
Alternative methods for local ablation—interventional pulmonology: a narrative review
Introduction
Lung cancer medicine has undergone major transformation in recent years and personalised therapies are becoming reality. The 8th edition TNM (tumour, node, metastasis) staging system (1) includes, for the first time, subgroups within M1, highlighting a potential survival difference in patients with a contralateral nodule or single extrathoracic metastasis from those with disseminated metastatic disease. Definitions of oligometastatic disease vary, and may occur at differing timepoints in the treatment journey. For example, a metastatic deposit may be evident at initial diagnosis or develop following treatment signalling recurrence. They may manifest as treatment-resistant lesions following systemic therapy or by limited progression in a small number of sites during treatment despite an otherwise positive response.
Curative-intent treatment options for isolated lung parenchymal lesions have similarly expanded. Whilst anatomical surgical resection remains the gold standard, anatomical and non-anatomical sublobar resection, stereotactic radiation and percutaneous ablative therapies are also viable options. Performance status, comorbidity, anatomic location and underlying lung conditions such as emphysema are amongst the important patient factors considered.
Percutaneous ablative techniques include thermoablative (heat based) radiofrequency ablation (RFA), microwave ablation (MWA), laser ablation, and cryoablation (cold based). These are all delivered under imaging guidance with variation in catheter dimensions and numbers. Necessarily traversing the visceral pleura and the requisite size of needle and intervention catheter, pneumothorax occurs in up to 50% of cases of percutaneous ablation (2). In a population of patients deemed unfit for surgical resection, the potential risk to them are high. Perhaps surprisingly, the proportion of patients with pneumothorax who require intercostal catheter insertion is only 5–10%, although some studies include primarily extrathoracic cancer metastases, and hence represent younger patients with less COPD incidence (3,4). Additional adverse effects (AE) include prolonged air leak due to bronchopleural fistula and occasional tumor implantation along insertion tracts. Delayed haemoptysis as a result of pseudoaneurysm has been described with RFA (5). Direct comparisons of efficacy are challenging due to heterogeneity of studies, different cancer cell types, sizes of lesions and lack of randomised controlled data, however all modalities demonstrate lower recurrence rates and/or longer cancer-related survival in smaller compared to larger lesions (size threshold definitions vary between 2 or 3 cm) (4,6,7). A narrative synthesis of 16 methodologically weak studies (mostly retrospective case series) described comparable 1- and 3-year survival rates between transthoracic RFA and SBRT, but SBRT has more favourable 5-year survival and lesser local recurrence (8). A meta-analysis comparing outcomes in patients with lung cancer or pulmonary metastases treated with percutaneous RFA or MWA demonstrated a greater 1-, 2-, 3-, 4- and 5-year overall survival with RFA but no difference otherwise, including median overall survival, progression free survival, complete ablation rate and complication rate (9).
Post treatment radiologic effects are well documented (10) with ground glass opacification, hypodensity due to necrosis, cavitation and fibrosis part of the natural course. Reactive hilar and mediastinal lymphadenopathy mimicking locoregional nodal disease is common and may persist for up to 1 year (11). FDG-PET avidity in thermoablative methods may see SUVmax values at or above pre-ablation levels for up to 1 year following ablation (12). PET/CT imaging is not as thoroughly studied in cryoablation.
Central airway malignancies are routinely managed bronchoscopically, using many platforms such as electrocautery, laser, argon plasma coagulation (APC), cryotherapy, photodynamic therapy, brachytherapy, intratumoural chemotherapy and mechanical debridement including rigid bronchoscopy and rotational microdebrider predominantly for debulking and palliative intent (13).
Extending bronchoscopic ablation towards the peripheral airways is thus a tantalising prospect, although in the past this has been limited because predicate bronchoscopic interventions are real-time bronchoscopically image guided, and the overall low accuracy of targeting smaller peripheral lesions, 20–30% yield in accurately reaching lesions <2 cm in the outer 1/3 of the lung parenchyma. The advances in technical capability to navigate peripherally for diagnostic reasons is well-established. Technological evolution has improved diagnostic yield (smaller bronchoscopes, radial endobronchial ultrasound (EBUS, with or without a guide sheath), virtual navigation bronchoscopy (VNB) by airway fly through reconstruction software or by real-time electromagnetic navigation bronchoscopy (ENB), and more recently bronchoscopic transparenchymal nodule access (BPTNA) (14), robotic bronchoscopy (15) and cone-beam CT (16). Pairing therapeutic platforms with navigational instruments is conceptually logical and may offer benefits in terms of higher yield, better tissue acquisition, lower risk of pneumothorax and control of bleeding complications.
Thus this review aimed to summarise and inform regarding available platforms, current evidence base and future research directions. We present the following article in accordance with the Narrative Review reporting checklist (available at https://dx.doi.org/10.21037/tlcr-20-1185).
Methods
In this narrative review of a subject that is fairly specific and narrow in scope, we initially queried Pubmed for indexed articles using the key words bronchoscopic tumor/cancer ablation, lung cancer high dose local therapy in the English language. There is absence of any prospective randomized studies in bronchoscopic tumor ablation interventions and a general paucity of high-quality prospective studies in this arena. For a updated review of the latest state of the art, we therefore also included abstract publications and meeting presentations, where possible published, with at least the abstract, in English. We also searched the US NIH ClinicalTrials.gov website (http://www.clinicaltrials.gov) and searched under the same terms, and several references are from personal communication with on-going studies’ principal investigators with full references to publications.
Established bronchoscopic tumor ablation platforms
Radiofrequency ablation (RFA)
‘Radiofrequency’ is defined as electromagnetic energy with frequencies below 30 MHz but most clinical devices are between 375 and 500 kHz. The electrode is grounded to the patient’s skin. Voltage is delivered by the radiofrequency generator between the electrode and grounding pad, resulting in an electric field that oscillates, akin to alternating current. The resultant effect is a shifting of electrons within adjacent structures and subsequent heat generation. Temperatures above 60 °C result in necrotic cell death, however, tissue carbonisation occurs at temperatures above 105 °C which counterproductively increases tissue impedance. For this reason, cooling (internal or external) has been incorporated. Likewise, bipolar devices have replaced monopolar. In lesions adjacent to large vessels, the cooling effect of flowing blood can dissipate heat, the “heat-sink” effect, resulting in a lower treatment volume.
RFA is the most advanced of the trans-bronchoscopic ablative technologies with a Japanese group publishing animal studies, multiple ‘ablate and resect’ human cohort studies with promising results (17-20). Their largest includes 28 procedures (23 initial and 5 retreatment) in 20 non-surgically appropriate patients utilising a CT guided bronchoscopic cooled-RFA catheter (1.67 mm diameter, 10 mm active tip, ablation time 50 seconds) performed between 2006 and 2012. This was an older, comorbid population, approximately 2:1 adenocarcinoma to squamous cell carcinoma and a median tumour size of 24 mm. All target lesions displayed an air-bronchus sign on thin-slice CT. There were no serious adverse events, 3 patients had unexpected hospitalisation with a febrile illness. Local control rate was demonstrated to be 82.6% and 5-year survival 61.5% (19).
