
A nomogram model based on peripheral blood lymphocyte subsets to assess the prognosis of non-small cell lung cancer patients treated with immune checkpoint inhibitors
Introduction
Lung cancer is one of the most common malignant tumors and the major cause of cancer-related death in the world (1). Lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) according to tissue type, with NSCLC accounting for about 85% of all lung cancer cases (1). Clinically, only a few patients with NSCLC can be diagnosed in the early stage (stage I or II), which allows treatment by surgical resection (2). However, more than 60% of NSCLC patients reach advanced stages or distant metastasis (stage III or IV) at the time of diagnosis, thus surgical resection may no longer be an option (2). Although many advances have been made in the clinical diagnosis and treatment of NSCLC in recent years, such as targeted therapy (3), the 5-year overall survival (OS) rate of NSCLC patients is still less than 20% (3,4). Therefore, it is urgent to improve the prognosis of NSCLC patients with novel therapies.
Immunotherapy is one of the most effective treatments for cancer. It acts through enhancing antitumor immune response and has also been applied to NSCLC treatment (5-7). Immune checkpoint inhibitors (ICIs) have been used in immunotherapy of NSCLC (8,9), with the most commonly used type being, monoclonal anti-programmed death receptor-1 (PD-1) antibody or ligand (PD-L1) antibody (e.g., nivolumab, pembrolizumab, atezolizumab) (10-13). Although no head-to-head clinical trials have been conducted, PD-1 inhibitors appear to be slightly superior to PD-L1 inhibitors in NSCLC (14-17). On the contrary, PD-L1 inhibitors have shown significant advantages over PD-1 inhibitors in SCLC and have been approved for first-line therapy (18). PD-1 is usually expressed on activated T cells and B cells, while numerous cancers including NSCLC express PD-L1 (19,20). The binding of PD-L1 to PD-1 suppresses tumor killing by abolishing effector T cell functioning via restricting T cell proliferation, migration, and release of cytokines (21). Monoclonal anti-PD-1 antibody blocks the association of PD-L1 on the surface of tumor cells with the PD-1 on activated T cells and B cells to enhance tumor cell killing by immune cells (8).
Growing evidence has revealed the predictive and prognostic value of peripheral blood lymphocyte subsets in patients with cancers. For instance, levels of CD4+ and CD3+ lymphocyte subsets in peripheral blood are potent predictive and prognostic indicators in patients with metastatic breast cancer (22). Besides, natural killer (NK) cell percentage in circulating blood has been identified to be a predictor of survival in colorectal cancer patients (23). Moreover, nomogram based on lymphocyte-to-monocyte ratio in peripheral blood could predict survival in patients with stage I NSCLC (24). In addition, PD1-positive CD4+ T cell count in peripheral blood can predict PFS in NSCLC patients receiving treatment with ICIs (25). However, the predictive and prognostic value of other peripheral blood lymphocyte subsets in NSCLC patients treated with ICIs remains unknown.
The primary aim of this study was thus to investigate the predictive and prognostic value of peripheral blood lymphocyte subsets in NSCLC patients treated with ICIs.
We present the following article in accordance with the TRIPOD reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-899).
Methods
Enrolment to the study
The flowchart was shown in Figure S1. This analysis included 82 patients diagnosed with stage IIIB–IV NSCLC who were treated at Zhejiang Cancer Hospital from August 2018 to November 2020. The brief inclusion criteria were as follows: patients older than 18 but younger than 75 years, an expected survival time of more than 12 weeks, histologically or cytologically confirmed stage IIIB–IV, ECOG performance status (PS) 0–2, and expected treatment with ICIs. The exclusion criteria were the following: patients with symptomatic central nervous system metastasis; lactating women; patients with active infection requiring systemic therapy; patients who could receive chest radiotherapy; radiotherapy and chemotherapy performed within 4 weeks before ICI treatment; patients with autoimmune disease that has required systemic treatment or any other disease requiring steroid therapy or immunosuppressive therapy within 7 days prior the first dose of ICIs; and patients who did not comply with the requirements of the study, obviously violated the protocol, or switched to other treatments midway. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All experimental procedures involving human participants were approved by the Ethics Committee of Zhejiang Cancer Hospital. Written informed consent was obtained from all participants enrolled in this study. Clinical data of patients were collected, including pathological classification, tumor-node-metastasis (TNM) staging, pathological diagnosis, survival time, treatment information, and others. Routine follow-up examinations including CT scan was conducted every 3 months.
Treatment
The patients who received any ICI agent as monotherapy or in combination with chemotherapy regardless of treatment line were permitted to enroll to this study. The choice of chemotherapy regimen was based on generally accepted standards of clinical practice.
ICI regimens used of patients were included pembrolizumab (n=23, 28.0%), nivolumab (n=9, 11.0%), sintilimab (n=30, 36.6%), carrelizumab (n=4, 4.9%) and treprizumab (n=16, 19.5%).
