
This article has an erratum available at: http://dx.doi.org/10.21037/tlcr-2022-1 the article has been update on 2022-03-21 at here.
Combing stereotactic body radiotherapy with checkpoint inhibitors after oligoprogression in advanced non-small cell lung cancer
Introduction
Lung cancer is the leading cause of cancer incidence and mortality worldwide (1). Programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors have fundamentally changed the first-line treatment pattern for advanced non-small cell lung cancer (NSCLC) (2). Despite the significant progress in checkpoint inhibitors (CPIs) therapy for advanced NSCLC, the majority of patients who have shown initial response to CPIs typically exhibit acquired resistance throughout treatment (3). Among them, 13–56% developed oligoprogression diseases (4-6), other than systematic progression. Patients with oligoprogression may benefit from a combination of systemic therapy and oligoprogression-directed local therapy (7). However, the safety and use of local therapy for oligoprogressed lesions following acquired resistance to CPIs have not been well evaluated.
Oligoprogression is a clinical scenario where patients with solid metastatic tumors initially respond to systemic therapy, but later progress to limited sites. Generally, oligoprogression is defined as an intermediate state between localized primary and multimetastatic cancers in which local therapy could achieve long-term survival or cure, without limiting primary lesions (8). A previous study has reported the efficacy of stereotactic body radiotherapy (SBRT) to treat early-stage lung cancer, and this treatment has become one of the effective treatments for the disease (9).
In addition, several studies have reported the significant efficacy of SBRT in treating pulmonary oligometastasis (10,11). SBRT can deliver high radiation doses to the tumor while minimizing radiation doses to the neighboring normal tissues, resulting in a high local tumor control rate with acceptable toxicity to normal tissues (7). SBRT is now a standard treatment of inoperable stage I lung cancer and advanced lung cancers with brain oligometastases. In addition, SBRT has been reported to be an effective strategy to delay further systemic treatment, especially in cases where oligoprogression has occurred, in other conditions such as lung and prostate cancer (12,13). Moreover, SBRT is active in chemotherapy-resistant diseases, and may enhance the immune response by releasing tumor neoantigens following cell killing, which allows synergistic venture between SBRT and immunotherapeutic approaches (14,15).
However, the efficacy and safety of SBRT for oligoprogressed lesions following acquired resistance to CPIs have not been well documented. In this study, we assessed the outcome of concurrent sequential immunotherapy in patients with advanced NSCLC who received SBRT on oligoprogressed lesions. Our preliminary results were presented at annual meeting of the American Society of Clinical Oncology in 2021 (16). We present the following article in accordance with the STROBE reporting checklist (available at https://dx.doi.org/10.21037/tlcr-21-682).
Methods
Patients characteristics of the study cohort
This is a retrospective study, and data of patients with advanced NSCLC were obtained from the Radiotherapy Center of Jinling Hospital between January 2015 and January 2021. Patients who received SBRT treatment for oligoprogressed lesions following acquired resistance to CPIs (anti-PD-1 or anti-PD-L1) were reviewed continuously. The inclusion criteria were: patients who were diagnosed with advanced NSCLC; at least 2 doses of CPI treatment; oligoprogression (defined as a condition characterized by a progression in a maximum of 2 metastatic sites, new metastases, or existing metastases); experienced objective response [partial (PR)/complete response (CR)] prior to oligoprogression. The exclusion criterions were: patients with systemic progression or who received systemic therapy other than immune CPIs during the development of oligo-progressive disease. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Ethics Committee of Jinling Hospital (number: 2021NZKY-025-01), and the written informed consent was waived for this retrospective analysis.
Stereotactic radiotherapy
Stereotactic Radiotherapy was performed (CyberKnife®, Accuray, Sunnyvale, CA, USA) using technology, which was previously reported by our group (17). To locate lesions in internal moving organs (such as lung, liver, and adrenal glands), one to three gold fiducials were implanted inside or near the tumor to define the tumor position and used for tumor tracking during SBRT. Approximately 1 week after fiducial placement, CT simulation was performed for treatment planning (BrillianceTM Big Bore, Philips, Netherlands). Different methods were used to track the lesions at different sites. Intracranial and head and neck tumors were tracked using six-dimensional skull tracking, and spinal metastases were tracked using the “X sight spine” tracking approach. The lesions of two patients in the upper lung were tracked using the “X sight lung” option. For other internal moving organs, synchronous respiration tracking (Synchrony) was employed to track the movement of the fiducials.