A recent porcine study with a novel catheter (1.4 mm diameter, 5 mm long electrode) with impedance sensoring and a proximal anchoring silicone balloon and distal filling of the target segment with conducting hypertonic saline (23.4%) were conducted. Two treated lobes of nine pigs with sacrifice and necropsy at one week, four weeks and twelve weeks. No immediate or delayed complications were observed and pathologic examination demonstrated necrotic tissue progressively replaced with fibrosis (21). A current in human study with this new device is ongoing, targeting <2 cm peripheral lesions, results pending (personal communication K Yoneda).
Microwave ablation (MWA)
MWA uses higher frequency electromagnetic waves (300 MHz–300 GHz) than RFA, although the therapeutic range for human oncologic therapies have been between 915 MHz–2.5 GHz. Rapid rotation of polarised water molecules creates heat, resulting in protein denaturation, cell membrane rupture and coagulation necrosis in quick succession. MWA results in a greater treatment volume effect than RFA as heat is generated further from the treatment probe, is less affected by tissue conductivity, results in less tissue carbonisation, and is less effected by heat-sink (22). In theory, MWA may have less limitation of ablation zone by air, an important consideration with ground glass and sub-solid nodules.
There is extensive literature of transcutaneous MWA of thoracic lesions (23) but a very limited experience with transbronchoscopic approach, even though flexible catheters have been developed for transbronchoscopic delivery. A number of porcine studies (24) has demonstrated feasibility of using between 1.4–1.9 mm diameter water-cooled prototype catheters to create ablation zones ranging from 14–40 mm in normal pig lungs, depending on energy level (24–100 watts) and time of application (5–10 minutes), as well as aeration status of the lung models. There has been only a single in-human experience presenting 3 patients with 4 non-primary lung cancer metastases to the lungs (colorectal and endometrial cancers). In the single armed observational report, all cases were guided by both Superdimension ENB and real-time Cone-Beam CT (CBCT) confirmation of catheter localization with the target lesions of median size 10.5 mm (7–13 mm). A special transbronchial puncture tool (Cross Country) was also needed to target lesions without an airway path (25). Treatment was at 100 W for 10 minutes, with post procedure CBCT demonstrating ground glass opacification. No immediate serious adverse events (pneumothorax or bleeding) were reported, although several subjects with lesions close to the pleura reported chest pain. There is no long-term follow-up of this case series to report on disease control (25). Extrapolating from the transcutaneous MWA experience, disease control has generally improved in the past decade from the 2000–2010 decade, and it is now standard application of 2.5 GHz. The currently available commercial transbronchoscopic MWA systems have paired navigation or robotic bronchoscopy systems, and given the need to confirm accurate localization of the catheter tips within target lesions for effective ablation, it is likely that transbronchoscopic MWA will be used in a platform system.
Photodynamic therapy (PDT)
PDT has been used in thoracic and other malignancies for over 30 years. An intraveneous photo-sensitising (PS) agents (Porfimer sodium – Photofrin a 1st generation and Talaporfirin – Laserphyrin a 2nd generation haematoporphyrin derivative) are preferentially taken up by tumour cells (to a lesser degree, normal tissue). After a timed interval when the tumoral concentration remains high whereas normal tissue levels are reduced, a diffuser laser fibre is positioned and laser shone upon the tumor resulting in a photochemical effect, releasing superoxide radicals which mediate cellular toxicity through direct cell killing, damage of tumor vasculature and possibly inducing host anti-tumour immune responses. Although a Laser light source is used, the therapeutic effect is not thermal. The activating laser light generally cannot travel through more than 5–10 mm of tissue. Until now, in the lung, this has confined treatment to superficial central airway lesions and palliation of central airway obstruction in conjunction with debulking methods (26).
Application of PDT for peripheral lung cancers guided by conventional bronchoscopes was first reported by Downie (27) with a subset of 2 of 7 typical carcinoids treated with Photofrin dosed 1–2 mg/kg and energized at 200 J/cm2. The two cancers 5 and 14 mm were targeted by using thin bronchoscope but without use of rEBUS or “navigation bronchoscopy” (28). Incorporation of a novel flexible optical fibre coupled with ENB allowed photodynamic treatment of peripheral lung lesions in dogs, however, residual viable tumour cells were noted beyond a 1.5 cm ‘kill zone.’ (29). Further innovation in creating a 1 mm Composite type Optical Fiberscope (COF) has allowed visually directed positioning of the laser fiber. A prospective clinical trial NCT03344861 for 10 patients scheduled for surgery to be treated with Photofrin and subsequently evaluated for safety and tissue response is opened in 11/2017, but no final results have been presented. In Japan, a multicenter Phase 1 dose escalation study in humans, using 40 mg/M2 of Talaporfirin (NPe6 Laserphyrin) in 7 patients with cT1N0 (stage IA) NSCLC, <20 mm (mean 16.7 mm), was conducted with an escalating energy dose from 50 J/cm2 in 3 and 100 J/cm2 in 4 (30). A diode laser emitting light at 664 nm (one of Talaporfirin’s peak absorption wavelength) is used for PS activation. At scheduled follow-ups by CT scans and repeat bronchoscopy at 6 months, 3/7 had complete response (CR) and 4/7 had stable disease (SD). Perhaps because of the much shorter half-life of the second generation photosensitizer, only 1 patient had grade I photosensitivity (30). Of note investigators employed ENB (SuperDimension system) with guide sheath, with follow-up confirmation with radial-EBUS and finally advancing the combined fiberscope for final positioning. In future treatment will likely shift to the second generation agents that has a quicker onset of action (light activation 4–6 hours after injection instead of 48 hours), shortened duration and effects of photosensitivity, and not needing at least two bronchoscopies.
Bronchial thermal vapour ablation (BTVA)
BTVA, a technology initially proposed as means for bronchoscopic lung volume reduction (31), involves a catheter being introduced into a target subsegmental airway and a tamponade balloon effectively sealing this off. A predetermined volume of heated water vapour is introduced through the catheter with resultant inflammatory response and subsequent lasting atelectasis. A porcine model (32) demonstrated a uniform field of necrosis obeying subsegmental anatomical boundaries. Pneumotocoeles developed more commonly with higher treatment energy.
A follow-up human cadaver study (33) targeting subsegmental airways with diameters 2–4 mm and varied treatment energy demonstrated anatomical containment and, in explanted lungs containing malignant lesions, tumours were completely contained within the ablation zone. Markedly fewer pneumatocoeles were identified than in porcine lungs (4 from 107 treatments).