The analysis of blood samples
Five mL of peripheral venous blood were collected from 82 patients with advanced NSCLC just 1 day before ICI treatment. Only 47 of the patients were willing to collect another 5 mL of peripheral venous blood at the time of the best curative effect. Next, 50 µL of anticoagulant whole blood was added into the test tube followed by 20 µL of premixed monoclonal antibody (Beckman Coulter, Brea, CA, USA) and homotypic control and incubated for 15 minutes at room temperature (RT) in the dark. Subsequently, the blood samples were treated by 500 µL of hemolysin for 15 minutes at RT in the dark. Next, 1 mL of sheath solution was added to stop hemolysis. Blood samples were then centrifuged for 5 minutes at 1,000 R/min, and supernatants were discarded. After being by phosphate buffer once, cells were resuspended in 500 µL of sheath solution and analyzed by a Beckman Coulter Cytomics FC500 Flow Cytometer.
Flow cytometry
Flow cytometry was performed as described previously (26) to detect lymphocyte subsets, including CD3+ T cells, CD3+CD4+ T cells, CD3+CD8+ T cells, CD4/CD8 ratio, NK cells (CD3−CD56+), B cells (CD19+), natural killer T (NKT) cells (CD3−CD56+), Ts cells (CD4+CD45RA+), helper T (Th) cells (CD4+CD45RA+), memory T cells (CD4+CD45RO+), activated T cells (CD45RA+CD45RO+). and activated CD8+ cells (CD8+CD38+). Antibodies used for flow cytometry were obtained from BD Biosciences (San Jose, CA, USA) and included CD3-FITC (#555332), CD4-FITC (#550628), CD8-FITC (#555366), CD19-FITC (#555412), CD56-PE (#55664), CD45RO-APC (#559865), CD45RA-PE (#555489), CD38-PE (#555460), FITC/PE/APC isotype controls (#555748; #555749; #555576). Cell Quest software (BD, Franklin Lakes, NJ, USA) was used to analyze the proportion of peripheral blood lymphocyte subsets.
Statistical analysis
Statistical analyses were performed using SPSS (version 21.0, IBM Corp., Armonk, NY, USA) and R (version 3.5.1, The R Foundation for Statistical Computing). All statistical tests were two-sided, and P values <0.05 were considered statistically significant. A correlation heat map was utilized to identify the association between the percentage of peripheral blood lymphocyte subsets and clinicopathological features. Besides, the compare of continuous variables between two groups was carried out using Mann-Whitney U test and the Student’s t-test. Moreover, the statistical differences among three or more groups were examined by Kruskal-Wallis test. Response Evaluation Criteria in Solid Tumors Version 1.1 (RECIST 1.1) was used to evaluate tumor response. Furthermore, the Kaplan-Meier curve and the log-rank test were performed to determine the differences in the survival rates between two groups. What’s more, progression-free survival (PFS) was calculated from the date of advanced disease diagnosis to the date of NSCLC recurrence or metastasis, while OS was determined from the date of advanced disease diagnosis to the date of death or censored at the date of the last follow-up. A univariate Cox regression analysis was performed based on the patients’ clinicopathological features and percentage of peripheral blood lymphocyte subsets in NSCLC patients. Finally, a nomogram was established according to the forward stepwise logistic regression analyses performed to evaluate prognostic factors for survival probability and survival time of NSCLC patients treated with ICIs.
Results
Characteristics of the patients
The clinicopathological features of 82 NSCLC patients are described in Table 1. Briefly, 16 (19.5%) female patients and 66 (80.5%) male patients were enrolled with a median age of 61.5 (range, 35–78) years. Among these NSCLC patients, 55 (67.1%) patients and 27 (32.9%) patients had a history of tobacco smoking and alcohol drinking, respectively. Moreover, the ECOG PS of 62 (75.6%) patients was 1. A total of 36 (43.9%) patients were diagnosed with adenocarcinoma while 38 (46.3%) patients were diagnosed with squamous cell carcinoma. Furthermore, 11 (13.4%) patients were diagnosed with stage IIIB–IIIC disease, and 71 (86.6%) with stage IV disease. All patients had received chemotherapy, while 42 (51.2%) patients received radiotherapy before ICI treatment. Additionally, 19 (23.2%) patients had developed brain metastases before ICI treatment. NSCLC patients. Only 28 (34.1%) patients received first-line therapy before ICI treatment. For ICI treatment, 27 (32.9%) patients received monotherapy and 55 (67.1%) patients were treated with combined immunotherapy and chemotherapy.