Gross tumor volume (GTV) was defined as the tumor volume delineated on simulation CT imaging and co-registered with MRI scan (for brain metastases) or PET-CT scan (if available). According to the disease site and organs-at-risk, a 1–3 mm margin was added to GTV to form the planning target volume (PTV). The dose was prescribed based on the isodose line and covered the PTV. Stereotactic Radiotherapy was delivered to a total dose of 30 to 50 Gy over 2 to 6 days. The dose equivalence was used as a linear-quadratic model and considered by assuming α/β=10 Gy for the tumor. The biologically effective dose (BED) ranged from 45–124.8 Gy, and the median BED was 64.2 Gy. The dose and fractionation schedules were developed based on the patient’s performance status, tumor size, and location. The dosimetry index of 34 oligoprogression sites during radiosurgery treatment is shown in Table S1.
Endpoints and assessment
Post-oligoprogression progression-free survival (PFS-PO) was defined as the time between the date of treatment of oligoprogression and the date of subsequent radiologic progression or death. Overall survival after oligoprogression (OS-PO) was defined as the time interval between the date of treatment of oligoprogression and the date of death or the last follow-up. Local control (LC) was defined for specific lesions as the time between the date of oligoprogression and subsequent radiologic progression. For previous treatment of CPIs, PFS was defined as the time between the first dose of CPI and the date of radiologic oligoprogression. Overall survival (OS) was defined as the time from the start of therapy with CPIs to death or last follow-up. Further analysis was performed to determine whether clinical variables were associated with prognosis.
Statistical analysis
Kaplan-Meier analysis was used to analyze PFS, PFS-PO, OS, OS-PO and LC. Variables related to clinical outcomes were analyzed using univariable Cox regression models. Comparisons of LC between subgroups of lesions were conducted using Fisher’s exact test. For all the analyses, the two-tailed P value <0.05 was considered statistically significant. All the statistical analyses were conducted using R version 4.0.3 (http://www.r-project.org).
Results
Patients characteristics
We reviewed a total of 177 patients with stage III–IV NSCLC who were treated with immunotherapy and SBRT between January 2015 and January 2021 at the Radiotherapy Center of Jinling Hospital. Among them, 49 received SBRT after immunotherapy. Twenty-five experienced oligoprogression after the initial objective response (PR/CR). Finally, 24 patients completed SBRT after oligoprogression due to acquired resistance were enrolled in our study (Figure 1). Patient characteristics are summarized in Table 1, and details are provided in Table S2. The median follow-up was 28.0 months (range, 12 to 65 months). Among all the patients, 15 (62.5%) were diagnosed with adenocarcinoma, and 20 (83.3%) were with stage IV. The lung immune prognostic index (LIPI) status was good (0) in 14 patients (58.3%) and PD-L1 expression was positive (≥1%, Tumor Proportion Score) in 13 (54.1%) patients. 3 (12.5%), 4 (16.7%) and 5 (20.8%) patients carried mutation of EGFR, KRAS and TP53, respectively.
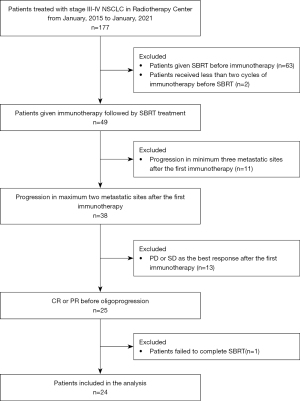
Table 1
| Variable | N (%) |
|---|---|
| Age (years), median (range) | 63 (37–82) |
| Male gender | 17 (71.8) |
| Pathology | |
| Adenocarcinoma | 15 (62.5) |
| Squamous cell carcinoma | 9 (37.5) |
| Stage | |
| III | 4 (16.7) |
| IV | 20 (83.3) |
| ECOG | |
| 0 | 1 (4.2) |
| 1 | 15 (62.5) |
| 2 | 8 (33.3) |
| Smoking History | |
| Never | 11 (45.8) |
| Former | 2 (8.4) |
| Always | 11 (45.8) |
| LIPI | |
| 0 | 14 (58.3) |
| 1 | 8 (33.3) |
| 2 | 2 (8.4) |
| PD-L1 | |
| Positive (≥1%) | 13 (54.1) |
| Negative (<1%) | 10 (41.7) |
| Unknown | 1 (4.2) |
| EGFR | |
| Mutant | 3 (12.5) |
| Wildtype | 17 (70.8) |
| Unknown | 4 (16.7) |
| ALK | |
| Wildtype | 20 (83.3) |
| Unknown | 4 (16.7) |
| KRAS | |
| Mutant | 4 (16.7) |
| Wildtype | 13 (54.1) |
| Unknown | 7 (29.2) |
| TP53 | |
| Mutant | 5 (20.8) |
| Wildtype | 10 (41.7) |
| Unknown | 9 (37.5) |
ECOG, performance score of Eastern Cooperative Oncology Group; LIPI, the lung immune prognostic index.
