
The clinical benefit of molecular re-assessments in management of progressive lung cancer
Introduction
Genomic analyses have resulted in a better understanding of the biology of non-small cell lung cancer (NSCLC) and in the successful development of molecularly targeted therapy (1,2).
Somatic BRAF mutations occur in 2% to 4% of all NSCLC (3,4), with approximately half of them showing a typical V600E mutation, associated with upregulation of MAPK signaling pathway (5). Even though monotherapy with BRAF-V600-specific inhibitors such as vemurafenib and dabrafenib shows clinical activity (6,7), combination treatment with BRAF- and MEK-inhibitors results in even higher response and progression-free survival rates and has thus been licensed by FDA and EMA (7,8).
In Melanoma, resistance to BRAF-inhibitor treatment is frequently caused by off-target mechanisms such as reactivation of MAPK signaling (9-11), PI3K-AKT pathway dysregulation and loss of CDKN2A (12,13). On-target resistance mechanisms are less common in this setting with only a few publications describing BRAF amplifications (14)and alternative BRAF splicing variants (15) as potential mechanisms. Combinations of the described alterations may occur in melanoma patients treated with combined BRAF/MEK inhibition (16).
In contrast, little is known about the mechanisms driving resistance to BRAF inhibitor or BRAF and MEK inhibitor treatment in NSCLC, with only a few case reports describing acquired KRAS, NRAS, MEK1 or PTEN mutations as potential off target mechanisms (17-19). Therefore, current guidelines recommend to switch lung cancer patients progressing on first-line BRAF/MEK inhibitor treatment to standard immune- and/or chemotherapy regimen (20).
In this case report, we describe the progressive disease of a patient with BRAF-V600E mutant metastatic adenocarcinoma of the left lung under combination therapy of dabrafenib and trametinib. We illustrate, that even in an apparently typical clinical course, a careful re-assessment of treatment options including renewed molecular diagnostic may be essential to optimize treatment outcome.
We present the following article in accordance with the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-996.
Case presentation
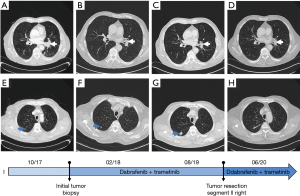
In September 2017, a 75-year-old male patient with a 55-year history of smoking presented with bone pain and increasing dyspnea. The initial computed tomography (CT) of thorax and abdomen showed a 33×33 mm central mass in the left lower lobe of the lung (Figure 1) with multiple lymph node and bone metastases, which were histologically proven to represent a TTF1-positive pulmonary adenocarcinoma (Figure 2). Next-generation sequencing (NGS) revealed the presence of a BRAF-V600E mutation and no other targetable driver mutations.
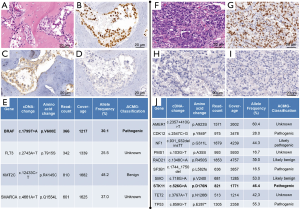
Expression of PD-L1 was restricted to less than 1 percent of the tumor cells.
Thus, a combination of dabrafenib (150 mg twice daily orally) and trametinib (2 mg/day orally) was started and resulted in partial response (PR) in subsequent CT examinations (decrease of the index lesion to 24×18 mm in Figure 1B). However, a single lesion in segment II of the contralateral lung, which was hardly visible in the initial CT-scan, progressed in size (to 20×15 mm in Figure 1G) after 18 months of treatment. At that point, we considered switching to immunochemotherapy. However, as the lesion in segment II (right) was the only progressive tumor manifestation and the patient was reluctant to start a chemotherapy containing regimen resection of this single lesion was offered. Histology demonstrated TTF1-positive adenocarcinoma (Figure 2F,G,H,I) compatible with the primary tumor. In an attempt to elucidate potential resistance mechanisms, we performed NGS-based panel sequencing. Much to our surprise, we were unable to find the initial BRAF-V600E mutation. Therefore, we performed additional sequencing on both tumor samples with identical commercially available, larger amplicon based NGS panels (Human Comprehensive Cancer Panel and Human Oncology Panel, Qiagen) for comprehensive molecular characterization and comparison of both specimen.