Table 1
| Characteristics | Number (%) |
|---|---|
| Age (years) | |
| <65 | 46 (56.1) |
| ≥65 | 36 (43.9) |
| Gender | |
| Female | 16 (19.5) |
| Male | 66 (80.5) |
| Smoking | |
| Yes | 55 (67.1) |
| No | 27 (32.9) |
| Drinking | |
| Yes | 27 (32.9) |
| No | 55 (67.1) |
| ECOG PS | |
| 0 | 19 (23.2) |
| 1 | 62 (75.6) |
| 2 | 1 (1.2) |
| Pathology | |
| Adenocarcinoma | 36 (43.9) |
| Squamous | 38 (46.3) |
| Other | 8 (9.8) |
| Stage | |
| IIIB–IIIC | 11 (13.4) |
| IV | 71 (86.6) |
| Chemotherapy | |
| Yes | 82 (100.0) |
| No | 0 (0.0) |
| Radiotherapy | |
| Yes | 42 (51.2) |
| No | 40 (48.8) |
| Brain metastases | |
| Yes | 19 (23.2) |
| No | 63 (76.8) |
| Therapeutic regimen for ICI treatment | |
| Monotherapy | 27 (32.9) |
| Combined therapy | 55 (67.1) |
| First-line therapy | |
| Yes | 28 (34.1) |
| No | 54 (65.9) |
| EGFR status | |
| Wildtype | 67 (81.7) |
| Mutation | 15 (18.3) |
NSCLC, non-small cell lung cancer; PS, performance status; ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor.
For epidermal growth factor receptor (EGFR) status, 67 (81.7%) patients had wildtype EGFR while 15 (18.3%) patients had mutated EGFR. As of the last follow‐up period on April 5, 2021, 41 (50.0%) patients had died.
Efficacy of immunotherapy in NSCLC patients
The analysis showed that confirmed objective response occurred in 27 patients NSCLC patients with immunotherapy [partial response (PR), n=27; stable disease (SD), n=41; progressive disease (PD), n=14], and the objective response rate (ORR) was 32.93%. Besides, the disease control rate (DCR) was 82.93% in NSCLC patients treated with immunotherapy.
Changes of peripheral blood lymphocyte subsets in NSCLC patients after ICI treatment
The proportion of peripheral blood lymphocyte subsets is shown in Figure 1 and Table 2. Results indicated that the CD4/CD8 ratio (P=0.036) and the percentage of B cells (P=0.024) was decreased after ICI treatment (Figure 1 and Table 2). The median of CD4/CD8 ratio and B cells decreased within the normal range after ICI treatment (Figure 1 and Table 2). These results suggested that ICI treatment led to changes in the composition of peripheral blood lymphocyte subsets.
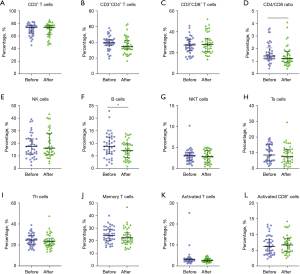
Table 2
| Lymphocyte subset | Percentage of NSCLC patients (median) | P value | |
|---|---|---|---|
| Before | After | ||
| CD3+ T cells | 71.6 | 74.0 | 0.860 |
| CD3+CD4+ T cells | 38.0 | 35.7 | 0.122 |
| CD3+CD8+ T cells | 26.5 | 27.4 | 0.194 |
| CD4/CD8 ratio | 1.3 | 1.2 | 0.036 |
| NK cells | 17.9 | 16.7 | 0.196 |
| B cells | 8.3 | 7.0 | 0.024 |
| NKT cells | 3 | 2.6 | 0.807 |
| Ts cells | 7.9 | 7.7 | 0.143 |
| Th cells | 24.3 | 22.2 | 0.416 |
| Memory T cells | 24.5 | 23.5 | 0.275 |
| Activated T cells | 3.5 | 2.8 | 0.205 |
| Activated CD8 cells | 6.2 | 6.8 | 0.503 |
NSCLC, non-small cell lung cancer; NK, natural killer; NKT, natural killer T; Th, helper T.
Correlation between peripheral blood lymphocyte subset percentage and clinicopathological features before ICI treatment
Correlation analysis demonstrated that the percentage of CD3+ T cells in NSCLC patients was negatively correlated with age (r=−0.36) and positively correlated with brain metastases (r=0.28) before ICI treatment (Figure 2). The proportion of CD3+CD4+ T cells in NSCLC patients was negatively correlated with EGFR status (r=−0.22) before ICI treatment (Figure 2). Furthermore, the CD4/CD8 ratio in NSCLC patients was positively correlated to pathology (r=0.24) before ICI treatment (Figure 2). The percentage of NK cells in NSCLC patients was positively correlated to age (r=0.48) but negatively correlated to brain metastases (r=−0.24) before ICI treatment (Figure 2). Meanwhile, the proportion of B cells in NSCLC patients was negatively correlated to age (r=−0.3), gender (r=−0.27), smoking (r=−0.27), and drinking (r=−0.28) but positively correlated with the therapeutic regimen (r=0.23) before ICI treatment (Figure 2). More importantly, the ratio of NKT cells in NSCLC patients was positively correlated to brain metastasis (r=0.24) and radiotherapy (r=0.34). The percentage of activated T cells and activated CD8+ cells was positively correlated to drinking (r=0.22) and age (r=−0.44) before ICI treatment, respectively (Figure 2).