The main features of immunotherapy and oligoprogression are listed in Table 2. Immunotherapy was used as first-line treatment in 16 (66.7%) patients. Four (16.7%) of patients received monotherapy, the others were combined with chemotherapy (11 patients), anti-angiogenesis (5 patients) and chemotherapy + anti-angiogenesis (4 patients), respectively. Six (25%) and 18 (69.2%) patients achieved CR and PR on immunotherapy, respectively. The 34 oligoprogression sites included brain (n=14, 41.2%), lung (n=10, 29.4%), lymph node (n=5, 14.8%), adrenal gland (n=3, 8.8%), liver (n=1, 2.9%), and cervical vertebra (n=1, 2.9%). After combining SBRT with the continued immunotherapy since oligoprogression, 7 patients added anti-angiogenesis or chemotherapy to the initial treatment. 17 patients (70.8%) received the same treatment as before, 7 (41.2%, 7/17) of them received monotherapy. Among 17 patients with combination strategy after oligoprogression, 1, 3 and 13 patients combined with chemotherapy + anti-angiogenesis, chemotherapy and anti-angiogenesis, respectively.
Table 2
| Variable | N (%) |
|---|---|
| Type of immunotherapy (CPI Strategy) | |
| Monotherapy | 4 (16.7) |
| Combination | 20 (83.3) |
| Lines of immunotherapy before oligoprogression | |
| 1 | 16 (66.7) |
| 2 | 5 (20.8) |
| 3 | 3 (12.5) |
| Response to immunotherapy before oligoprogression | |
| CR | 6 (25.0) |
| PR | 18 (75.0) |
| No. of oligoprogression | |
| 1 | 14 (58.3) |
| 2 | 10 (41.7) |
| Site of oligoprogression | |
| Brain | 14 (41.2) |
| Lung | 10 (29.4) |
| Lymph node | 5 (14.8) |
| Adrenal gland | 3 (8.8) |
| Liver | 1 (2.9) |
| Cervical vertebra | 1 (2.9) |
| Pattern of oligoprogression | |
| New metastasis | 16 (47.1) |
| Existing metastasis | 18 (52.9) |
| Type of immunotherapy after oligoprogression (CPI Strategy-PO) | |
| Monotherapy | 7 (29.2) |
| Combination | 17 (70.8) |
| Change of CPI Strategy | |
| Consistent Strategy (continue previous treatment or maintenance strategy) | 17 (70.8) |
| Monotherapy to combination | 3 (8.8) |
| Adjusted combination | 4 (16.7) |
Values presented are n (%) unless otherwise noted. CPI, checkpoint inhibitor; CR, complete response; PR, partial response; CPI Strategy-PO, strategy of checkpoint inhibitor post oligoprogression.
Outcomes analyses
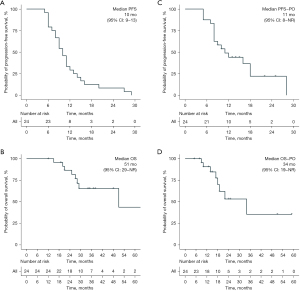
Among the patient experiencing the previous line of immunotherapy, the median PFS was 10 months (95% CI: 9–13) (Figure 2A). The median OS from the first time of immunotherapy was 51 months (95% CI: 29–NA) (Figure 2B). The median PFS-PO and OS-PO post oligoprogression were 11 months (95% CI: 8–NA) and 34 months (95% CI: 19–NA) (Figure 2C,2D). The treatment landscape of each patient is shown in Figure 3. The univariable analysis (Tables S3,S4) showed that pathology of squamous predicts shorter PFS/OS/OS-PO, LIPI (≥1) predicted shorter PFS and OS-PO, and positive PD-L1 at baseline was associated with prolonged OS and OS-PO. Meanwhile, EGFR mutations were associated with a higher risk of PFS-PO. No difference was observed in treatment line and strategy (mono vs. combination) on PFS/PFS-PO/OS/OS-PO. Patients with modified strategy after oligoprogression showed no difference in PFS-PO (16 vs. 11 months, P=0.979) and OS-PO (34 vs. 22 months, P=0.663) compared with those continued with the same treatment or maintenance treatment.

