
Intermittent chest tube clamping decreases chest tube duration time and drainage volume after lung cancer surgery in patients without air leak: an open-label, randomized controlled trial
Introduction
Chest tube insertion is routinely required after lung surgery to monitor air leaks and hemothorax. Typically, chest tube removal occurs prior to patient discharge. Thus, chest tube duration strongly affects not only the patient’s comfort and mobility but also their length of stay (LOS) (1,2). Further research is required on optimal chest tube management to shorten chest tube use duration and hospital stay, thereby reducing the economic burden placed on the healthcare system without increasing the risk to patients (3,4). Currently, variable chest tube management protocols are being implemented to efficiently drain air and fluid from the chest cavity, including the use of an electronic chest drainage system or more permissive fluid criteria for chest tube withdrawal (5-8).
Provocative clamping has played an important role in the management of difficult or high-risk patients (9,10). In a preliminary study, we modified the chest tube management protocol by combining intermittent chest tube clamping with gravity drainage (11). Using a propensity score matching analysis, our previous retrospective data demonstrated that intermittent chest tube clamping reduced the duration of chest tube drainage and postoperative hospital stay after lung cancer surgery. However, observational researches are prone to various biases and structural limitations, which can be overcome by randomized clinical trials under ideal conditions among highly selected populations.
Therefore, we conducted a randomized clinical trial to determine whether intermittent chest tube clamping decreases chest tube duration and total drainage volume after lung cancer surgery in patients without air leak. We present the following article in accordance with the CONSORT reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-150/rc).
Methods
Patient selection and enrollment
A single-institution, open-label, randomized, parallel, controlled clinical trial (NCT03379350) involving 2 participating thoracic surgeons (N.W. and S.Y.) was conducted. Patients with resectable lung cancer scheduled to undergo lobectomy and systematic mediastinal lymph node dissection/sampling at the Department of Thoracic Surgery II, Peking University Cancer Hospital & Institute were identified as potential candidates for inclusion in the trial. Patients with the following conditions were excluded from the trial because of the dramatic effects of these conditions on chest tube drainage duration: bronchoplasty and/or pulmonary arterioplasty, air leakage, bleeding, chylothorax, atelectasis, liver cirrhosis, renal insufficiency, wound infection, pyrexia, costectomy, or severe subcutaneous emphysema. All patients underwent routine preoperative staging. PET/CT examination should be performed as much as possible. If PET/CT examination cannot be performed, chest computed tomography, brain magnetic resonance imaging, abdominal ultrasonography, and bone scintigraphy can be used instead. Fiberoptic bronchoscopy is recommended for patients with centrally located tumors. Pulmonary function testing and cardiac assessment are mandatory as part of the preoperative evaluation to screen high-risk patients.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of Peking University Cancer Hospital & Institute (No. 2017KT27), and all patients provided informed consent.
Randomization and blinding
The randomization sequence was generated by an independent statistician (Y. Z.) using a computerized system (SAS software, version 9.4; SAS Institute, Cary, NC, USA). A blinded non-investigator (XG.Z.) was responsible for the treatment allocation using sequentially, opaque, sealed envelopes. The thoracic surgeons, sub-investigators, and patients were not blinded due to the nature of the intervention. However, the data collectors and outcome assessors were blinded to group assignment.
Chest tube management protocols
All the patients underwent lateral thoracotomy or video-assisted thoracoscopic surgery. No pleural tents or buttressed staple lines were used in this study. At the end of the operation, the lung parenchyma was submerged in sterile saline to test for air leakage, and a single 24-Fr chest tube was enclosed in each patient. All patients were postoperatively managed with gravity drainage (water seal only, without suction) during the first 12–24 h (depending on the surgery completion time). Once the re-expansion of the lung was confirmed via radiography the morning of postoperative day 1 (POD 1) and no air leak was detected, the patients were randomly assigned (1:1) to intermittent chest tube clamping (the clamping group) or continuous gravity drainage (the control group). In the clamping group, the chest tube was clamped and the patients were checked by nurses every 6 h. If the patients had no compliance issues, their clamps were removed for 30 min the following morning to record the drainage volume; this procedure was repeated every 24 h. The criterion for chest tube removal was a drainage volume ≤250 mL in 24 h.