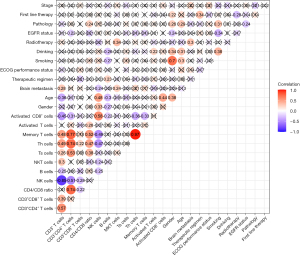
The above results suggested that the percentage of CD3+ T cells, NK cells, and NKT cells in NSCLC patients before ICI treatment might be potential predictors for brain metastases, while the proportion of CD3+CD4+ T cells in NSCLC patients before ICI treatment might be a potential indicator for EGFR status. Moreover, the CD4/CD8 ratio in NSCLC patients before ICI treatment might be correlated with pathology. Furthermore, the ratio of B cells in NSCLC patients before ICI treatment might be affected by the therapy used before ICI treatment, while the percentage of NKT cells in NSCLC patients before ICI treatment may corelate with previous use of radiotherapy.
Correlation between the change of peripheral blood lymphocyte subset percentage and clinicopathological features after ICI treatment
The change of peripheral blood lymphocyte subset percentage after ICI treatment might be affected by clinicopathological features. Results indicated that changes in the percentage of CD3+CD8+ T cells, B cells, and activated CD8+ cells were related to age (Figure 3 and Table 3). Compared with NSCLC patients over the age of 65 years, the percentage of CD3+CD8+ T cells and activated CD8+ cells increased, while the ratio of B cells decrease in NSCLC patients under the age of 65 years after ICI treatment (Figure 3 and Table 3). Moreover, the change in the percentage of NKT cells was related to radiotherapy (Figure 3 and Table 3). Compared with NSCLC patients treated without radiotherapy, the percentage of NKT cells decreased in NSCLC patients receiving radiotherapy after ICI treatment (Figure 3 and Table 3). Also, the change in the percentage of CD3+CD4+ T cells was related to first-line therapy (Figure 3 and Table 3). Compared with NSCLC patients treated without first-line therapy, NSCLC patients receiving first-line therapy after ICI treatment had an increased percentage of CD3+CD4+ T cells (Figure 3 and Table 3). These results suggested that age, radiotherapy, and first-line therapy might affect the change of peripheral blood lymphocyte subset percentage after ICI treatment.

Table 3
| Variables | Age | Radiotherapy | First-line therapy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| z (after-before) | P | z (after-before) | P | z (after-before) | P | ||||||
| ≥65 | <65 | No | Yes | No | Yes | ||||||
| ΔCD3+ T cells | −2.20 | 1.00 | 0.139 | −1.55 | 1.00 | 0.179 | −1.00 | −0.80 | 0.173 | ||
| ΔCD3+CD4+ T cells | 1.85 | −1.70 | 0.358 | −3.30 | 0.70 | 0.058 | −3.90 | 1.70 | 0.014 | ||
| ΔCD3+CD8+ T cells | −2.35 | 3.80 | 0.004 | −0.50 | 1.60 | 0.845 | 2.05 | 0.20 | 0.171 | ||
| ΔCD4/CD8 ratio | 0.14 | −0.26 | 0.126 | −0.17 | −0.20 | 0.732 | −0.25 | 0.06 | 0.088 | ||
| ΔNK cells | 2.40 | 0.80 | 0.756 | 1.90 | 0.80 | 0.366 | 1.80 | 0.00 | 0.297 | ||
| ΔB cells | 0.45 | −1.70 | 0.005 | −1.95 | −1.20 | 0.715 | −0.80 | −2.20 | 0.057 | ||
| ΔNKT cells | −0.60 | −0.10 | 0.235 | 0.70 | −0.50 | 0.010 | −0.20 | −0.30 | 0.634 | ||
| ΔTs cells | 0.10 | −1.10 | 0.069 | 0.05 | −1.10 | 0.824 | −0.60 | −0.40 | 0.825 | ||
| ΔTh cells | −1.00 | 0.50 | 0.318 | −1.25 | 0.90 | 0.282 | −1.00 | 0.70 | 0.138 | ||
| ΔMemory T cells | −0.60 | 0.40 | 0.268 | −1.45 | 0.80 | 0.193 | −0.85 | 0.00 | 0.123 | ||
| ΔActivated T cells | −0.20 | −0.20 | 0.543 | −0.20 | −0.20 | 0.472 | −0.40 | 0.00 | 0.155 | ||
| ΔActivated CD8 cells | −1.90 | 1.60 | 0.007 | 0.45 | 1.00 | 0.708 | 1.30 | 0.40 | 0.699 | ||
ICI, immune checkpoint inhibitor; NK, natural killer; NKT, natural killer T; Th, helper T.