Among all oligoprogression sites, the best response was CR in 11 (32.4%) sites, PR in 17 (50.0%) sites, SD in 3 (8.8%) sites and PD in 3 (8.8%) sites. The median LC was 43 months (95% CI: 7.7–78.3) (Figure 4). The 1-year and 2-year LC rates were 100% and 81.8%, respectively. For the most common observed lesion of brain, the best response was CR in 5 (35.7%) sites, PR in 8 (57.1%) sites, and SD in 1 (7.1%) site. No difference was found in ORR and LC between the brain and non-brain lesions.
Among all the patients who received local therapy at the time of oligoprogression, Grade 3–4 and Grade 1–2 adverse events (AEs) occurred in 5/24 patients and 10/24 patients, respectively (Table 3). 6 patients experienced radiation-related AEs, 5 with grade 1–3 radiation-induced pneumonia, and 1 with grade 3 radiation-induced brain edema.
Table 3
| Adverse event category | CPI based treatment related (n) | RT related (n) | Total (n, %) | |||
|---|---|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |||
| Pneumonia | 0 | 1 | 4 | 1 | 6 (25%) | |
| Kidney damage | 2 | 0 | 0 | 0 | 2 (8.3%) | |
| Hypophysitis | 2 | 0 | 0 | 0 | 2 (8.3%) | |
| Thyroiditis | 2 | 0 | 0 | 0 | 2 (8.3%) | |
| Skin reaction | 1 | 1 | 0 | 0 | 2 (8.3%) | |
| Hypothyroidism | 1 | 0 | 0 | 0 | 1 (4.2%) | |
| Stubborn brain edema | 0 | 0 | 0 | 1 | 1 (4.2%) | |
| Hemoptysis | 0 | 1 | 0 | 0 | 1 (4.2%) | |
CPI, checkpoint inhibitor; RT, radiotherapy.
Discussion
The use of CPIs has significantly improved the outcome of advanced lung cancer patients, especially for the subset of patients with an initial response. However, acquired resistance occurs in most patients, of which oligoprogression is most common (3,5). Currently, there are no standard strategies to overcome acquired resistance, thus exploring potential effective approaches is critical. Several studies have discussed prolonged survival by continuing CPIs beyond progression or combing with local therapy (5,6,18,19). To the best of our knowledge, our study first reported the efficacy and safety of combing SBRT to the ongoing CPIs in NSCLC patients who developed oligoprogression after initial response.
Previous studies have shown that patients with oligoprogression often developed resistance later than (median PFS: 6.4–13 months) those with systemic progression (3,4,6,20). Therefore, our PFS of 10 months before oligoprogression showed a comparable baseline of enrolled patients with previous observations. After acquired resistance (PR/CR to initial CPI treatment), the median OS for all patients neglecting patterns of progression (oligo- or systemic) and following treatment strategies was 18.9 months (3). Specifically, treatment beyond progression (TBP) (after resistance) of CPIs tended to achieve longer OS (12.9–17.8 months) post-progression than those who stopped CPIs immediately after PD (3.7–4.3 months) (18,21). Our OS-PO at 34 months brought a promising survival for patients after progression. Among TBP after oligoprogression, combing with local therapy was often administrated (5,20). However, a comprehensive summary of survival by combing SBRT to CPIs after oligoprogression due to acquired resistance was lacking, thus similar studies were reviewed. Campbell et al. (22) reported a disease control rate at 57.14%, mPFS at 4.1 months and mOS at 7.6 months after combing SBRT with CPIs on one of the progressed sites (≥2 measurable sites) among patients progressed on immunotherapy. Xu et al. (6) reported a PFS2(defined as the time from the first cycle of immunotherapy to the second progression or death) of 15 months and an OS of 26.4 months by combing radiotherapy with CPIs after progression. One difference to the present study is that, patients with best response of stable disease to CPIs were included, its impact on survival post progression is unknown. Our results revealed 11 months’ PFS-PO and 34 months’ OS-PO in oligoprogressed patients after PR/CR to initial CPI treatment. Our results indicate that combing SBRT with CPIs might be a promising strategy to overcome acquired resistance for patients with oligoprogression disease and result in considerable survival benefits.