The regions of interest were amplified using the above mentioned amplicon panels according to the protocol “QIAseq Targeted DNA V3 Panel, May 2017” (Qiagen). Tumor cell content of both samples was at least 70%. The bioinformatics evaluation was performed with Biomedical Workbench from CLC (12.0.3) using a customized analysis algorithm with the following filters: coverage >/=200, allele frequency >/=10%. HG19 was used as reference genome.
The initial left-sided tumor (Figure 2E) and the resected right-sided lesion (Figure 2J) showed completely distinct molecular profiles with not a single shared variant, arguing for the latter being a second independent BRAF-wildtype NSCLC rather than a metastasis of the initial BRAF-mutated cancer.
Given the complete resection of the second primary BRAF-wildtype NSCLC, the well-tolerated and effective TKI combination therapy was continued. The patient remained in stable PR at last follow-up, 34 months after treatment initiation and 12 months after resection of the single progressing lesion in segment II right (index lesion 26×16 mm; Figure 1D).
Ethical statement
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
Almost 60% of all patients with NSCLC harbor an oncogenic driver mutation with nearly half of them being therapeutically targetable (1). Targeted therapies have, in comparison to conventional chemotherapy, resulted in impressive improvements in response rates, survival and patient-reported quality of life (22). However, the treatment of advanced NSCLC with typical driver mutations remains a challenge due to the invariable development of on- and off-target resistance (23).
While molecular re-assessment guided selection of targeted second-line treatment has been established for more frequent molecular subtypes of NSCLC (24,25), BRAF-mutated patients progressing on BRAF/MEK-inhibition are usually switched to immuno(chemo)therapy (20). This seems reasonable as response rates to immuno(chemo)therapy in BRAF-mutated patients are comparable to the non-mutated situation (26-28). However, an increase in the frequency of re-biopsies with renewed molecular diagnostic will be necessary to elucidate and potentially target resistance mechanism in this rare mutation subtype. Our case illustrates that this is of special importance in patients with “oligo-progression”, which may represent local clonal evolution (29,30) but also the emergence of a second primary malignancy. Even though the genetic information obtained for both tumors is limited by the size of the applied NGS-panels, we believe that the grade of molecular disparity clearly argues against clonal evolution in our case. The STK11 mutation identified in the second BRAF-wildtype NSCLC of our patient has been associated with decreased activity of immune checkpoint-inhibitor based treatment. Therefore following the current clinical standard by switching to this treatment modality might not have had the desired effect in this individual (31).
Keeping in mind the limitations of a single case report, we think that the course of our patient illustrates, that molecular re-assessment of tumor material obtained at time of progression might have a tremendous impact on an individual patient’s outcome and should therefore be considered on a routine basis.
Acknowledgments
Funding: This work was supported by the Stiftung Deutsche Krebshilfe within the National Network Genomic Medicine Lung Cancer, nNGM.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at http://dx.doi.org/10.21037/tlcr-20-996
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at http://dx.doi.org/10.21037/tlcr-20-996). FCS reports personal fees from Takeda Pharma Vertrieb GmbH und Co. KG, personal fees from Pfizer Pharma GmbH, non-financial support from Eli Lilly and Company, outside the submitted work. DEA reports personal fees from Astra Zeneca, personal fees from Roche, personal fees from MSD, outside the submitted work. MW reports personal fees and non-financial support from Novartis, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work. The authors ensure that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. [Crossref] [PubMed]
- Clinical Lung Cancer Genome Project (CLCGP). A Genomics-Based Classification of Human Lung Tumors. Sci Transl Med 2013;5:209ra153 [PubMed]