Importantly, this technology does not require the same degree of bronchoscopic navigation accuracy as a catheter applied directly towards the lesion. It is comparable to needing to find the right street, rather than the correct house, turning into the driveway and parking in the garage! In addition, the lung volume reduction effect may also benefit patients with significant emphysema and hyperinflation in the lobe containing the target malignancy. This is especially relevant given not only the shared risk factor of cigarette smoking but the greater likelihood of cancers developing in emphysematous areas of lungs (34).
A recent 6 patient pilot ablate and resect study demonstrated feasibility and was well tolerated. Histologic results from the 5 patients who proceeded to resection suggested well demarcated thermal injury margins with expected necrotic change in keeping with theoretically effective ablation. Further research is necessary for determining optimal catheter location, dose and the effect of intra-lesional scarring and pleural contact (35).
Cryotherapy
Cryotherapy has been utilised in varied forms for over 100 years (36) with dermal applications common and prostate cancer a previous area of interest. Effective tissue destruction relies upon four criteria: monitoring, rapid cooling, slow thawing and repetition of the freeze-thaw cycle. Increasing availability of bronchoscopic cryoprobes has led to their use in large airway malignant processes (for diagnostic and therapeutic purposes) (37,38) as well as peripheral transbronchial biopsy for diagnosis of interstitial (39) and malignant disease, with reusable 1.9 mm and 2.4 mm and thinner single use 1.7 mm and 1.1 mm probes. The 1.9 and 2.4 mm probes have been paired with radial EBUS (40) and guide sheath procedures (40-42) to assist in identification of biopsy site and improve the diagnostic yield. Complications are greater with transbronchial cryobiopsy compared to conventional forcep biopsies, particularly significant bleeding and pneumothorax.
Transthoracic cryotherapy has been performed with CT guidance with high rates of treatment success (43,44). CT scan post procedure allows for visualisation of ice ball formation and thus approximation of treatment zone (44).
Whilst percutaneous cryotherapy has established efficacy and tools are commercially available for a bronchoscopic approach, no human studies of bronchoscopic cryotherapy for peripheral lung cancers have been published. Even though the thinner and disposable probes are more flexible, they may still be too stiff for routine navigation to small peripheral lesions. Development of Bronchoscopic Trans-Parenchymal Nodule Access (BTPNA) (14) with image guided positioning of guide sheath, insertion of larger and more rigid instruments may abrogate this limitation. While reliable tumor destruction using cryotherapy especially of larger lesions may be less reliable than the heat based therapies, published observations of cryotherapy treated central airway lung cancer, followed by external beam radiotherapy (EBRT) versus EBRT alone (45,46) and experimental animal studies of cryotherapy followed by chemotherapy (47) suggest an adjunctive role for cryotherapy followed by SBRT or systemic chemotherapy.
Brachytherapy
Conventional fractionated EBRT (External Beam Radiotherapy) and SBRT (Stereotactic Body Radiotherapy) are both tele-radiotherapy, the delivery of ionizing radiation from a distant non-contact source. In contrast brachytherapy is the placement of the radiation source directly in contact, or at a very short distance from the target. The treatment source can be implanted as seeds or needles into cancer tissue and left in place or a high dose rate (HDR) source can be transported to the target lesion and treated according to dosimetric calculations. Unlike most of the other LRTs (Localized Radical Therapy) for limited peripheral lung cancer, where treatments were first introduced by percutaneous approaches and where the predominance of experience lies, bronchoscopic brachytherapy for lung cancers or for thoracic indications have been carried out since the 1920s (48), and peri-operative placement of radon seeds to the bronchial stump for residual disease first described in 1933 (49). However most brachytherapy has been for bronchoscopically visible disease in the navigable “central airways” (central 1/3 to navigable fifth to sixth generation airways). As a potentially curative therapy, because of the normally limited effective depth of dose that falls radially to r2, it was limited to central carcinoma-in-situ. Treatment was also limited by the formation of post treatment fistulas (tracheal/bronchial – esophageal/mediastinal/pleural space) and more ominously and often fatal airway-vascular erosions, with mortality from massive hemoptysis in the 12.5–15% ranges (50). As with percutaneous therapies, application of brachytherapy targeting non-bronchoscopically visible peripheral lesions awaited the availability of CT scans for navigation or real-time guidance. Successful positioning of 6Fr catheters guided by CT bronchoscopy (prior to intraoperative cone-beam CTs) allowed treatment with r192 HDR (High Dose Rate) sources delivering single 15 Gy and 24 Gy in two treatments to two unresectable patients with 18×26 and 19×19 mm lesions, with no recurrence nor progression at 18 months (51). A 13 patients series also out of Japan, where bronchoscopy pioneers developed much of the early bronchoscopic navigation to small peripheral lesions using thin bronchoscope and peripheral radial EBUS, reported satisfactory outcome (52). In this non-randomized mixed approaches series, 5 patients were treated with transthoracic brachytherapy via a 21 guage needle, and 8 patients were treated trans-bronchoscopically. Selection of approach was based on size of lesion, and likelihood of pneumothorax. The 7 evaluable brachytherapy patients were treated with 25 Gy in multiple fractions, five with five fractions of 5 Gy and two with two fractions of 12.5 Gy versus single fraction of 20 Gy via the transthoracic approach. 1/5 percutaneous and none of the BBRx patients had a pneumothorax, most subjects experienced “mild pneumonitis”. Combined response of 25% CR, 33% PR and 42% SD was reported, with projected overall 5 years survival of 50%, and 60–70% for T1N0 cases. There were three instances of disease recurrence at 12, 13 and 32 months (52). Unlike application of brachytherapy for central airway lesions where the depth of penetration for cell kill is limited by concerns of complications, lesion target volume V100 treated have ranged in this largest series from 20±15 mL for percutaneous versus 49±37 mL with bronchoscopic catheter delivery, with better control for smaller lesions. The ENB system was subsequently adapted to localizing another inoperable peripheral lesion (53). Intraprocedural confirmation of catheter position was done using rEBUS, and follow-up 3D/4D CT planning targeted the lesion with 15 Gy (53). Updated guidelines for thoracic brachytherapy for lung cancers have been developed to standardize imaging guidelines, and recognizing the still limited data, recommend practice performance within confines of clinical trials (54).
Advantages and disadvantages of the above are summarised in Table 1.