Meanwhile, consistent with concerns on TBP (6,23), predictive biomarkers were warranted to identify patients more likely to benefit from TBP. Our study confirmed that patients with better LIPI were more likely to benefit from CPI’s (24) (Tables S3,S4), as well as patients with positive PD-L1 expression or adenocarcinoma. Besides, they not only had better benefits with higher PD-L1 expression in the TBP group, but also they tended to develop more oligoprogression than systemic progression compared with lower PD-L1 expression (4). These results suggest that immunologic tumor control could be an essential prerequisite for both occurrence of oligoprogression and benefits for TBP. Furthermore, consistent with a previous study (25), patients with EGFR mutation tented to have poor PFS-PO (mPFS-PO, 8 vs. 12 months). While no difference in PFS, OS and OS-PO induced by EGFR mutation was observed, the small number of 3 EGFR-mutated patients was insufficient to confirm this finding. Notably, we did not observe the difference of PFS/PFS-PO/OS/OS-PO between groups with varying treatment strategies either (monotherapy vs. combination, using a modified strategy or not after oligoprogresssion), the limited sample size might be insufficient to clarify their impact. Analysis with a larger sample size is critical to identify the predive ability to confounding factors.
Our results also achieved considerable benefit of local control (mLC at 43 months, 1- and 2-year LC rates at 100% and 81.8%, respectively), with no difference between intra- and extra-cranial lesions. Since response and local control of specific lesions were barely reported, these results may be helpful for patients and physicians before administration.
Our results might partially attribute the considerable re-response and survival benefits to CPIs after combing SBRT to the synergy of radiotherapy and immunotherapy (26). Resistance of CPIs might be induced by tumor-mediated immunosuppression (27), defects in antigen presentation (28), altered interferon signaling, additional inhibitory checkpoints (29) etc. Radiotherapy has shown potential in reshaping the immune microenvironment (26,30), the mechanisms included triggering type I IFN production, upregulating MHC-I expression, increasing tumor-infiltrating immune cells (22,31,32), etc., thus restored the response to CPIs. Currently, radiotherapy has become a standard intervention for patients with radical lung cancer. It is also widely explored in metastatic cancer, with concurrent systemic therapy or as an additional intervention to overcome progressions (10,11,30,33,34). Our current findings and contemporaneous studies (5,6), have put forward radiotherapy and its potential to derive considerable clinical benefits in patients with oligoprogression after CPIs.
In addition, the toxicity of concurrent radiotherapy is a major concern (35). Among radiotherapies, SBRT enables the delivery of radiation with millimeter precision and allows a high tumoricidal dose with minimizing dose to neighboring tissues. Accumulating data suggested that SBRT and immunotherapy have non-intersecting and complementary toxicity profiles, the reported moderate and significant actual toxicities were rare and relatively safe (30,35). Our study reported that 25% of the patients had radioactive adverse events, and 20.8% developed 3–4 grades of AEs, which were clinically not significant and well managed by symptomatic treatment. The dosage and toxicity profiles were comparable to reports in concurrent SBRT and CPIs therapies (30,34,36), confirming its safety in patients with oligoprogression. Taken together, combing SBRT with CPIs has brought considerable safety and acceptable clinical outcome. Moreover, given the potential synergistic effect with immunotherapy, it might be a promising combing strategy of CPIs for metastatic cancers not restricted to oligoprogression. However, further studies are needed to validate the proper timing, dosages and scenarios.
Meanwhile, the results of present study should be cautiously interpreted due to its intrinsic limitations. First, the retrospective design with a small number of patients might introduce patient selection bias and distort the results estimate. Second, the baseline characterizes are heterogeneous, such as different CPIs and treatment lines. In addition, patients without radiotherapy could not be systematically reviewed due to the radiotherapy limitation in our department. A prospective study design and comparing patients treated with and without SBRT after acquired resistance, as well as a comparison between different combining strategy (with chemotherapy vs. anti-angiogenesis), may comprehensively clarify the benefit of combing SBRT.