Correlation between clinicopathological features or peripheral blood lymphocyte subsets and survival
The median PFS was 8.5 (range, 6.3–10.8) months (Table 4). However, all percentages of peripheral blood lymphocyte subsets before ICI treatment were not prognostic factors for PFS in NSCLC patients with ICI treatment (Table 5). Analysis found that the median OS in NSCLC patients with immunotherapy was 18.4 (range, 10.8–37.9) months. Univariate analysis indicated that age (P=0.023), physical status score (P=0.006), therapeutic regimen (P=0.004; Table 6), and the percentage of B cells (P=0.04) before ICI treatment were independent prognostic factors for OS in NSCLC patients with ICI treatment (Table 7). To further determine the prognostic value of peripheral blood lymphocyte subsets in NSCLC patients treated with ICIs, survival analysis was performed. Results revealed that NSCLC patients with a low percentage of B cells had shorter OS than did those with a high percentage of B cells before ICI treatment (Figure 4), which was consistent with the result of univariate Cox regression analyses. Thus, the percentage of B cells before ICI treatment level in tumor tissue were related to long-term survival in NSCLC patients treated with ICIs.
Table 4
| Characteristics | P value | HR | 95% CI |
|---|---|---|---|
| Age (years) | |||
| <65 | – | – | – |
| ≥65 | 0.318 | 1.29 | 0.78–2.12 |
| Gender | |||
| Female | – | – | – |
| Male | 0.886 | 0.95 | 0.51–1.79 |
| Smoking | |||
| No | – | – | – |
| Yes | 0.737 | 1.09 | 0.65–1.85 |
| Drinking | |||
| No | – | – | – |
| Yes | 0.20 | 0.71 | 0.42–1.20 |
| ECOG PS | |||
| 0 | – | – | – |
| 1 | 0.18 | 1.52 | 0.82–2.82 |
| 2 | 0.254 | 3.31 | 0.42–25.98 |
| Pathology | |||
| Adenocarcinoma | – | – | – |
| Squamous | 0.457 | 1.22 | 0.73–2.04 |
| Other | 0.593 | 0.75 | 0.26–2.15 |
| Stage | |||
| IIIB–IIIC | – | – | – |
| IV | 0.23 | 0.65 | 0.32–1.32 |
| Radiotherapy | |||
| No | – | – | – |
| Yes | 0.897 | 0.97 | 0.59–1.59 |
| Brain metastases | |||
| No | – | – | – |
| Yes | 0.479 | 0.82 | 0.47–1.43 |
| Therapeutic regimen for ICI treatment | |||
| Monotherapy | – | – | – |
| Combined therapy | 0.256 | 0.74 | 0.44–1.24 |
| First-line therapy | |||
| No | – | – | – |
| Yes | 0.247 | 1.37 | 0.80–2.34 |
| EGFR status | |||
| Wildtype | – | – | – |
| Mutation | 0.741 | 1.11 | 0.59–2.09 |
PFS, progression-free survival; PS, performance status; ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; HR, hazard ratio; CI, confidence interval.
Table 5
| Lymphocyte subset | P value | HR | 95% CI |
|---|---|---|---|
| CD3+ T cells | |||
| High | – | – | – |
| Low | 0.50 | 0.86 | 0.52–1.41 |
| CD3+CD4+ T cells | |||
| High | – | – | – |
| Low | 0.20 | 1.35 | 0.82–2.22 |
| CD3+CD8+ T cells | |||
| High | – | – | – |
| Low | 0.80 | 0.93 | 0.57–1.53 |
| CD4/CD8 ratio | |||
| High | – | – | – |
| Low | 0.50 | 1.18 | 0.72–1.94 |
| NK cells | |||
| High | – | – | – |
| Low | 0.80 | 0.93 | 0.56–1.52 |
| B cells | |||
| High | – | – | – |
| Low | 0.90 | 0.98 | 0.60–1.61 |
| NKT cells | |||
| High | – | – | – |
| Low | 0.40 | 0.81 | 0.49–1.34 |
| Ts cells | |||
| High | – | – | – |
| Low | 0.50 | 0.86 | 0.52–1.42 |
| Th cells | |||
| High | – | – | – |
| Low | 0.60 | 1.15 | 0.70–1.89 |
| Memory T cells | |||
| High | – | – | – |
| Low | 0.80 | 1.08 | 0.66–1.78 |
| Activated T cells | |||
| High | – | – | – |
| Low | 0.50 | 1.20 | 0.73–1.98 |
| Activated CD8 cells | |||
| High | – | – | – |
| Low | 0.60 | 0.87 | 0.53–1.42 |
The median percentage of positive cells was used as a cutoff to define low and high level. PFS, progression-free survival; NK, natural killer; NKT, natural killer T; Th, helper T; HR, hazard ratio; CI, confidence interval.