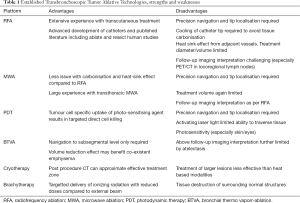
Full table
Investigational technologies
Bronchoscopic laser interstitial thermal therapy (BLITT)
Laser, like some of the previously discussed technologies, is used effectively for rapid tissue necrosis in central airways (55). The small aperture of a laser makes for precise targeting under direct visual guidance, not suited to peripheral lung lesions. BLITT, a novel laser delivery fibre with a rounded tip and wide aperture was used on in vivo and ex vivo porcine lungs as a proof of concept and dose finding study with necropsy at day three (10 ablations performed). Treatment demonstrated a dose related area of central char with variable degrees of necrosis surrounding. One pneumothorax occurred (56). There is a registered single center prospective clinical study NCT03707925 of safety and post treatment pathology effects of BLITT in humans. Following a common ablate and resect protocols, patients scheduled for surgical excision of primary lung cancer or metastatic carcinomas will undergo BLITT guided by rEBUS and ConeBeamCT, and post treatment changes evaluated in the explanted lungs. No results of the 15 subjects study that has been enrolling for two years since 9/2018 have been reported.
Irreversible electroporation (IRE)
Electroporation is an interesting but little known technology to the interventional pulmonology community. Repeated application of high voltage but short duration electrical pulses result in cell membranes disruption due to formation of nanoscale pores (80–490 nm) in the phospholipid bilayers (57-59). The exact mechanism of action is not entirely clear, and the membrane leakage can be reversible and hence the effects transient (transient aqueous pore hypothesis) (58), or for tumor ablation made irreversible (IRE). The major distinction between IRE and other power generated ablative therapies such as RFA and electrocautery is that even though potentially very high electrical voltages, up to 4,000 volts are applied to generate this field, it has minimal thermal effects (58,60). Furthermore the depth of cellular apoptosis and necrosis is limited to the electric field created between two electrodes, and the edge of tissue ablation can be sharply defined (57,59). The non-thermal ablation and tight targeting result in reduced collateral complications of luminal scarring or damage to delicate neural and vascular structures. It is also not susceptible to “heat-sink” effects common to other thermal ablations. The minimal scarring makes it possible to treat subjects previously treated with SBRT or who have very limited lung reserves. On the negative side, even though treatment time can be short, because applied current can induce muscular contractions, subjects require neuromuscular blockade and hence general anesthesia. To generate the current field requires placement of multiple electrodes, at least two, to three to generate a volume covered by the IRE field. This may be especially challenging in the lungs where respiratory changes will alter the volumetric parameters, unlike for needles placed in liver, pancreas or prostate, organs most often treated. The percutaneous placement of multiple needle electrodes, each of which may cause a pneumothorax is another challenge.
Two human studies of percutaneous IRE are reported. In 2012 Usman demonstrated feasibility in two cases (NSCLC and a metastatic synovial sarcoma case). These patients were treated each with multiple transcutaneous needle probes at 2,800 V and 90 pulses. There was no pneumothorax nor significant AE, but both cancers recurred locally within 6 months (61). The second is a phase II prospective multicenter trial of IRE on lungs with metastatic malignancies. The planned 36 patients trial was stopped after 23 enrollees, because of lack of overall efficacy and significant complications of pneumothoraces (62). While there was 30% deemed complete responses (CR) and another 8% either SD or PR, a 61% majority demonstrated disease progression during the short median follow-up of 12 months. Pneumothoraces developed in 11/23 (48%), 8 (i.e., 73%) of whom required chest tubes, or 35% of the entire cohort of treated patients. In additional needle tract seeding was noted in 13% (62).
To avoid limitations of multiple transthoracic insertions for pulmonary use, preliminary work on bronchoscopic IRE include in-vivo porcine with deployment of an expandible four pronged basket electrode for treatment of endobronchial or peribronchial cancers (60). In the timed animal sacrifices at 4 hours to 2 weeks, there was no delayed mucosal damage or scarring, but there was peribronchial parenchymal apoptosis, suggesting the potential of application for submucosal non penetrating tumors. Of note the same study by Kodama has an in-vitro component of combining platinum chemotherapy with IRE application of increasing voltage (500 to 2,000 V) to LC cell lines, demonstrating a voltage dependent synergistic effect on cell kill (60). The concept of electrochemotherapy (ECT), that of applying electroporation to increase cell permeability and hence sensitivity to chemotherapy is well established (63,64), and with recognized potential for LC application (65). In addition, studies on the alteration in immune expression and response of tumor xenografts to immunotherapies, including checkpoint inhibitors suggest neo-adjuvant benefits when combined with systemic therapy or abscopal effect with improved combination radiotherapy treatments (66,67).
As device development for IRE applications proceed for intraluminal uses, expandable balloons (68) and electrode arrays deployable under direct vision (69) may be adaptable for transbronchoscopic treatment of peripheral malignant nodules. This include either stand-alone IRE, for endo and peri-bronchial central primary lung cancers, especially Carcinoma-in-situ or unresectable central airways diseases, or as ECT, an adjuvant to systemic or locally deposited chemotherapy (70,71).
Intra-tumoral injection of cytotoxic compounds (ITC)
All the techniques reviewed in this paper have as their primary intent cancer control with improved patient quality of life and duration of survival, most often in patients with limited physiologic reserve. An attractive concept would be local intratumoral delivery of high dose therapies that can concentrate supratherapeutic dose where needed with less systemic toxicities. Thus far high dose cytotoxic therapies have mostly been percutaneously directed injections, although transurethral/intravesicular treatment of uroepithelial cancers and direct intra-cavitary delivery of high dose cytotoxics (intrapleural and peritoneal chemotherapies), immunotherapies (bladder BCG, intrapleural InterLeukin-2, Interferon) and even photoactivated therapies in the pleura have all been tried. In comparison, the record of direct transbronchoscopic delivery of cancer cytotoxic therapies have consisted of limited case series.
Initial publications from the mid 1980s highlighted endobronchial injections into invasive cancers causing central airways obstruction, compounds injected included alcohols and chemotherapeutics such as 5 Fluorouracil (5FU) that are not specifically targeting lung cancers, but likely caused cell death by their astringent properties (72). More recent case series used cisplatin that is a widely used chemotherapeutic for lung cancer (73-75). There has also been attempts to boost tumor suppression by the intratumoral injection of gene therapies such as p53 (tumor suppressor gene), although the longer-term outcome in these early 1980s studies were not encouraging (76-78). In addition to the ITC injections into bronchoscopically visible tumor obstructing the airways, more recent efforts have used convex probe linear EBUS guided injections of cisplatin into regional lymph nodes (70,71,79).
As with other transbronchoscopic therapies for isolated peripheral malignancies, whether primary lung cancers, oligometastases from LC or extrapulmonary primaries or second primary LCs, the overall scope of experience of transbronchoscopic ITC into peripheral pulmonary malignancies, whether primary or metastatic, remain limited.
