
Phase I study of afatinib plus bevacizumab in patients with advanced non-squamous non-small cell lung cancer harboring EGFR mutations
Introduction
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) monotherapies result in improvements in the progression-free survival (PFS) and response rates (RRs) compared to those with platinum-doublet chemotherapies in patients with non-small cell lung cancer (NSCLC) harboring EGFR mutations (1-6). However, most patients with NSCLC harboring EGFR mutations who are administered EGFR-TKI monotherapies eventually acquire resistance and relapse approximately one year after the initiation of treatment (1-6). To improve their outcomes, combination therapies using EGFR-TKIs have actively been developed in recent years.
Bevacizumab is a promising candidate as a combination drug with EGFR-TKIs. This compound is a monoclonal antibody that targets vascular endothelial growth factor A, a key factor in tumor-associated angiogenesis. Saito et al. performed a phase III study to measure the combination of erlotinib (a first-generation EGFR-TKI) and bevacizumab versus erlotinib monotherapy, the former resulted in longer PFS than that associated with erlotinib monotherapy (median PFS: 16.9 months with erlotinib plus bevacizumab and 13.3 months with erlotinib monotherapy) (7). Additionally, several phase II studies have supported the efficacy of bevacizumab with EGFR-TKIs (8,9). However, most of these studies used first-generation EGFR-TKIs. There are few data corresponding to second- or third- generation EGFR-TKIs plus bevacizumab.
Afatinib is a second-generation EGFR-TKI. This compound irreversibly binds to and blocks not only EGFR, but also human EGFR-related 2 and 4 tyrosine kinases. In a phase IIB trial, afatinib significantly prolonged the PFS in patients with NSCLC harboring EGFR mutations compared with first-generation EGFR-TKIs (10). In a combined analysis of phase III trials to compare afatinib with platinum-doublet chemotherapy, afatinib significantly improved the PFS and overall survival (OS) (11), while first-generation EGFR-TKIs did not improve the OS (12,13). Based on these results, it is suggested that afatinib achieves better outcomes than those achieved by first-generation EGFR-TKIs.
With this background, a combination therapy of afatinib with bevacizumab is expected to be a promising combination for patients with NSCLC harboring EGFR mutations. However, there are few data of the adequate dose and feasibility of this combination therapy. Therefore, we performed a phase I study to assess the combination therapy of afatinib plus bevacizumab.
The authors present the following article in accordance with the TREND reporting checklist (available at http://dx.doi.org/10.21037/tlcr-20-824).
Methods
Study design
This open-label, phase I study was performed at two facilities (Juntendo University Hospital and Kurume University Hospital). The pharmacokinetic study was performed at the Department of Clinical Pharmacokinetics and Pharmacodynamics of Keio University School of Medicine. The study protocol was approved by the institutional review boards of Juntendo University Hospital (registration ID 14-134) and Kurume University Hospital (registration ID 14243). Written informed consent was obtained from all patients prior to the study. This study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and all relevant Japanese laws and regulations. This study was registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN 000016029).
The purpose of this study was to assess the feasibility and recommended dose (RD) of afatinib with bevacizumab in patients with advanced NSCLC harboring EGFR mutations. The primary endpoints were the maximum tolerated dose (MTD) and RD. The secondary endpoints were the toxicity, objective RR, PFS, OS, and afatinib exposure at steady state.
This study comprised of a dose-finding cohort and expansion cohort. The dose-finding cohort was performed in a 3+3 manner. In the expansion cohort, 10–20 patients received the RD derived from the results of the dose-finding cohort.
Afatinib was orally administered by continuous once-daily dosing in combination with bevacizumab (15 mg/kg) intravenously administered on day 1 every 3 weeks until disease progression or an unacceptable adverse event (AE) was noted. In the dose-finding cohort, the initial afatinib dose was 30 mg/day. If the tolerability at 30 mg/day was determined, the dose was escalated to 40 mg/day. If the tolerability at 30 mg/day was not determined, the following dose was de-escalated to 20 mg/day. Tolerability was evaluated based on the frequency of dose-limiting toxicities (DLTs) confirmed by the end of cycle 1.
The following toxicities were defined as DLTs: grade 3 or higher diarrhea, skin toxicity, nausea, vomiting, hemorrhage, hypertension, and other non-hematological toxicities under appropriate supportive therapy; grade 2 diarrhea, nausea, and vomiting which continued for 4 days or more under appropriate supportive therapy; grade 4 hematological toxicities; grade 3 or higher febrile neutropenia; and other toxicities that needed discontinuation of afatinib for 8 days or more.