Table 6
| Characteristics | P value | HR | 95% CI |
|---|---|---|---|
| Age (years) | |||
| <65 | – | – | – |
| ≥65 | 0.023 | 0.43 | 0.21–0.89 |
| Gender | |||
| Female | – | – | – |
| Male | 0.695 | 1.21 | 0.46–3.16 |
| Smoking | |||
| No | – | – | – |
| Yes | 0.476 | 1.34 | 0.60–3.00 |
| Drinking | |||
| No | – | – | – |
| Yes | 0.254 | 1.52 | 0.74–3.10 |
| ECOG PS | |||
| 0 | – | – | – |
| 1 | 0.132 | 2.24 | 0.78–6.44 |
| 2 | 0.006 | 25.28 | 2.51–254.64 |
| Pathology | |||
| Adenocarcinoma | – | – | – |
| Squamous | 0.288 | 1.52 | 0.70–3.28 |
| Other | 0.176 | 2.21 | 0.70–6.98 |
| Stage | |||
| IIIB–IIIC | – | – | – |
| IV | 0.319 | 0.64 | 0.26–1.55 |
| Radiotherapy | |||
| No | – | – | – |
| Yes | 0.268 | 0.67 | 0.33–1.36 |
| Brain metastases | |||
| No | – | – | – |
| Yes | 0.455 | 0.71 | 0.29–1.74 |
| Therapeutic regimen for ICI treatment | |||
| Monotherapy | – | – | – |
| Combined therapy | 0.004 | 0.35 | 0.17–0.71 |
| First-line therapy | |||
| No | – | – | – |
| Yes | 0.269 | 0.63 | 0.28–1.43 |
| EGFR status | |||
| Wildtype | – | – | – |
| Mutation | 0.392 | 0.63 | 0.22–1.81 |
OS, overall survival; PS, performance status; ICI, immune checkpoint inhibitor; EGFR, epidermal growth factor receptor; HR, hazard ratio; CI, confidence interval.
Table 7
| Lymphocyte subset | P value | HR | 95% CI |
|---|---|---|---|
| CD3+ T cells | |||
| High | – | – | – |
| Low | 0.86 | 0.94 | 0.46–1.91 |
| CD3+CD4+ T cells | |||
| High | – | – | – |
| Low | 0.55 | 0.8 | 0.39–1.63 |
| CD3+CD8+ T cells | |||
| High | – | – | – |
| Low | 0.57 | 1.23 | 0.61–2.50 |
| CD4/CD8 ratio | |||
| High | – | – | – |
| Low | 0.82 | 0.92 | 0.45–1.87 |
| NK cells | |||
| High | – | – | – |
| Low | 0.09 | 0.53 | 0.25–1.11 |
| B cells | |||
| High | – | – | – |
| Low | 0.04 | 2.15 | 1.03–4.52 |
| NKT cells | |||
| High | – | – | – |
| Low | 0.99 | 1.01 | 0.50–2.04 |
| Ts cells | |||
| High | – | – | – |
| Low | 0.86 | 0.94 | 0.46–1.91 |
| Th cells | |||
| High | – | – | – |
| Low | 0.80 | 0.91 | 0.45–1.85 |
| Memory T cells | |||
| High | – | – | – |
| Low | 0.48 | 0.78 | 0.38–1.58 |
| Activated T cells | |||
| High | – | – | – |
| Low | 0.37 | 1.38 | 0.68–2.80 |
| Activated CD8 cells | |||
| High | – | – | – |
| Low | 0.22 | 0.64 | 0.31–1.31 |
The median percentage of positive cells was used as a cutoff to define low and high level. OS, overall survival; NK, natural killer; NKT, natural killer T; Th, helper T; HR, hazard ratio; CI, confidence interval.

A nomogram model based on peripheral blood lymphocyte subsets
A nomogram model was developed to assess the survival probability and the expected survival time of NSCLC patients treated with ICIs based on age, percentage of B cells, and therapeutic regimen (Figure 5). As shown in the nomogram, the prognosis of NSCLC patients with a high percentage of B cells was better after immunotherapy (Figure 5). These results were consistent with those of survival analysis, suggesting that the nomogram comprising the percentages of peripheral blood lymphocyte subsets could help to assess the survival probability and the expected survival time of NSCLC patients treated with ICIs.

Discussion
In this study, 82 NSCLC patients who underwent ICI treatment were recruited. Results indicated that the CD4/CD8 ratio and the percentage of B cells were decreased after ICI treatment. In addition, the percentage of CD3+ T cells, NK cells, and NKT cells before ICI treatment was associated with brain metastases; the proportion of CD3+CD4+ T cells before ICI treatment was related to EGFR status; the CD4/CD8 ratio before ICI treatment was correlated to pathology; the ratio of B cells before ICI treatment was related to therapeutic regimen; and the percentage of NKT cells before ICI treatment was associated with radiotherapy. Furthermore, univariate analyses and survival analysis revealed that a low percentage of B cells predicted a poor OS for NSCLC patients with ICI treatment.
In tumors, the activity and function of antitumor T cells or B cells are impaired by the association of PD-L1 on the surface of cancer cells with the PD-1 on T cells and B cells (19,20,27). This study indicated that the proportion of peripheral blood lymphocyte subsets were changed after ICI treatment. For example, the CD4/CD8 ratio was decreased after ICI treatment, suggesting that the level of antitumor CD8+ T effect cells was elevated after ICI treatment. These results suggest that ICI treatment might attenuate the impairment of the immune system in NSCLC patients through blocking PD-L1. Similarly, some studies have demonstrated the effects of ICIs on lymphocyte subsets. For example, ICIs restore the function of tissue-resident memory T cells in tumor immune surveillance (28). Besides, PD-1-PD-L1 blockade suppresses the escape of solid cancer cells from B-cell-mediated cytotoxicity (29). Yet, the percentage of B cells was also reduced after ICI treatment in the present study. B cells are usually recognized as the major effector cells in killing cancer cells (30,31). However, some studies have revealed that B cells also play a protumorigenic role. For instance, B cells recruited by hypoxia inducible factor-1 alpha (HIF1α)-suppressed CXCL13 promotes the development of pancreatic neoplasia (32,33). Thus, B cells in NSCLC might exert protumorigenic effects. Furthermore, less activated B cells are required after ICI treatment as the efficiency of B cells is improved.