If intolerable abnormal symptoms occurred after clamping the tube, such as dyspnea, pneumothorax, and severe subcutaneous emphysema, the clamp was removed for 30 minutes. The clamp could be reused after symptoms disappeared. Such patients were placed under more rigorous surveillance after re-clamping, whereby the medical staff checked the patients every 2–4 h to detect abnormal symptoms in time. A radiograph was required to rule out the presence of a pneumothorax if needed. The chest tube management protocols are shown in Figure 1. The criteria for chest tube removal were as follows: (I) drainage volume ≤250 mL in 24 h; (II) an absence of air leakage and intrathoracic hemorrhage; and (III) no signs of purulent pleural effusion and atelectasis.
Follow-up
All clinical data were recorded. Pleural drainage volume was recorded every day after surgery until chest tube was removed. Hemoglobin and serum albumin levels were recorded on POD 1, before discharge, and 1 month after surgery.
Outcomes
The primary outcome in this study was chest tube drainage duration (days from the operation until chest tube removal). The secondary outcomes were total drainage volume (sum of the daily pleural drainage), postoperative hospital stay (days from the operation until discharge), the decrease of serum albumin and hemoglobin levels, and postoperative complications. The rates of tube reinsertion for pneumothorax or delayed pleural effusion, pyrexia and dyspnea were recorded as safety indicators.
Sample size calculation
The minimum required sample size was estimated using the PASS software (version 11.0, NCSS, LLC, Kaysville, UT, USA). The assumed chest tube drainage duration was 3.0±1.0 days in the control group according recent data of our team (unpublished data). A 0.5-day reduction of chest tube duration was considered clinically meaningful in the clamping group. A total of 168 patients were required to achieve a power of 90% and an alpha value of 5%. With an estimated 7% loss, 90 patients were needed per group.
Statistical analysis
The continuous variables with a normal distribution are expressed as mean ± standard deviation, and the continuous variables with non-normal distribution are expressed as the median and interquartile range. The categorical variables are expressed as frequencies and percentages. After confirming homogeneity using the Levene’s test, normally distributed data were analyzed using Student’s t-test. The Mann-Whitney U test was used to analyze non-normally distributed data. Use Pearson’s chi-square (χ2) test or Fisher’s exact test to compare proportions, as needed. The intention-to-treat analysis (ITT) involved all participants who were randomized, and the per-protocol (PP) analysis involved participants who adhered to the assigned chest tube management. When calculating the decrease of plasma albumin and hemoglobin levels, participants with missing data were excluded. All tests of statistical significance were two-sided and the threshold of statistical significance was set at P<0.05. SPSS software (version 26.0, SPSS, Chicago, IL, USA) was used for all analyses.
Results
Patients characteristics
The CONSORT flow diagram is shown in Figure 2. Between July 2017 and May 2021, 209 patients with lung cancer who underwent lobectomy and systematic mediastinal lymph node dissection/sampling became potential candidates for the trial. Twenty-nine patients were excluded before randomization, and a total of 180 participants were randomized to the clamping group (n=90) or the control group (n=90). Of them, 12 were subsequently withdrawn from the study for the following reasons: chylothorax (n=4), air leakage (n=3), pyrexia (n=2), and due to bleeding concerns (n=3). As Table 1 shows, no significant differences in patient characteristics were observed between the 2 groups. Overall, there were 107 (59.4%) females and 73 (40.6%) males in the cohort, with a mean age of 59.1±9.7 years at recruitment. The median chest tube drainage duration was 2 [2, 3] days, and the median postoperative stay was 5 [4, 5] days. The mean pleural drainage volume was 587.3±434.6 mL.