Patient selection
The eligibility criteria were as follows: histologically or cytologically diagnosed stage IIIB/IV or postoperative recurrent non-squamous NSCLC with EGFR mutations (exon 21 L858R, exon 19 deletion, exon 18 G719X, exon21 L861Q; with or without exon 20 T790M), age of 20–74 years, with measurable lesions, Eastern Cooperative Oncology Group performance status of 0 or 1, expected survival of ≥90 days, adequate organ function (hemoglobin concentration ≥9.0 g/dL, platelet count ≥100,000/mm3, aspartate aminotransferase and alanine aminotransferase levels 100 IU/L, serum total bilirubin concentration 1.5 mg/dL, serum creatinine concentration 1.5 mg/dL, prothrombin time international normalized ratio 1.5, proteinuria 1+), and SpO2 ≥95% in room air.
The major exclusion criteria were as follows: active concurrent malignancies; pulmonary fibrosis or interstitial pneumonitis; untreated symptomatic brain metastasis; uncontrolled pleural effusion or ascites; a history of hemoptysis (≥2.5 mL) or continuous history of hemosputum; or a high risk of complications relevant to hemorrhage, severe infection, or severe drug allergies.
Assessment of safety and efficacy
The severity of AE was evaluated based on the Common Terminology Criteria for Adverse Events version 4.0. Antitumor effect was evaluated radiologically (using computed tomography or magnetic resonance imaging) every 6 weeks. All responses were determined based on the Response Evaluation Criteria in Solid Tumors version 1.1.
Statistical analysis
OS was defined as the period from the date of enrollment to death from any cause. PFS was defined as the period from the date of enrollment to the date of the verification of disease progression or death from any cause. OS and PFS were evaluated using the Kaplan-Meier method. All analyses were conducted using JMP 10 for Windows statistical software (SAS Institute Japan Inc., Tokyo, Japan).
Pharmacokinetic studies
Blood samples were collected before the administration of afatinib from day 8 to day 15 as steady state. Peripheral blood samples (3 mL) were collected into vacuum tubes without anticoagulants and centrifuged at 3,000 rpm for 10 min at room temperature, and serum was frozen and stored at –20 °C until analysis.
The afatinib concentration was determined using an ultra-performance liquid chromatography tandem mass spectrometry technique modified from a previously reported method and developed specifically for this study (14). The concentration range of the standard curves was 0.5–100 ng/mL. The bias and precision of the quality control samples were both less than 15%. At the lower limit of assay quantification, the bias and precision were both less than 20%, in accordance with the guidelines provided in the Food and Drug Administration Guidance for Industry Bioanalytical Method Validation (15). Inter-day and intra-day variabilities in precision (expressed as coefficients of variation) ranged from 6.0% to 8.8% and from 2.8% to 4.0%, respectively. The average accuracies ranged from 96.0% to 104.3%.
Results
Patient characteristics
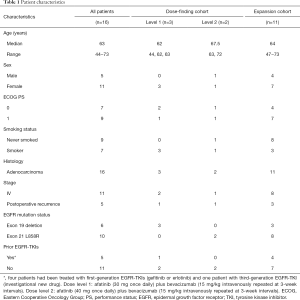
Between May 2015 and October 2016, 17 patients were enrolled: three patients at dose level 1 (afatinib at 30 mg/day and bevacizumab at 15 mg/kg) and three patients at dose level 2 (afatinib at 40 mg/day and bevacizumab at 15 mg/kg) in the dose-finding cohort. One patient was excluded from the main analysis because it was found that the patient had been treated with concomitant hyperthermia therapy at another institution after enrollment. Eleven patients were administered the RD (afatinib at 30 mg/day and bevacizumab at 15 mg/kg) in the expansion cohort. The patient characteristics are showed in Table 1. Six patients had EGFR exon 19 deletions, and 10 patients had exon 21 L858R mutations. Five patients had a treatment history with EGFR-TKIs.
Full table
Evaluation of DLTs in the dose-finding cohort
No DLTs occurred at dose level 1. At dose level 2, two of two patients experienced DLTs (grade 3 diarrhea; n=2) in cycle 1. At this point, even with another four patients not experiencing DLTs, the incidence of DLTs at dose level 2 was higher than 30%. From these results, we decided that dose level 2 was greater than the MTD, and dose level 1 was appropriate for the RD. Therefore, we administered patients in the expansion cohort with afatinib at a dose of 30mg once daily plus bevacizumab at a dose of 15 mg/kg intravenously repeated at 3-week cycles. All DLTs recovered soon after the discontinuation of afatinib. Two patients who developed DLTs were able to continue this combination therapy with a reduced dose of afatinib.