- Cardarella S, Ogino A, Nishino M, et al. Clinical, Pathologic, and Biologic Features Associated with BRAF Mutations in Non-Small Cell Lung Cancer. Clin Cancer Res 2013;19:4532-40. [Crossref] [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical Characteristics of Patients With Lung Adenocarcinomas Harboring BRAF Mutations. J Clin Oncol 2011;29:2046-51. [Crossref] [PubMed]
- Yao Z, Yaeger R, Rodrik-Outmezguine VS, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature 2017;548:234-8. [Crossref] [PubMed]
- Hyman DM, Puzanov I, Subbiah V, et al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. N Engl J Med 2015;373:726-36. [Crossref] [PubMed]
- Planchard D, Kim TM, Mazieres J, et al. Dabrafenib in patients with BRAFV600E-positive advanced non-small-cell lung cancer: A single-arm, multicentre, open-label, phase 2 trial. Lancet Oncol 2016;17:642-50. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Wagle N, Emery C, Berger MF, et al. Dissecting Therapeutic Resistance to RAF Inhibition in Melanoma by Tumor Genomic Profiling. J Clin Oncol 2011;29:3085-96. [Crossref] [PubMed]
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973-7. [Crossref] [PubMed]
- Nissan MH, Pratilas CA, Jones AM, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res 2014;74:2340-50. [Crossref] [PubMed]
- Paraiso KHT, Xiang Y, Rebecca VW, et al. PTEN Loss Confers BRAF Inhibitor Resistance to Melanoma Cells through the Suppression of BIM Expression. Cancer Res 2011;71:2750-60. [Crossref] [PubMed]
- Shi H, Hugo W, Kong X, et al. Acquired Resistance and Clonal Evolution in Melanoma during BRAF Inhibitor Therapy. Cancer Discov 2014;4:80-93. [Crossref] [PubMed]
- Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies V600E B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun 2012;3:724. [Crossref] [PubMed]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480:387-90. [Crossref] [PubMed]
- Moriceau G, Hugo W, Hong A, et al. Tunable-Combinatorial Mechanisms of Acquired Resistance Limit the Efficacy of BRAF/MEK Cotargeting but Result in Melanoma Drug Addiction. Cancer Cell 2015;27:240-56. [Crossref] [PubMed]
- Rudin CM, Hong K, Streit M. Molecular Characterization of Acquired Resistance to the BRAF Inhibitor Dabrafenib in a Patient with BRAF-Mutant Non-Small-Cell Lung Cancer. J Thorac Oncol 2013;8:e41-2. [Crossref] [PubMed]
- Niemantsverdriet M, Schuuring E, Elst AT, et al. KRAS Mutation as a Resistance Mechanism to BRAF/MEK Inhibition in NSCLC. J Thorac Oncol 2018;13:e249-51. [Crossref] [PubMed]
- Facchinetti F, Lacroix L, Mezquita L, et al. Molecular mechanisms of resistance to BRAF and MEK inhibitors in BRAFV600E non–small cell lung cancer. Eur J Cancer 2020;132:211-23. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology. 2020. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
- Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405-24. [Crossref] [PubMed]
- von Verschuer U, Schnell R, Tessen HW, et al. Treatment, outcome and quality of life of 1239 patients with advanced non-small cell lung cancer – final results from the prospective German TLK cohort study. Lung Cancer 2017;112:216-24. [Crossref] [PubMed]
- Rotow J, Bivona TG. Understanding and targeting resistance mechanisms in NSCLC. Nat Rev Cancer 2017;17:637-58. [Crossref] [PubMed]
- Jänne PA, Chih-Hsin Yang J, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- Gainor JF, Dardaei L, Yoda S, et al. Molecular mechanisms of resistance to first- and second-generation ALK inhibitors in ALK -rearranged lung cancer. Cancer Discov 2016;6:1118-33. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann Oncol 2019;30:1321-8. [Crossref] [PubMed]
- Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and Safety of Anti–PD-1 Immunotherapy in Patients With Advanced NSCLC With BRAF, HER2, or MET Mutations or RET Translocation: GFPC 01-2018. J Thorac Oncol 2020;15:628-36. [Crossref] [PubMed]
- Jamal-Hanjani M, Wilson GA, McGranahan N, et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N Engl J Med 2017;376:2109-21. [Crossref] [PubMed]
- Califano R, Romanidou O, Mountzios G, et al. Management of NSCLC Disease Progression after First-Line EGFR Tyrosine Kinase Inhibitors: What Are the Issues and Potential Therapies? Drugs 2016;76:831-40. [Crossref] [PubMed]
- Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 Deficiency Promotes Neutrophil Recruitment and Proinflammatory Cytokine Production to Suppress T-cell Activity in the Lung Tumor Microenvironment. Cancer Res 2016;76:999-1008. [Crossref] [PubMed]