To date, no studies have revealed the relationship between circulating CD3+ T cells, NK cells, or NKT cells and brain metastases; circulating CD3+CD4+ T cells and EGFR status; CD4/CD8 ratio in peripheral blood and pathology; peripheral blood B cells and therapeutic regimen; or peripheral blood NKT cells and radiotherapy. Thus, this study might provide novel indicators for brain metastases, pathology, therapeutic regimen, and radiotherapy in NSCLC.
In addition, the present study found that the percentage of B cells was negatively correlated with activated CD8+ cells, which exert the immune killing effect on tumor cells (34). The percentage of B cells was also negatively correlated to age. A growing body of evidence indicates that immunity decreases with age (35). Therefore, the above studies suggest that B cells may suppress activated CD8+ cells to kill tumor cells in NSCLC patients with ICI treatment but may decrease with age. This result suggests that B cells may play a protumorigenic role in younger NSCLC patients. By contrast, the percentage of NK cells was positively correlated to activated CD8+ cells and age. Thus, NK cells may collaborate with activated CD8+ cells to kill tumor cells in NSCLC patients with ICI treatment and may increase with age, and the percentage of NK cells might be a potential indicator for the killing efficiency of ICI treatment in older patients.
Numerous studies have indicated the prognostic value of peripheral blood lymphocyte subsets in cancer patients (36-38). However, the prognostic roles of peripheral blood lymphocyte subsets in NSCLC patients treated with ICIs is unclear. This study demonstrated that the percentage of B cells before ICI treatment was an independent prognostic factor for OS in NSCLC patients with ICI treatment. Despite this, all percentages of peripheral blood lymphocyte subsets before ICI treatment were not prognostic factors for PFS in NSCLC patients with ICI treatment. A recent study has found that the percentage of NK cells and the CD4/CD8 ratio in peripheral blood before ICI treatment was associated with PFS in lung cancer patients treated with ICIs (39). Therefore, assessing the correlation between peripheral blood lymphocyte subsets and PFS in NSCLC patients treated with ICIs is needed, which requires the recruiting of more NSCLC patients in future study.
Although this study demonstrated the predictive and prognostic value of peripheral blood lymphocyte subsets in NSCLC patients with ICI treatment, it still had a few limitations. First, the follow-up period (median 18.4 months) of this study was relatively short. Second, the correlation between percentages of peripheral blood lymphocyte subsets after ICI treatment and clinicopathological features, PFS or OS, was not identified. Third, the association between peripheral blood lymphocyte subsets and PFS in NSCLC patients’ needs to be clarified through an analysis of a greater sample of NSCLC patients in future studies.
Conclusions
ICI treatment induces changes in the percentage of peripheral blood lymphocyte subsets, and this may have prognostic value for brain metastases, radiotherapy, EGFR status, pathology, and therapeutic regimen for NSCLC patients treated with ICIs. However, due to the limitations of our study, further research is needed to verify these findings and explore their implications. In addition, a further study to enlarge the sample size and have more robust results for the validation of the nomogram model will be performed.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81802995) and the Zhejiang Province Public Welfare Funds (LGF19H280004).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-899
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-899
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-899). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All experimental procedures involving human participants were approved by the Ethics Committee of Zhejiang Cancer Hospital. Written informed consent was obtained from all participants enrolled in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Travis WD, Brambilla E, Burke AP, et al. Introduction to The 2015 World Health Organization Classification of Tumors of the Lung, Pleura, Thymus, and Heart. J Thorac Oncol 2015;10:1240-2. [Crossref] [PubMed]
- Osmani L, Askin F, Gabrielson E, et al. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin Cancer Biol 2018;52:103-9. [Crossref] [PubMed]
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Ge X, Zhang Z, Zhang S, et al. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res 2020;9:2391-400. [Crossref] [PubMed]
- Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 2019;75:39-51. [Crossref] [PubMed]
- Suresh K, Naidoo J, Lin CT, et al. Immune Checkpoint Immunotherapy for Non-Small Cell Lung Cancer: Benefits and Pulmonary Toxicities. Chest 2018;154:1416-23. [Crossref] [PubMed]