AEs in all patients
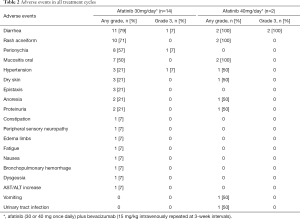
Table 2 shows the AEs in all treatment courses. The numbers of treatment cycles were median of 12.5 (range, 4–28) cycles at afatinib 30 mg/day, and 6 and 30 cycles at afatinib 40 mg/day. All 16 patients experienced AEs during the treatment (Table 2). There were no grade 4 or 5 toxicities. The frequent (>30%) AEs were diarrhea, rash acneiform, perionychia, mucositis oral, and hypertension. Grade 3 toxicities included diarrhea (18.8%), perionychia (6.3%), and hypertension (6.3%). There were no treatment-related deaths or cases of interstitial lung disease in this phase I study.
Full table
Pharmacokinetics
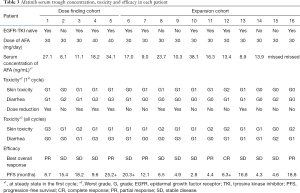
Fourteen of 16 patients could be evaluated for the serum trough concentration of afatinib at steady state (Ctrough ss). The median Ctrough ss was 15.1 ng/mL (range, 8.1–38.1 ng/mL) in 12 patients receiving 30 mg/day. The Ctrough ss in two patients receiving 40 mg/day were 18.2 and 34.1 ng/mL, respectively. A dose-proportional increase in the Ctrough ss was not observed. The Ctrough ss, toxicity, and efficacy corresponding to each patient are shown in Table 3.
Full table
Antitumor activities
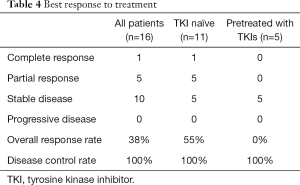
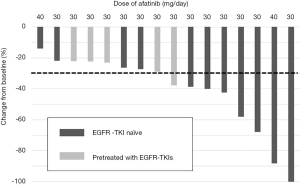
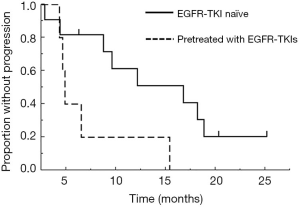
The RRs and disease control rates were 55% and 100% in EGFR-TKI-naïve patients and 0% and 100% in patients previously-treated with EGFR-TKIs, respectively (Table 4). A waterfall plot for the 16 patients is presented in Figure 1. At the data cut-off date, the median follow-up time was 20.3 months. The median PFSs were 16.8 months (95% confidence interval: 4.4–18.8 months) in EGFR-TKI-naïve patients and 4.9 months (95% confidence interval: 4.3–15.4 months) in patients pretreated with EGFR-TKIs (Figure 2). The antitumor effects of EGFR exon 21 L858R and exon 19 deletion are shown in Figures S1-S4. Of the 16 patients, only two patients died of disease progression before data cutoff.
Full table
Resistance mechanisms
Of 16 patients, 13 had disease progression at the time of data cutoff. The most common site of progression manifesting as lesions was the intrathoracic lesion (10 patients). Only three patients had disease progression due to distant metastatic lesions: bone lesions in two patients and a brain lesion in one patient. Eight patients underwent re-biopsy after acquiring resistance to the combination therapy of afatinib with bevacizumab. T790M mutations were identified in three of eight patients (38%). Gene mutations other than EGFR were not evaluated in this study.
Discussion
In this phase I trial, afatinib at a dose of 30 mg/day and bevacizumab at a dose of 15 mg/kg were defined as the RD. In another phase I study of the combination therapy of afatinib with bevacizumab in Japan, a similar dose was reported to be tolerable (16). Based on these results, afatinib at a dose of 30 mg once daily plus bevacizumab at a dose of 15 mg/kg intravenously repeated at 3-week intervals is considered an appropriate dose in Japanese patients.
