions
It is obvious that greater local control is necessary to further improve the outcome of patients with marginally resectable-unresectable stage III disease, even in the immunotherapy era. We have shown that existing studies suggest that salvage surgery can be considered, especially for those with late local recurrence or those with a metabolic response. However, it is difficult to establish the role of salvage surgery in this setting because of the lack of phase III studies, due to considerable heterogeneity of patients and the high level of patient selection. Considering this, the accumulation of empirical evidence, preferably in a prospective fashion, is warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Mariano Provencio) for the series “Multimodal management of locally advanced N2 non-small cell lung cancer” published in Translational Lung Cancer Research. The article has undergone external peer review.
Peer Review File: Available online http://dx.doi.org/10.21037/tlcr-20-453
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available online http://dx.doi.org/10.21037/tlcr-20-453). The series “Multimodal management of locally advanced N2 non-small cell lung cancer” was commissioned by the editorial office without any funding or sponsorship. TM serves as an unpaid editorial board member of Translational Lung Cancer Research from Sep 2019 to Sep 2021. TM reports grants and personal fees from AstraZeneca, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Chugai, personal fees from Pfizer, personal fees from Novartis, personal fees from Bristol-Myers Squibb, personal fees from Eli Lilly, personal fees from Merck Sharp and Dohme, grants from Daiichi Sankyo, grants from Taiho, grants from Ono Pharmaceutical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bauman JE, Mulligan MS, Martins RG, et al. Salvage lung resection after definitive radiation (>59 Gy) for non-small cell lung cancer: surgical and oncologic outcomes. Ann Thorac Surg 2008;86:1632-8; discussion 1638-9. [Crossref] [PubMed]
- Casiraghi M, Maisonneuve P, Piperno G, et al. Salvage Surgery After Definitive Chemoradiotherapy for Non-small Cell Lung Cancer. Semin Thorac Cardiovasc Surg 2017;29:233-41. [Crossref] [PubMed]
- Yang CF, Meyerhoff RR, Stephens SJ, et al. Long-Term Outcomes of Lobectomy for Non-Small Cell Lung Cancer After Definitive Radiation Treatment. Ann Thorac Surg 2015;99:1914-20. [Crossref] [PubMed]
- Sonobe M, Yutaka Y, Nakajima D, et al. Salvage Surgery After Chemotherapy or Chemoradiotherapy for Initially Unresectable Lung Carcinoma. Ann Thorac Surg 2019;108:1664-70. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- National Comprehensive Cancer Network: Non-Small Cell Lung Cancer (Version2.2018) Accessed July 28, 2020. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- Detterbeck FC, Lewis SZ, Diekemper R, et al. Executive Summary: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:7S-37S.
- Koshy M, Fedewa SA, Malik R, et al. Improved survival associated with neoadjuvant chemoradiation in patients with clinical stage IIIA(N2) non-small-cell lung cancer. J Thorac Oncol 2013;8:915-22. [Crossref] [PubMed]
- Senan S, Brade A, Wang LH, et al. PROCLAIM: Randomized Phase III Trial of Pemetrexed-Cisplatin or Etoposide-Cisplatin Plus Thoracic Radiation Therapy Followed by Consolidation Chemotherapy in Locally Advanced Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:953-62. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Gray JE, Villegas A, Daniel D, et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020;15:288-93. [Crossref] [PubMed]
- European Medicines Agency. Durvalumab (Imfinzi). Summary of product characteristics 2018. Accessed July 28, 2020. Available online: https://www.ema.europa.eu/en/documents/product-information/imfizi-epar-product-information_en.pdf
- Dickhoff C, Dahele M, Paul MA, et al. Salvage surgery for locoregional recurrence or persistent tumor after high dose chemoradiotherapy for locally advanced non-small cell lung cancer. Lung Cancer 2016;94:108-13. [Crossref] [PubMed]
- Shimada Y, Suzuki K, Okada M, et al. Feasibility and efficacy of salvage lung resection after definitive chemoradiation therapy for Stage III non-small-cell lung cancer. Interact Cardiovasc Thorac Surg 2016;23:895-901. [Crossref] [PubMed]
- Sawada S, Suehisa H, Ueno T, et al. Eight cases of salvage pulmonary resection for residual disease or isolated local recurrence detected after definitive chemoradiotherapy for N2 Stage-IIIA lung cancer. Asian J Surg 2017;40:95-9. [Crossref] [PubMed]
- Schreiner W, Dudek W, Lettmaier S, et al. Long-Term Survival after Salvage Surgery for Local Failure after Definitive Chemoradiation Therapy for Locally Advanced Non-small Cell Lung Cancer. Thorac Cardiovasc Surg 2018;66:135-41. [Crossref] [PubMed]
- Romero-Vielva L, Viteri S, Moya-Horno I, et al. Salvage surgery after definitive chemo-radiotherapy for patients with Non-Small Cell Lung Cancer. Lung Cancer 2019;133:117-22. [Crossref] [PubMed]
- Committee for Scientific Affairs TJAfTS, Shimizu H, Endo S, et al. Thoracic and cardiovascular surgery in Japan in 2016: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2019;67:377-411. [Crossref] [PubMed]
- Rosen JE, Hancock JG, Kim AW, et al. Predictors of mortality after surgical management of lung cancer in the National Cancer Database. Ann Thorac Surg 2014;98:1953-60. [Crossref] [PubMed]
- Paramanathan A, Solomon B, Collins M, et al. Patients treated with platinum-doublet chemotherapy for advanced non--small-cell lung cancer have inferior outcomes if previously treated with platinum-based chemoradiation. Clin Lung Cancer 2013;14:508-12. [Crossref] [PubMed]
- Griffioen GH, Dahele M, de Haan PF, et al. High-dose, conventionally fractionated thoracic reirradiation for lung tumors. Lung Cancer 2014;83:356-62. [Crossref] [PubMed]
- McAvoy S, Ciura K, Wei C, et al. Definitive reirradiation for locoregionally recurrent non-small cell lung cancer with proton beam therapy or intensity modulated radiation therapy: predictors of high-grade toxicity and survival outcomes. Int J Radiat Oncol Biol Phys 2014;90:819-27. [Crossref] [PubMed]
- Chao HH, Berman AT, Simone CB 2nd, et al. Multi-Institutional Prospective Study of Reirradiation with Proton Beam Radiotherapy for Locoregionally Recurrent Non-Small Cell Lung Cancer. J Thorac Oncol 2017;12:281-92. [Crossref] [PubMed]
- Junker K, Langner K, Klinke F, et al. Grading of tumor regression in non-small cell lung cancer: morphology and prognosis. Chest 2001;120:1584-91. [Crossref] [PubMed]
- Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol 2012;7:825-32. [Crossref] [PubMed]