In summary, our data preliminarily suggest that combing SBRT to the continuing CPIs in patients with oligoprogression after required resistance on CPIs is feasible and safe and has brought considerable survival benefit. Patients with adenocarcinoma, LIPI (≥1) and positive PD-L1 tended to achieve better survival improvement. Our findings may assist decision-making when continuing CPIs beyond progression and provide a comprehensive reference for the efficacy and safety of combing SBRT. At the same time, further investigations with larger simple size and control cohort are warranted to comprehensively evaluate the clinical outcome and potential predictive biomarkers.
Acknowledgments
We thank Mingzhe Xiao and Chuang Qi from Jiangsu Simcere Diagnostics for their kind assistance.
Funding: This study was supported by grants from Foundation of Nanjing City (No. SZDZK2016012).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://dx.doi.org/10.21037/tlcr-21-682
Data Sharing Statement: Available at https://dx.doi.org/10.21037/tlcr-21-682
Peer Review File: Available at https://dx.doi.org/10.21037/tlcr-21-682
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://dx.doi.org/10.21037/tlcr-21-682). Yong Song serves as an Editor-in-Chief of Translational Lung Cancer Research. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). It was approved by the Ethics Committee of Jinling Hospital (Number: 2021NZKY-025-01), and the written informed consent was waived for this retrospective analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thandra KC, Barsouk A, Saginala K, et al. Epidemiology of lung cancer. Contemp Oncol (Pozn) 2021;25:45-52. [Crossref] [PubMed]
- Sui H, Ma N, Wang Y, et al. Anti-PD-1/PD-L1 Therapy for Non-Small-Cell Lung Cancer: Toward Personalized Medicine and Combination Strategies. J Immunol Res 2018;2018:6984948. [Crossref] [PubMed]
- Schoenfeld AJ, Rizvi H, Memon D, et al. Acquired resistance to PD-1 blockade in NSCLC. J Clin Oncol 2020;38:9621. [Crossref]
- Rheinheimer S, Heussel CP, Mayer P, et al. Oligoprogressive Non-Small-Cell Lung Cancer under Treatment with PD-(L)1 Inhibitors. Cancers (Basel) 2020;12:1046. [Crossref] [PubMed]
- Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical Features and Management of Acquired Resistance to PD-1 Axis Inhibitors in 26 Patients With Advanced Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:831-9. [Crossref] [PubMed]
- Xu Y, Li H, Fan Y. Progression Patterns, Treatment, and Prognosis Beyond Resistance of Responders to Immunotherapy in Advanced Non-Small Cell Lung Cancer. Front Oncol 2021;11:642883. [Crossref] [PubMed]
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 2019;393:2051-8. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol 1995;13:8-10. [Crossref] [PubMed]
- Arcidiacono F, Aristei C, Marchionni A, et al. Stereotactic body radiotherapy for adrenal oligometastasis in lung cancer patients. Br J Radiol 2020;93:20200645. [Crossref] [PubMed]
- Kobayashi N, Abe T, Noda SE, et al. Stereotactic Body Radiotherapy for Pulmonary Oligometastasis from Colorectal Cancer. In Vivo 2020;34:2991-6. [Crossref] [PubMed]
- Klement RJ, Hoerner-Rieber J, Adebahr S, et al. Stereotactic body radiotherapy (SBRT) for multiple pulmonary oligometastases: Analysis of number and timing of repeat SBRT as impact factors on treatment safety and efficacy. Radiother Oncol 2018;127:246-52. [Crossref] [PubMed]
- Mazzola R, Fersino S, Ferrera G, et al. Stereotactic body radiotherapy for lung oligometastases impacts on systemic treatment-free survival: a cohort study. Med Oncol 2018;35:121. [Crossref] [PubMed]
- Triggiani L, Alongi F, Buglione M, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. Br J Cancer 2017;116:1520-5. [Crossref] [PubMed]
- Rodríguez-Ruiz ME, Vanpouille-Box C, Melero I, et al. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol 2018;39:644-55. [Crossref] [PubMed]
- Formenti SC, Rudqvist NP, Golden E, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845-51. [Crossref] [PubMed]