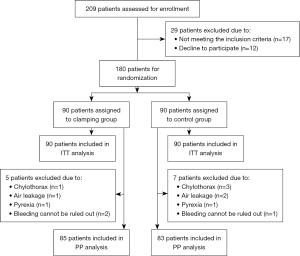
Table 1
| Characteristics | Clamping group (n=90) | Control group (n=90) | P value |
|---|---|---|---|
| Males/females | 39/51 | 34/56 | 0.448 |
| Age, mean ± SD, years | 58.1±9.7 | 60.0±9.5 | 0.175 |
| Left/right | 35/55 | 39/51 | 0.545 |
| Upper/lower* | 55/35 | 49/41 | 0.365 |
| Neoadjuvant chemotherapy, n (%) | 7 (7.8) | 5 (5.6) | 0.550 |
| VATS, n (%) | 81 (90.0) | 80 (88.9) | 0.808 |
| SMLD/SS | 47/43 | 57/33 | 0.131 |
| Pleural adhesion, n (%) | 4 (4.4) | 9 (10.0) | 0.150 |
*, right middle lobectomies were counted as lower. SD, standard deviation; VATS, video-assisted thoracoscopic surgery; SMLD, systematic mediastinal lymph node dissection; SS, systematic mediastinal lymph node sampling.
Primary and secondary outcomes in the intention-to-treat analysis
Table 2 shows the results of the intention-to-treat analysis. After randomization, 90 patients were assigned to each of the study groups. Based on our data, the chest tube drainage duration was significantly shorter in the clamping group than the control group {2 [2, 3] vs. 3 [2, 3] days; P=0.009}. For the secondary outcomes, the total drainage volume was much less in the clamping group than the control group (516.7±410.9 vs. 657.8±448.2 mL; P=0.029). However, the postoperative stay was similar between the 2 group {5 [4, 5] vs. 5 [4, 5] days; P=0.660}.
Table 2
| Characteristics | Clamping group (n=90) | Control group (n=90) | P value |
|---|---|---|---|
| Chest tube duration, median [IQR], days | 2 [2, 3] | 3 [2, 3] | 0.009 |
| Total drainage volume, mean ± SD, mL | 516.7±410.9 | 657.8±448.2 | 0.029 |
| Postoperative stay, median [IQR], days | 5 [4, 5] | 5 [4, 5] | 0.660 |
IQR, interquartile range; SD, standard deviation.
Primary and secondary outcomes in the per-protocol analysis
In the per-protocol analysis, 12 patients who experienced complications were excluded; 85 and 83 patients were randomly assigned to the clamping and control groups, respectively. As Table 3 shows, the chest tube drainage duration of the clamping group remained significantly shorter than that of the control group {2 [2, 3] vs. 3 [2, 3] days; P=0.007}. The total drainage volume was also lower in the clamping group than the control group (437.8±213.9 vs. 604.8±352.8 mL; P=0.001). Additionally, the LOS did not differ between the 2 groups {5 [4, 5] vs. 5 [4, 5] days; P=0.589}. To assess the effect on the plasma albumin and hemoglobin levels, we included patients with available laboratory data at POD 1, discharge and 1 month after surgery (Tables S1,S2). Figure 3 shows the differences in plasma albumin and hemoglobin decline between the 2 groups at various times. The clamping group showed a major improvement in plasma albumin decline at discharge compared to the control group (7.7±2.9 vs. 9.0±5.2 g/L; P=0.040), as well as a marginal improvement in hemoglobin decline (16.8±9.0 vs. 20.4±14.4 g/L; P=0.050). The differences in plasma albumin and hemoglobin decline were comparable between the 2 groups on POD 1 and 1 month after surgery.
Table 3
| Characteristics | Clamping group (n=85) | Control group (n=83) | P value |
|---|---|---|---|
| Chest tube duration, median [IQR], days | 2 [2, 3] | 3 [2, 3] | 0.007 |
| Total drainage volume, mean ± SD, mL | 437.8±213.9 | 604.8±352.8 | 0.001 |
| Postoperative stay, median [IQR], days | 5 [4, 5] | 5 [4, 5] | 0.589 |
IQR, interquartile range; SD, standard deviation.