Immuno-oncology has been in the headlines since the elucidation of the Cytotoxic T-Lymphocyte (CTLA-4) and Programmed Death/Ligand (PD-1/PD-L1) pathways and development of targeted therapies in this field. However long preceeding the current attention, and based on effects on melanoma, thoracic oncologists had been interested in immune stimulation with Bacillus Calmette-Guerin for primary lung cancer as well (80,81). In 1986, Matthay conducted a large 88 NSCLC patients study prospectively randomizing patients scheduled for LC resection surgery to transbronchoscopic injection (TBBI) with 5×106 viable M bovis organism 2–3 weeks pre-surgery versus immediate thoracic surgery (81). At an era when effective systemic chemotherapy for lung cancer was not established, stage I–III patients were considered for surgery. The paper is very short on technical details, regarding type of bronchoscope used, and only mentioned that lesions were as small as 20 mm, an early transbronchoscopic needle (Meditech, no guage) needle used to inject the BCG bolus under fluoroscopic guidance. Aside from a single pneumothorax in the 48 subjects randomized to TBBI, mild and transient toxicities of fever and malaise was noted. Radiographically, planar CXR after the procedure showed perilesional haziness. Because there was no statistical improvement in outcome, either of cancer recurrence or long-term survival when analyzed by stage of cancer or by cell types (44 squamous, 31 adenocarcinomas, 1 mixed and 9 large cell), there was no immediate follow-up interests (81). With the renewed focus in secondary abscopal effects, that is the stimulation of the immune system to fight cancer in the whole body as a result of local therapy, whether by radiation or now various lung local ablative therapies, there is interest in treating peripheral tumors with ICT as well, provided the usual challenges of accurate navigation and reliable dosing and distribution of intratumoral injections can be addressed.
Technology implementation
The technological developments to this point provide an exciting stepping stone, as highlighted by a number of similarly themed recent reviews (16,82). As one author detailed, we are not ready for ‘prime-time’ but a number of registered trials are currently recruiting human subjects in safety and feasibility studies with RFA, MWA, BTVA, BLITT, PDT (16,82).
As with most aspects of lung cancer diagnosis and management, the evaluation of suspected solitary lung cancers or oligometastatic disease is best under the auspices of a multidisciplinary tumor board. To investigate and eventually to implement alternative potentially more effective and less morbid tumor ablation technologies depends greatly on the interest and resources of the specific stakeholders, clinicians and administrators, of an institution. With rare exceptions of large flagship research institutions, selection of a limited number of appropriate offerings where focused practice of a sufficient number of cases make most sense. A selection of ‘enablers and barriers’ are presented in Table 2 and discussed below.
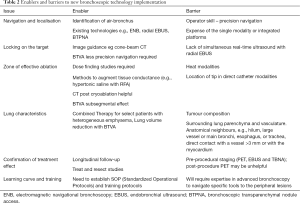
Full table
Enablers
Methods for accurate navigation and lesion identification are long standing limitations in the past, but improved image processing and ready to use soft-ware applications (VirtualNB), combined with multimodal real-time image confirmation of treatment localization at targets (r-EBUS, ConeBeamCT) are now well established procedures in interventional pulmonology. Overlay of PET/CT on live-cone beam CT has been described as a technique for further precision placement of both diagnostic and ablative tools within regions of highest PET avidity (83). Exciting new developments such as robotic bronchoscopy (15) and transparenchymal nodule access (BTPNA) (14) expand capabilities within the field even further.
The implementation of lung cancer screening programs is anticipated to result in an increase in early stage lung cancer diagnosis. While smaller stage lung cancers are more curable, combined with overall better case survival of even advanced diseases with molecularly targeted therapies and immunotherapies, this only means a larger cohort of subjects will survive their initial primary lung cancer to develop second primary lung cancers (SPLC) and or oligometastases. Add to this an aging comorbid population, effective, lung sparing, low complication procedures will have an increasing role to play.
Barriers
The established treatment modalities of surgical resection, SBRT and percutaneous ablative methods will result in a small number of patients with amenable disease eligible for experimental bronchoscopic ablative therapies.
The standard of practice is to embrace evidence based approaches with preference towards well conducted Randomized Controlled Trials (RCT). With some bronchoscopic interventions, sham controlled studies, such as in Bronchial Thermoplasty for Reactive Airways Diseases can address how to blind the non-procedural medical team and patients, however sham interventions would be unethical in active cancers. With the number of emerging methods and variation of technology within each method there will be further heterogeneity of results, making definitive conclusions of comparative efficacy challenging. Whilst this may also be seen as an enabler having competition between technology manufacturers, it will likely result in a reduced ability to conduct adequately powered, timely, high quality clinical research.
For best performance of navigation and targeting treatment of smaller peripheral lesions, experienced investigators at well-endowed institutions often use a “platform” of multiple advanced technologies, incorporating advanced radiomics for navigation, often with special hardware such as electromagnetic or other position sensors, special endoscopes for manual or robotic steering, and final positional confirmation by real-time CBCT or r-EBUS. The equipment and software required for many modalities will likely relegate these therapies to large research institutions, and when disseminated for more general practice, still limited by affordability to financially stronger institutions and payors willing to reimburse for the charges. Currently largely absent are cost-benefit (Quality per Life Year gained Vs cost) calculations.
As previously discussed, imaging assessment post treatment is anticipated to be challenging for up to 1 year.
Future research
Further development and optimisation of delivery devices is continuing, along with early human studies. As outlined above, some technologies have been demonstrated upon healthy animal lung with few utilising animal tumor models. Sheep with ovine pulmonary adenocarcinoma (caused by jaagsiekte sheep retrovirus) may provide one such avenue (84).
Initial human studies must incorporate a subset of ablate and resect methodology in order to demonstrate these modalities as genuine curative options in both primary and oligometastatic disease. Identification of biomarkers [such as circulating tumour DNA (ctDNA) (85)] or radiologic features [such as novel PET tracers (86)] to assess treatment efficacy will also be of great benefit to both clinicians and patients. In time, high-quality randomised controlled trials should be sought to establish the exact role of each ablative technique in the modern multidisciplinary treatment of lung cancer.
Further work is also required regarding how ablative therapies could be combined with each other and/or systemic therapies. Sequential application of two different platforms may provide the most precise treatments tailored to the individual. For instance, in a patient with a tumour displaying an air bronchus sign in a hyperinflated, emphysematous upper lobe, catheter-directed thermoablation, followed by thermal vapour ablation, may result in a ‘double hit’ to the tumour as well as achieving lung volume reduction. It remains to be seen whether tumour and tissue damage caused by ablative therapies evokes a similar systemic synergistic tumour killing (abscopal) effect with immunotherapy observed in patients receiving radiotherapy with immunotherapy (87).
Combining current advances may allow for a ‘diagnose and treat’ approach (85). One such example would be robotic (15) or ultraslim bronchoscopy utilising virtual navigation with rEBUS, ENB or both for lesion identification, with cone-beam CT confirmation, followed by peripheral transbronchial needle aspiration (TBNA) and rapid onsite cytologic evaluation (ROSE). If diagnostic, proceed to treatment, if not, additional sampling is required.