- Qu J, Jiang M, Wang L, et al. Mechanism and potential predictive biomarkers of immune checkpoint inhibitors in NSCLC. Biomed Pharmacother 2020;127:109996. [Crossref] [PubMed]
- Lin A, Wei T, Meng H, et al. Role of the dynamic tumor microenvironment in controversies regarding immune checkpoint inhibitors for the treatment of non-small cell lung cancer (NSCLC) with EGFR mutations. Mol Cancer 2019;18:139. [Crossref] [PubMed]
- Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol 2020;21:655-63. [Crossref] [PubMed]
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2019;381:2020-31. [Crossref] [PubMed]
- Herbst RS, Giaccone G, de Marinis F, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med 2020;383:1328-39. [Crossref] [PubMed]
- Hanna NH, Robinson AG, Temin S, et al. Therapy for Stage IV Non-Small-Cell Lung Cancer With Driver Alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol 2021;39:1040-91. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Jotte R, Cappuzzo F, Vynnychenko I, et al. Atezolizumab in Combination With Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results From a Randomized Phase III Trial. J Thorac Oncol 2020;15:1351-60. [Crossref] [PubMed]
- Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019;20:924-37. [Crossref] [PubMed]
- Duan J, Cui L, Zhao X, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2020;6:375-84. [Crossref] [PubMed]
- Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014;94:107-16. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Butte MJ, Keir ME, Phamduy TB, et al. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111-22. [Crossref] [PubMed]
- Yang J, Xu J, E Y, et al. Predictive and prognostic value of circulating blood lymphocyte subsets in metastatic breast cancer. Cancer Med 2019;8:492-500. [Crossref] [PubMed]
- Tang YP, Xie MZ, Li KZ, et al. Prognostic value of peripheral blood natural killer cells in colorectal cancer. BMC Gastroenterol 2020;20:31. [Crossref] [PubMed]
- Gao J, Ren Y, Guo H, et al. A new method for predicting survival in stage I non-small cell lung cancer patients: nomogram based on macrophage immunoscore, TNM stage and lymphocyte-to-monocyte ratio. Ann Transl Med 2020;8:470. [Crossref] [PubMed]
- Inomata M, Kado T, Okazawa S, et al. Peripheral PD1-positive CD4 T-Lymphocyte Count Can Predict Progression-free Survival in Patients With Non-small Cell Lung Cancer Receiving Immune Checkpoint Inhibitor. Anticancer Res 2019;39:6887-93. [Crossref] [PubMed]
- Chen Y, Jin Y, Hu X, et al. Effect of chemoradiotherapy on the proportion of circulating lymphocyte subsets in patients with limited-stage small cell lung cancer. Cancer Immunol Immunother 2021;70:2867-76. [Crossref] [PubMed]
- Huang AH, Wang HB, Wu ZF, et al. Infiltrating regulatory T cells promote invasiveness of liver cancer cells via inducing epithelial-mesenchymal transition. Transl Cancer Res 2019;8:2405-15. [Crossref]
- Mami-Chouaib F, Blanc C, Corgnac S, et al. Resident memory T cells, critical components in tumor immunology. J Immunother Cancer 2018;6:87. [Crossref] [PubMed]
- Goodman A, Patel SP, Kurzrock R. PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev Clin Oncol 2017;14:203-20. [Crossref] [PubMed]
- Tokunaga R, Naseem M, Lo JH, et al. B cell and B cell-related pathways for novel cancer treatments. Cancer Treat Rev 2019;73:10-9. [Crossref] [PubMed]
- Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol Immunol 2017;14:662-74. [Crossref] [PubMed]
- Pylayeva-Gupta Y, Das S, Handler JS, et al. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov 2016;6:247-55. [Crossref] [PubMed]
- Lee KE, Spata M, Bayne LJ, et al. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov 2016;6:256-69. [Crossref] [PubMed]
- Guo Y, Xie YQ, Gao M, et al. Metabolic reprogramming of terminally exhausted CD8+ T cells by IL-10 enhances anti-tumor immunity. Nat Immunol 2021;22:746-56. [Crossref] [PubMed]
- Zhang X, Meng X, Chen Y, et al. The Biology of Aging and Cancer: Frailty, Inflammation, and Immunity. Cancer J 2017;23:201-5. [Crossref] [PubMed]
- Wu Y, Ye S, Goswami S, et al. Clinical significance of peripheral blood and tumor tissue lymphocyte subsets in cervical cancer patients. BMC Cancer 2020;20:173. [Crossref] [PubMed]
- Wang YY, Zhou N, Liu HS, et al. Circulating activated lymphocyte subsets as potential blood biomarkers of cancer progression. Cancer Med 2020;9:5086-94. [Crossref] [PubMed]
- Luo Z, Wang Y, Lou Y, et al. Unfavorable clinical implications of peripheral blood CD44+ and CD54+ lymphocytes in patients with lung cancer undergoing chemotherapy. Int J Biol Markers 2018;33:208-14. [Crossref] [PubMed]
- Li P, Qin P, Fu X, et al. Associations between peripheral blood lymphocyte subsets and clinical outcomes in patients with lung cancer treated with immune checkpoint inhibitor. Ann Palliat Med 2021;10:3039-49. [Crossref] [PubMed]
(English Language Editor: J. Gray)