None of the patients required tube reinsertion for pneumothorax or delayed pleural effusion in either group. 1 patient with pyrexia in each group was excluded from the study. Only 1 patient in the clamping group developed dyspnea, which was relieved soon after removing the clamping, with no signs of pneumothorax and subcutaneous emphysema. No additional discomfort was recorded after re-clamping.
Discussion
Despite the rapid improvement of minimally invasive surgery and enhanced recovery after surgery (ERAS) programs, chest tube management continues to be a key element of postoperative care after major pulmonary resection. However, its role in postoperative pain and impaired pulmonary function also needs to be considered. Further, it may also contribute to the economic burden by prolonging the LOS (12,13). Thus, safe conditions for early chest tube removal should be explored.
The criteria for chest tube withdrawal have been refined and now include 3 factors: objective air leak quantification, fluid output threshold extension, and suction drainage. Pfeuty et al. removed the chest tube on POD 0 in 45% of patients who have undergone thoracoscopic major pulmonary resection based on a digital drainage device protocol (14). This demonstrated that air leak quantification of <20 mL/min for at least 4 h without a fluid threshold can serve as a safe condition for early chest tube removal. However, unlike Pfeuty et al., some trials still use the daily drainage amount as a criterion for chest tube removal. Moreover, the extension of the fluid output threshold varies dramatically among different trials. Cerfolio et al. considered 450 mL/day of non-chylous drainage as the maximum amount of daily pleural drainage at which chest tube removal could be attempted (7), which appears to be the most radical fluid output threshold recommended in the literature. However, it should be noted that 5% of patients in their study were readmitted within 60 days of discharge (7). In real-world settings, the thoracic community has widely adopted a more restrictive criterion for the fluid output threshold. Further, due to external suction, there is currently no consensus on optimal chest tube management. Gocyk et al. found that non-suction drainage was more effective than suction drainage in terms of drainage volume, drainage duration, and the incidence of persistent air leakage (15). Conversely, Brunelli et al. found that patients who received suction suffered fewer postoperative complications than those who did not (16).
Due to the substantial divergence in the aforementioned refinements, a universal chest tube management protocol is yet to be developed. In some cases, the choice of an inappropriate criterion could induce adverse consequences, such as pleural effusion followed by thoracentesis, due to the excessive fluid output threshold (17). We have been working on developing a management protocol to reduce the chest tube drainage duration in patients with lung cancer. The clamping of a pleural drain used to be a strategy in the presence of a prolonged air leak. In practice, we have observed a decrease in fluid output after clamping, which was confirmed using a propensity score matching analysis in our retrospective study (11). The current study was then conducted to overcome the limitations of a retrospective study through a prospective randomized trial with a sufficiently large sample size. Intermittent chest tube clamping allows for a more definitive assessment of the feasibility of chest tube removal based on removal simulation. Under this approach, conversion back to draining remains an option if the patients experience any discomfort, and thus prevents premature removal. Our protocol could serve as an option until consensus is reached as to the optimal chest tube removal protocol.
The mechanism by which clamping contributes to drainage volume reduction is of interest. Pleural fluid is mainly drained via the lymphatic stomata in the parietal pleura (18). Lymphatics can increase the flow rate in response to an increase in pleural liquid volume (19) and pressure (20). No liquid pressure data were measured in our study; however, it was apparent that the pleural liquid volume and pressure increased after chest tube clamping. The increase in lymphatic flow facilitated the reabsorption processes, causing a decrease in drainage volume, thus reducing the chest tube drainage duration. Additionally, the pleural egress of albumin due to lymphatic clearance has been demonstrated in previous study (21). This theory was validated in our study, as plasma albumin decline at discharge significantly improved in the clamping group. Due to albumin reabsorption, further albumin loss was prevented; thus, the physiological functions of albumin were less disturbed. Further, the better maintenance of nutritional status due to clamping also complied with the requirements of ERAS.