Given the expansion of technology platforms and pairings, and the still limited number of cases appropriate for such advanced bronchoscopic ablations, it is very unlikely that there will be sufficiently powered prospective randomized studies enrolling sufficient numbers before technologies change to make meaningful statements regarding superiority or at least non-inferiority of treatments. There should be sufficient interest and urgency for key experts to come together and develop consensus recommendations regarding prospective data gathering protocols such that in future, the necessary data sets will be available for post-hoc analysis.
Conclusions
The future is bright for bronchoscopic ablative therapies in oligometastatic lung disease as well as peripheral primary lung cancers. Some or all of the above discussed therapies will come to complement current curative intent care. Continued collaborative, well designed studies will allow more advanced human studies and another feather in the cap of personalised lung cancer care.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-20-1185
Provenance and Peer Review: This article was commissioned by the Guest Editors (Maurizio Infante & Thierry Berghmans) for the series “Oligometastatic NSCLC: definition and treatment opportunities” published in Translational Lung Cancer Research. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-20-1185). The series “Oligometastatic NSCLC: definition and treatment opportunities” was commissioned by the editorial office without any funding or sponsorship. KMF serves as an unpaid editorial board member of Translational Lung Cancer Research. GO and KMF’s institution have received in-kind support from Olympus with loan bronchoscope equipment to undertake a research study for a duration of 18 months. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Moore W, Talati R, Bhattacharji P, et al. Five-year survival after cryoablation of stage I non-small cell lung cancer in medically inoperable patients. J Vasc Interv Radiol 2015;26:312-9. [Crossref] [PubMed]
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 2007;243:268-75. [Crossref] [PubMed]
- Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer 2015;121:3491-8. [Crossref] [PubMed]
- Borghol S, Alberti N, Frulio N, et al. Pulmonary artery pseudoaneurysm after radiofrequency ablation: report of two cases. Int J Hyperthermia 2015;31:1-4. [Crossref] [PubMed]
- Healey TT, March BT, Baird G, et al. Microwave Ablation for Lung Neoplasms: A Retrospective Analysis of Long-Term Results. J Vasc Interv Radiol 2017;28:206-11. [Crossref] [PubMed]
- Yashiro H, Nakatsuka S, Inoue M, et al. Factors affecting local progression after percutaneous cryoablation of lung tumors. J Vasc Interv Radiol 2013;24:813-21. [Crossref] [PubMed]
- Bilal H, Mahmood S, Rajashanker B, et al. Is radiofrequency ablation more effective than stereotactic ablative radiotherapy in patients with early stage medically inoperable non-small cell lung cancer? Interact Cardiovasc Thorac Surg 2012;15:258-65. [Crossref] [PubMed]
- Yuan Z, Wang Y, Zhang J, et al. A Meta-Analysis of Clinical Outcomes After Radiofrequency Ablation and Microwave Ablation for Lung Cancer and Pulmonary Metastases. J Am Coll Radiol 2019;16:302-14. [Crossref] [PubMed]
- Chheang S, Abtin F, Guteirrez A, et al. Imaging Features following Thermal Ablation of Lung Malignancies. Semin Intervent Radiol 2013;30:157-68. [Crossref] [PubMed]
- Sharma A, Digumarthy SR, Kalra MK, et al. Reversible locoregional lymph node enlargement after radiofrequency ablation of lung tumors. AJR Am J Roentgenol 2010;194:1250-6. [Crossref] [PubMed]
- McLoney ED, Isaacson AJ, Keating P. The Role of PET Imaging Before, During, and After Percutaneous Hepatic and Pulmonary Tumor Ablation. Semin Intervent Radiol 2014;31:187-92. [Crossref] [PubMed]
- Chaddha U, Hogarth DK, Murgu S. Bronchoscopic Ablative Therapies for Malignant Central Airway Obstruction and Peripheral Lung Tumors. Ann Am Thorac Soc 2019;16:1220-9. [Crossref] [PubMed]
- Herth FJ, Eberhardt R, Sterman D, et al. Bronchoscopic transparenchymal nodule access (BTPNA): first in human trial of a novel procedure for sampling solitary pulmonary nodules. Thorax 2015;70:326-32. [Crossref] [PubMed]
- Agrawal A, Hogarth DK, Murgu S. Robotic bronchoscopy for pulmonary lesions: a review of existing technologies and clinical data. J Thorac Dis 2020;12:3279-86. [Crossref] [PubMed]
- Sabath BF, Casal RF. Bronchoscopic ablation of peripheral lung tumors. J Thorac Dis 2019;11:2628-38. [Crossref] [PubMed]
- Tsushima K, Koizumi T, Tanabe T, et al. Bronchoscopy-guided radiofrequency ablation as a potential novel therapeutic tool. Eur Respir J 2007;29:1193-200. [Crossref] [PubMed]
- Tanabe T, Koizumi T, Tsushima K, et al. Comparative study of three different catheters for CT imaging-bronchoscopy-guided radiofrequency ablation as a potential and novel interventional therapy for lung cancer. Chest 2010;137:890-7. [Crossref] [PubMed]
- Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-Guided Cooled Radiofrequency Ablation as a Novel Intervention Therapy for Peripheral Lung Cancer. Respiration 2015;90:47-55. [Crossref] [PubMed]