- Wang Z, Li J, Zhou H, et al. Efficacy of stereotactic body radiotherapy for oligoprogression on PD-1 axis inhibitors in advanced non-small cell lung cancer: A single-center retrospective study. J Clin Oncol 2021;39:e21065. [Crossref]
- Li J, Wang Z, Li AM, et al. Analysis of the efficacy, safety and survival factors of stereotactic body radiation therapy in patients with recurrence of pancreatic cancer. Transl Oncol 2020;13:100818. [Crossref] [PubMed]
- Ricciuti B, Genova C, Bassanelli M, et al. Safety and Efficacy of Nivolumab in Patients With Advanced Non–small-cell Lung Cancer Treated Beyond Progression. Clin Lung Cancer 2019;20:178-185.e2. [Crossref] [PubMed]
- Artal-Cortes A, Mazieres J, Fehrenbacher L, et al. Evaluation of non-classical response by immune-modified RECIST and efficacy of atezolizumab beyond disease progression in advanced NSCLC: Results from the randomized Phase II study POPLAR. Anna Oncol 2017;28:ii35. [Crossref]
- Kagawa Y, Furuta H, Uemura T, et al. Efficacy of local therapy for oligoprogressive disease after programmed cell death 1 blockade in advanced non-small cell lung cancer. Cancer Sci 2020;111:4442-52. [Crossref] [PubMed]
- Genova C, Rijavec E, Rossi G, et al. Overall survival (OS) of selected patients (Pts) with non-small cell lung cancer (NSCLC) receiving nivolumab beyond progression. Ann Oncol 2017;28:vi61. [Crossref]
- Campbell AM, Cai WL, Burkhardt D, et al. Final Results of a Phase II Prospective Trial Evaluating the Combination of Stereotactic Body Radiotherapy (SBRT) with Concurrent Pembrolizumab in Patients with Metastatic Non-Small Cell Lung Cancer (NSCLC). Int J Radiat Oncol Biol Phys 2019;105:S36-7. [Crossref]
- Kazandjian D, Keegan P, Suzman DL, et al. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol 2017;44:3-7. [Crossref] [PubMed]
- Ruiz-Bañobre J, Areses-Manrique MC, Mosquera-Martínez J, et al. Evaluation of the lung immune prognostic index in advanced non-small cell lung cancer patients under nivolumab monotherapy. Transl Lung Cancer Res 2019;8:1078-85. [Crossref] [PubMed]
- Lee CK, Man J, Lord S, et al. Checkpoint Inhibitors in Metastatic EGFR-Mutated Non-Small Cell Lung Cancer—A Meta-Analysis. J Thorac Oncol 2017;12:403-7. [Crossref] [PubMed]
- Sharabi AB, Lim M, DeWeese TL, et al. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol 2015;16:e498-509. [Crossref] [PubMed]
- Sharabi AB, Nirschl CJ, Kochel CM, Nirschl TR, Francica BJ, Velarde E, et al. Stereotactic Radiation Therapy Augments Antigen-Specific PD-1-Mediated Antitumor Immune Responses via Cross-Presentation of Tumor Antigen. Cancer Immunol Res 2015;3:345-55. [Crossref] [PubMed]
- Gettinger S, Choi J, Hastings K, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov 2017;7:1420-35. [Crossref] [PubMed]
- Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017;168:707-23. [Crossref] [PubMed]
- Lin AJ, Roach M, Bradley J, et al. Combining stereotactic body radiation therapy with immunotherapy: current data and future directions. Transl Lung Cancer Res 2019;8:107-15. [Crossref] [PubMed]
- Wang X, Schoenhals JE, Li A, et al. Suppression of Type I IFN Signaling in Tumors Mediates Resistance to Anti-PD-1 Treatment That Can Be Overcome by Radiotherapy. Cancer Res 2017;77:839-50. [Crossref] [PubMed]
- McLaughlin M, Patin EC, Pedersen M, et al. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 2020;20:203-17. [Crossref] [PubMed]
- Comito F, Leslie I, Boos L, et al. Oligoprogression After Checkpoint Inhibition in Metastatic Melanoma Treated With Locoregional Therapy: A Single-center Retrospective Analysis. J Immunother 2020;43:250-5. [Crossref] [PubMed]
- Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of Pembrolizumab After Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients With Advanced Non–Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol 2019;5:1276. [Crossref] [PubMed]
- Amin NP, Remick J, Agarwal M, et al. Concurrent Radiation and Immunotherapy: Survey of Practice Patterns in the United States. Am J Clin Oncol 2019;42:208-14. [Crossref] [PubMed]
- Tian S, Switchenko JM, Buchwald ZS, et al. Lung Stereotactic Body Radiation Therapy and Concurrent Immunotherapy: A Multicenter Safety and Toxicity