The recommended phase II dose of afatinib monotherapy is 50 mg once daily, according to a phase I trial (17). However, afatinib at 50 mg/day has been judged as intolerable in several phase II trials (18,19). Therefore, afatinib at 40 mg/day has been selected for phase III trials to evaluate the efficacy of afatinib (5,6), and this dose has been approved as the standard dose of afatinib monotherapy globally, including in Japan. However, afatinib at 40 mg/day may be more toxic for Japanese patients than for other populations. In a Japanese subgroup of the LUX-Lung 3 trial, a phase III study that measured afatinib against platinum-doublet chemotherapy for patients with NSCLC harboring EGFR mutations, AEs that required dose reductions developed in 75.9% of patients treated with afatinib at 40 mg/day (20). Additionally, there were no significant differences in the efficacies between afatinib at <40 and ≥40 mg/day in a combined analysis of two phase III trials of afatinib monotherapy (21). Afatinib at 30 mg/day may be an optimal dose for Japanese patients with EGFR-mutant NSCLC, regardless of whether it is administered with or without bevacizumab.
Large inter-individual variability in the Ctrough ss was observed. It has been reported that the severity of diarrhea and rash are correlated with the exposure of afatinib (22,23). However, the Ctrough ss in two patients who developed DLTs involving grade 3 diarrhea (18.2 and 34.1 ng/mL, respectively) were not significantly higher compared to that in other patients. Therefore, there are inconsistencies in the dose-exposure and exposure-toxicity relationships, while we decided that the RD is 30 mg/day.
The median PFSs associated with afatinib plus bevacizumab were 16.8 months in EGFR-TKI-naïve patients and 4.9 months in patients previously-treated with EGFR-TKIs in our study. By comparison, the median PFS associated with afatinib monotherapy in patients with treatment-naïve advanced NSCLC with EGFR mutations has been reported to be 11.0–13.6 months (5,6,10). These data suggest the positive effect of bevacizumab on afatinib. Currently, a randomized phase II trial to compare the efficacies of afatinib and afatinib plus bevacizumab in patients with untreated NSCLC harboring EGFR mutations is ongoing (CRB6180001). Meanwhile, a phase II study to assess the outcomes of afatinib plus bevacizumab in patients pretreated using EGFR-TKIs has already been reported. In this trial, the RR, disease control rate, and median PFS associated with afatinib plus bevacizumab were reported as 18.8%, 90.7%, and 6.3 months, respectively (24). This combination therapy might be beneficial as an EGFR-TKI re-challenge therapy.
Re-biopsy was conducted in 8 patients once disease progression was detected following this combination therapy, and 3 patients (38%) acquired EGFR exon 20 T790M mutations. Meanwhile, the frequency of secondary T790M mutations is 33.3–48.8% in patients treated with afatinib monotherapy (25,26). However, there are limited data corresponding to the resistance mechanisms of afatinib with bevacizumab, and the effect of bevacizumab on the frequency of T790M mutations is unclear. Recently, several reports have suggested the promising effect of osimertinib in patients with EGFR-mutated NSCLC who have acquired resistance to afatinib by secondary T790M mutations (27,28). Further examination of resistance mechanisms of afatinib plus bevacizumab is expected.
Conclusions
In conclusion, the RD of the combination therapy of afatinib plus bevacizumab is afatinib at a dose of 30 mg/day plus bevacizumab at a dose of 15 mg/kg q3w, and this combination at the RD is well tolerated in Japanese patients with advanced non-squamous NSCLC harboring EGFR mutations. The efficacy of this combination therapy seems to be promising, and larger-scale studies are warranted to assess the toxicity and efficacy of this combination therapy further.
Acknowledgments
We greatly appreciate for all the participating patients, their families and caregivers, investigators, and site staffs.