- Koizumi T, Kobayashi T, Tanabe T, et al. Clinical experience of bronchoscopy-guided radiofrequency ablation for peripheral-type lung cancer. Case Rep Oncol Med 2013;2013:515160 [Crossref] [PubMed]
- Yoneda KY, Herth F, Spangler T, et al. Long-term Survival Results following Endobronchial RF Ablation in a Healthy-Porcine Model. Annu Int Conf IEEE Eng Med Biol Soc 2020;2020:5252-8. [PubMed]
- Pillai K, Akhter J, Chua TC, et al. Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 2015;94:e580 [Crossref] [PubMed]
- Nelson DB, Tam AL, Mitchell KG, et al. Local Recurrence After Microwave Ablation of Lung Malignancies: A Systematic Review. Ann Thorac Surg 2019;107:1876-83. [Crossref] [PubMed]
- Yuan HB, Wang XY, Sun JY, et al. Flexible bronchoscopy-guided microwave ablation in peripheral porcine lung: a new minimally-invasive ablation. Transl Lung Cancer Res 2019;8:787-96. [Crossref] [PubMed]
- Lau K, Spiers A, Pritchett M, et al. P1.05-06 Bronchoscopic Image-Guided Microwave Ablation of Peripheral Lung Tumours – Early Results. J Thorac Oncol 2018;13:S542. [Crossref]
- Simone CB 2nd, Friedberg JS, Glatstein E, et al. Photodynamic therapy for the treatment of non-small cell lung cancer. J Thorac Dis 2012;4:63-75. [PubMed]
- Downie GH, Qureshi A, Loewen GM, et al. Endobronchial Ablation of Typical. Carcinoid Tumor With Photodynamic Therapy. J Bronchol 2007;14:10-4. [Crossref]
- Downie GH, McGuire FR. Peripheral photodynamic therapy for lung carcinoid tumors. Chest 2010;138:S263A. [Crossref]
- Musani AI, Veir JK, Huang Z, et al. Photodynamic therapy via navigational bronchoscopy for peripheral lung cancer in dogs. Lasers Surg Med 2018;50:483-90. [Crossref] [PubMed]
- Usuda J, Inoue T, Tsuchida T, et al. Clinical trial of photodynamic therapy for peripheral-type lung cancers using a new laser device in a pilot study. Photodiagnosis Photodyn Ther 2020;30:101698 [Crossref] [PubMed]
- Snell G, Herth FJ, Hopkins P, et al. Bronchoscopic thermal vapour ablation therapy in the management of heterogeneous emphysema. Eur Respir J 2012;39:1326-33. [Crossref] [PubMed]
- Henne E, Ferguson JS, Mest R, et al. Thermal Vapor Ablation for Lung Lesions in a Porcine Model. Respiration 2015;90:146-54. [Crossref] [PubMed]
- Ferguson JS, Henne E. Bronchoscopically Delivered Thermal Vapor Ablation of Human Lung Lesions. J Bronchology Interv Pulmonol 2019;26:108-13. [Crossref] [PubMed]
- Sanchez-Salcedo P, Zulueta JJ. Lung cancer in chronic obstructive pulmonary disease patients, it is not just the cigarette smoke. Curr Opin Pulm Med 2016;22:344-9. [Crossref] [PubMed]
- Steinfort DP, Christie M, Antippa P, et al. Bronchoscopic Thermal Vapour Ablation for Localized Cancer Lesions of the Lung: A Clinical Feasibility Treat-and-Resect Study. Respiration 2021;100:432-42. [Crossref] [PubMed]
- Theodorescu D. Cancer cryotherapy: evolution and biology. Rev Urol 2004;6:S9-S19. [PubMed]
- Schumann C, Hetzel M, Babiak AJ, et al. Endobronchial tumor debulking with a flexible cryoprobe for immediate treatment of malignant stenosis. J Thorac Cardiovasc Surg 2010;139:997-1000. [Crossref] [PubMed]
- Troy LK, Hetzel J. Lung cryobiopsy and interstitial lung disease: What is its role in the era of multidisciplinary meetings and antifibrotics? Respirology 2020;25:987-96. [Crossref] [PubMed]
- Troy LK, Grainge C, Corte TJ, et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respir Med 2020;8:171-81. [Crossref] [PubMed]
- Imabayashi T, Uchino J, Yoshimura A, et al. Safety and Usefulness of Cryobiopsy and Stamp Cytology for the Diagnosis of Peripheral Pulmonary Lesions. Cancers (Basel) 2019;11:410. </jrn>. [Crossref] [PubMed]
- Schuhmann M, Bostanci K, Bugalho A, et al. Endobronchial ultrasound-guided cryobiopsies in peripheral pulmonary lesions: a feasibility study. Eur Respir J 2014;43:233-9. [Crossref] [PubMed]
- Herth FJ, Mayer M, Thiboutot J, et al. Safety and Performance of Transbronchial Cryobiopsy for Parenchymal Lung Lesions. Chest 2021; Epub ahead of print. [Crossref] [PubMed]
- de Baere T, Tselikas L, Woodrum D, et al. Evaluating Cryoablation of Metastatic Lung Tumors in Patients--Safety and Efficacy: The ECLIPSE Trial--Interim Analysis at 1 Year. J Thorac Oncol 2015;10:1468-74. [Crossref] [PubMed]
- Hinshaw JL, Littrup PJ, Durick N, et al. Optimizing the protocol for pulmonary cryoablation: a comparison of a dual- and triple-freeze protocol. Cardiovasc Intervent Radiol 2010;33:1180-5. [Crossref] [PubMed]
- Asimakopoulos G, Beeson J, Evans J, et al. Cryosurgery for malignant endobronchial tumors: analysis of outcome. Chest 2005;127:2007-14. [Crossref] [PubMed]
- Vergnon JM, Schmitt T, Alamartine E, et al. Initial combined cryotherapy and irradiation for unresectable non-small cell lung cancer. Preliminary results. Chest 1992;102:1436-40. [Crossref] [PubMed]
- Forest V, Peoc'h M, Campos L, et al. Benefit of a combined treatment of cryotherapy and chemotherapy on tumour growth and late cryo-induced angiogenesis in a non-small-cell lung cancer model. Lung Cancer 2006;54:79-86. [Crossref] [PubMed]
- . Yankauer. Two cases of lung tumor treated bronchoscopically. NY Med J 1922;21:741.