Funding: This study was funded by Department of Respiratory Medicine, Juntendo University Graduate School of Medicine, and Boehringer Ingelheim.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-824
Data Sharing Statement: Available at http://dx.doi.org/10.21037/tlcr-20-824
Peer Review File: Available at http://dx.doi.org/10.21037/tlcr-20-824
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tlcr-20-824). RK reports grants and personal fees from Boehringer Ingelheim and AstraZeneca, personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, and Pfizer, outside the submitted work. TS reports grants and personal fee from MSD and Boehringer Ingelheim, personal fees from Nichi-Iko Pharmaceutical Co., Daiichi Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, Eli Lilly, Novartis, and Taiho Pharmaceutical, outside the submitted work. CKI reports personal fees from Chugai Pharmaceutical and Ono Pharmaceutical, outside the submitted work. TT reports personal fees from AstraZeneca, Chugai Pharmaceutical, MSD and Boehringer Ingelheim, outside the submitted work. KY reports personal fees from Chugai Pharmaceutical, outside the submitted work. HI reports grants and personal fees from Boehringer Ingelheim, personal fees from Ono Pharmaceutical, Chugai Pharmaceutical, AstraZeneca and MSD, outside the submitted work. KA reports personal fees from Chugai Pharmaceutical, AstraZeneca, Merck Sharp and Dohme, Bristol-Myers Squibb, and Ono Pharmaceutical, outside the submitted work. KT reports grants and personal fees from AstraZeneca, Boehringer Ingelheim, and Chugai Pharmaceutical, grants from MSD, and Bristol-Myers Squibb, personal fees from Eli Lilly, Astellas, Ono Pharmaceutical, Taiho Pharmaceutical, Shionogi, Novartis, Pfizer, Glaxo Smith Kline, and Actelion, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study protocol was approved by the institutional review boards of Juntendo University Hospital (registration ID 14-134) and Kurume University Hospital (registration ID 14243). Written informed consent was obtained from all patients prior to the study. This study was conducted in compliance with the Declaration of Helsinki (as revised in 2013) and all relevant Japanese laws and regulations. This study was registered at the University Hospital Medical Information Network Clinical Trial Registry (UMIN 000016029).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019;20:625-635. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone of with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harhouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 2015;10:486-91. [Crossref] [PubMed]
- Park K, Tan EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin-paclitaxel for chemo-naïve non-small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol 2013;24:54-9. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann Oncol 2015;26:1877-83. [Crossref] [PubMed]
- Bouchet S, Chauzit E, Ducint D, et al. Simultaneous determination of nine tyrosine kinase inhibitors by 96-well solid-phase extraction and ultra performance LC/MS-MS. Clin Chim Acta 2011;412:1060-7. [Crossref] [PubMed]
- Validation. Rockville, Food and Drug Administration. Guidance for Industry: Bioanalytical Method US Department of Health and Human Services. FDA, Center for Drug Evaluation and Research; 2001.
- Ninomiya T, Nogami N, Kozuki T, et al. A phase I trial of afatinib and bevacizumab in chemo-naïve patients with advanced non-small-cell lung cancer harboring EGFR mutations: Okayama Lung Cancer Study Group Trial 1404. Lung Cancer 2018;115:103-8. [Crossref] [PubMed]
- Yap TA, Vidal L, Adam J, et al. Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 2010;28:3965-72. [Crossref] [PubMed]
- Yang JCH, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 2012;13:539-48. [Crossref] [PubMed]
- Katakami N, Atagi S, Goto K, et al. LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 2013;31:3335-41. [Crossref] [PubMed]
- Kato T, Yoshioka H, Okamoto I, et al. Afatinib versus cisplatin plus pemetrexed in Japanese patients with advanced non-small cell lung cancer harboring activating EGFR mutations: subgroup analysis of LUX-Lung 3. Cancer Sci 2015;106:1202-11. [Crossref] [PubMed]
- Yang JCH, Sequist LV, Zhou C, et al. Effect of dose adjustment on the safety and efficacy of afatinib for EGFR mutation-positive lung adenocarcinoma: post hoc analyses of the randomized LUX-Lung 3 and 6 trials. Ann Oncol 2016;27:2103-10. [Crossref] [PubMed]
- Wind S, Schmid M, Erhardt J, et al. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet 2013;52:1101-9. [Crossref] [PubMed]
- Niebecker R, Maas H, Staab A, et al. Modeling exposure-driven event time courses in oncology exemplified by afatinib. CPT Pharmacometrics Syst Pharmacol 2019;8:230-9. [Crossref] [PubMed]
- Hata A, Katakami N, Kaji R, et al. Afatinib plus bevacizumab combination after acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant non-small cell lung cancer: multicenter, single-arm, phase 2 trial (ABC study). Cancer 2018;124:3830-8. [Crossref] [PubMed]
- Lin YT, Chen JS, Liao WY, et al. Clinical outcomes and secondary epidermal growth factor receptor (EGFR) T790M mutation among first-line gefitinib, erlotinib and afatinibi-treated non-small cell lung cancer patients with activating EGFR mutations. Int J Cancer 2019;144:2887-96. [Crossref] [PubMed]
- Lee K, Kim Y, Jung H, et al. Repeat biopsy procedures and T790M rates after afatinib, gefitinib, or erlotinib therapy in patients with lung cancer. Lung Cancer 2019;130:87-92. [Crossref] [PubMed]
- Park K, Bennouna J, Boyer M, et al. Sequencing of therapy following first-line afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2019;132:126-31. [Crossref] [PubMed]
- Hochmair MJ, Morabito A, Hao D, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol 2018;14:2861-74. [Crossref] [PubMed]