- Graham EA, Singer JJ. Successful removal of an entire lung for carcinoma of the bronchus. JAMA 1933;101:1371-4. [Crossref] [PubMed]
- Soror T, Kovacs G, Furschke V, et al. Salvage treatment with sole high-dose-rate endobronchial interventional radiotherapy (brachytherapy) for isolated endobronchial tumor recurrence in non-small-cell lung cancer patients: a 20-year experience. Brachytherapy 2019;18:727-32. [Crossref] [PubMed]
- Kobayashi T, Kaneko M, Sumi M, et al. CT-assisted transbronchial brachytherapy for small peripheral lung cancer. Jpn J Clin Oncol 2000;30:109-12. [Crossref] [PubMed]
- Imamura F, Ueno K, Kusunoki Y, et al. High-dose-rate brachytherapy for small-sized peripherally located lung cancer. Strahlenther Onkol 2006;182:703-7. [Crossref] [PubMed]
- Harms W, Krempien R, Grehn C, et al. Electromagnetically navigated brachytherapy as a new treatment option for peripheral pulmonary tumors. Strahlenther Onkol 2006;182:108-11. [Crossref] [PubMed]
- Stewart A, Parashar B, Patel M, et al. American Brachytherapy Society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 2016;15:1-11. [Crossref] [PubMed]
- Ernst A, Feller-Kopman D, Becker HD, et al. Central airway obstruction. Am J Respir Crit Care Med 2004;169:1278-97. [Crossref] [PubMed]
- Casal RF, Walsh G, McArthur M, et al. Bronchoscopic Laser Interstitial Thermal Therapy: An Experimental Study in Normal Porcine Lung Parenchyma. J Bronchology Interv Pulmonol 2018;25:322-9. [Crossref] [PubMed]
- Savic LJ, Chapiro J, Hamm B, et al. Irreversible Electroporation in Interventional Oncology: Where We Stand and Where We Go. Rofo 2016;188:735-45. [Crossref] [PubMed]
- Weaver JC. Electroporation: a general phenomenon for manipulating cells and tissues. J Cell Biochem 1993;51:426-35. [Crossref] [PubMed]
- Wendler JJ, Fischbach K, Ricke J, et al. Irreversible Electroporation (IRE): Standardization of Terminology and Reporting Criteria for Analysis and Comparison. Pol J Radiol 2016;81:54-64. [Crossref] [PubMed]
- Kodama H, Vroomen LG, Ueshima E, et al. Catheter-based endobronchial electroporation is feasible for the focal treatment of peribronchial tumors. J Thorac Cardiovasc Surg 2018;155:2150-9.e3. [Crossref] [PubMed]
- Usman M, Moore W, Talati R, et al. Irreversible electroporation of lung neoplasm: a case series. Med Sci Monit 2012;18:CS43-7. [Crossref] [PubMed]
- Ricke J, Jurgens JH, Deschamps F, et al. Irreversible electroporation (IRE) fails to demonstrate efficacy in a prospective multicenter phase II trial on lung malignancies: the ALICE trial. Cardiovasc Intervent Radiol 2015;38:401-8. [Crossref] [PubMed]
- Mir LM, Orlowski S, Belehradek J Jr, et al. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur J Cancer 1991;27:68-72. [Crossref] [PubMed]
- Orlowski S, Belehradek J Jr, Paoletti C, et al. Transient electropermeabilization of cells in culture. Increase of the cytotoxicity of anticancer drugs. Biochem Pharmacol 1988;37:4727-33. [Crossref] [PubMed]
- Jahangeer S, Forde P, Soden D, et al. Review of current thermal ablation treatment for lung cancer and the potential of electrochemotherapy as a means for treatment of lung tumours. Cancer Treat Rev 2013;39:862-71. [Crossref] [PubMed]
- Li X, Xu K, Li W, et al. Immunologic response to tumor ablation with irreversible electroporation. PLoS One 2012;7:e48749 [Crossref] [PubMed]
- Narayanan JSS, Ray P, Hayashi T, et al. Irreversible Electroporation Combined with Checkpoint Blockade and TLR7 Stimulation Induces Antitumor Immunity in a Murine Pancreatic Cancer Model. Cancer Immunol Res 2019;7:1714-26. [Crossref] [PubMed]
- Witt CM, Sugrue A, Padmanabhan D, et al. Intrapulmonary Vein Ablation Without Stenosis: A Novel Balloon-Based Direct Current Electroporation Approach. J Am Heart Assoc 2018;7:e009575 [Crossref] [PubMed]
- Izzo F, Ionna F, Granata V, et al. New Deployable Expandable Electrodes in the Electroporation Treatment in a Pig Model: A Feasibility and Usability Preliminary Study. Cancers (Basel) 2020;12:515. [Crossref] [PubMed]
- Khan F, Anker CJ, Garrison G, et al. Endobronchial ultrasound-guided transbronchial needle injection for local control of recurrent non-small cell lung cancer. Ann Am Thorac Soc 2015;12:101-4. [Crossref] [PubMed]
- Mori V, Roy GS, Bates JHT, et al. Cisplatin Pharmacodynamics Following Endobronchial Ultrasound-Guided Transbronchial Needle Injection into Lung Tumors. Sci Rep 2019;9:6819. [Crossref] [PubMed]
- Celikoğlu F, Celikoglu SI. Intratumoural chemotherapy with 5-fluorouracil for palliation of bronchial cancer in patients with severe airway obstruction. J Pharm Pharmacol 2003;55:1441-8. [Crossref] [PubMed]
- Celikoglu F, Celikoglu SI, Goldberg EP. Bronchoscopic intratumoral chemotherapy of lung cancer. Lung Cancer 2008;61:1-12. [Crossref] [PubMed]
- Hohenforst-Schmidt W, Zarogoulidis P, Darwiche K, et al. Intratumoral chemotherapy for lung cancer: re-challenge current targeted therapies. Drug Des Devel Ther 2013;7:571-83. [PubMed]
- Seymour CW, Krimsky WS, Sager J, et al. Transbronchial needle injection: a systematic review of a new diagnostic and therapeutic paradigm. Respiration 2006;73:78-89. [Crossref] [PubMed]
- Griscelli F, Opolon P, Saulnier P, et al. Recombinant adenovirus shedding after intratumoral gene transfer in lung cancer patients. Gene Ther 2003;10:386-95. [Crossref] [PubMed]
- Neyns B, Noppen M. Intratumoral gene therapy for non-small cell lung cancer: current status and future directions. Monaldi Arch Chest Dis 2003;59:287-95. [PubMed]
- Weill D, Mack M, Roth J, et al. Adenoviral-mediated p53 gene transfer to non-small cell lung cancer through endobronchial injection. Chest 2000;118:966-70. [Crossref] [PubMed]
- Mehta HJ, Begnaud A, Penley AM, et al. Treatment of isolated mediastinal and hilar recurrence of lung cancer with bronchoscopic endobronchial ultrasound guided intratumoral injection of chemotherapy with cisplatin. Lung Cancer 2015;90:542-7. [Crossref] [PubMed]
- Hayata Y OK, Ogava I, et al. Immunotherapy for lung cancer cases using BCG and BCG cell-wall skeleton. Gann Monogr Cancer Res 1978;21:151-60.
- Matthay RA, Mahler DA, Beck GJ, et al. Intratumoral Bacillus Calmette-Guerin immunotherapy prior to surgery for carcinoma of the lung: results of a prospective randomized trial. Cancer Res 1986;46:5963-8. [PubMed]
- Steinfort DP, Herth FJF. Bronchoscopic treatments for early-stage peripheral lung cancer: Are we ready for prime time? Respirology 2020;25:944-52. [Crossref] [PubMed]
- Abi-Jaoudeh N, Mielekamp P, Noordhoek N, et al. Cone-beam computed tomography fusion and navigation for real-time positron emission tomography-guided biopsies and ablations: a feasibility study. J Vasc Interv Radiol 2012;23:737-43. [Crossref] [PubMed]
- Youssef G, Wallace WA, Dagleish MP, et al. Ovine pulmonary adenocarcinoma: a large animal model for human lung cancer. ILAR J 2015;56:99-115. [Crossref] [PubMed]
- Hellmann MD, Nabet BY, Rizvi H, et al. Circulating Tumor DNA Analysis to Assess Risk of Progression after Long-term Response to PD-(L)1 Blockade in NSCLC. Clin Cancer Res 2020;26:2849-58. [Crossref] [PubMed]
- Szyszko TA, Yip C, Szlosarek P, et al. The role of new PET tracers for lung cancer. Lung Cancer 2016;94:7-14. [Crossref] [PubMed]
- Zhou J, Huang Q, Huang Z, et al. Combining immunotherapy and radiotherapy in lung cancer: a promising future? J Thorac Dis 2020;12:4498-503. [Crossref] [PubMed]