In this study, the safety of clamping was again demonstrated. No severe adverse events related to chest tube clamping were observed. Clamping also did not cause thoracocentesis after chest tube removal. Only 1 patient had mild dyspnea after clamping, which was relieved immediately after chest tube unclamping. Thus, the safety and convenience properties shown in this study could have remarkable advantages in the application of the procedure.
However, our study had several limitations. If pleural fluid pressure had been measured before and after clamping, the mechanisms involved could be better explained. In addition, the protein content change of the pleural drainage fluid after clamping could have provided direct evidence that our chest tube withdrawal protocol preserved better nutritional status for patients by strengthening the reabsorption of proteins, including albumin.
In conclusion, our study indicates that chest tube clamping decreases the chest tube drainage duration and drainage volume without causing adverse effects. Chest tube clamping also slows the decline in plasma albumin levels after major pulmonary resection. Thus, the approach is worthy of a wider application to facilitate ERAS.
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group. The authors would like to thank all the patients who were willing to participate in this study. We are grateful to Yan Zhang and Xiugeng Zhou (Peking University Cancer Hospital & Institute, Beijing, China) for their support in implementing this study. We would also like to thank Elixigen Co. (Huntington Beach, CA, USA) for proofreading the manuscript. We would also like to thank Editage (www.editage.cn) for editing the English-language manuscript.
Funding: This work was supported by funding from Capital’s Funds for Health Improvement and Research (No. 2020-2-2154), the National Natural Science Foundation of China (No. 81972842), the Beijing Natural Science Foundation (No. 7192036), the Beijing Municipal Administration of Hospital’s Ascent Plan (No. DFL20191101), the Beijing Municipal Administration of Hospitals Incubating Program (No. PX2021044), and the Science Foundation for The Excellent Youth Scholars of Beijing (No. 2017000021469G235).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-150/rc
Trial Protocol: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-150/tp
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-150/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-150/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the institutional review board of the Peking University Cancer Hospital & Institute (No. 2017KT27), and all patients provided informed consent.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lim E. The devil is in the details: Managing chest drains and interpreting negative randomized trial data. J Thorac Cardiovasc Surg 2015;150:1252-3. [Crossref] [PubMed]
- Xing T, Li X, Liu J, et al. Early removal of chest tubes leads to better short-term outcome after video-assisted thoracoscopic surgery lung resection. Ann Transl Med 2020;8:101. [Crossref] [PubMed]
- Czerny M, Fleck T, Salat A, et al. Sealing of the mediastinum with a local hemostyptic agent reduces chest tube duration after complete mediastinal lymph node dissection for stage I and II non-small cell lung carcinoma. Ann Thorac Surg 2004;77:1028-32. [Crossref] [PubMed]
- Pompili C, Brunelli A, Salati M, et al. Impact of the learning curve in the use of a novel electronic chest drainage system after pulmonary lobectomy: a case-matched analysis on the duration of chest tube usage. Interact Cardiovasc Thorac Surg 2011;13:490-3; discussion 493. [Crossref] [PubMed]
- Pompili C, Detterbeck F, Papagiannopoulos K, et al. Multicenter international randomized comparison of objective and subjective outcomes between electronic and traditional chest drainage systems. Ann Thorac Surg 2014;98:490-6; discussion 496-7. [Crossref] [PubMed]
- Schmid S, Kaafarani M, Baldini G, et al. Implication of a novel postoperative recovery protocol to increase day 1 discharge rate after anatomic lung resection. J Thorac Dis 2021;13:6399-408. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg 2008;135:269-73. [Crossref] [PubMed]
- Bjerregaard LS, Jensen K, Petersen RH, et al. Early chest tube removal after video-assisted thoracic surgery lobectomy with serous fluid production up to 500 ml/day. Eur J Cardiothorac Surg 2014;45:241-6. [Crossref] [PubMed]
- Gupta N. Pneumothorax: is chest tube clamp necessary before removal? Chest 2001;119:1292-3. [Crossref] [PubMed]
- Becker JC, Zakaluzny SA, Keller BA, et al. Clamping trials prior to thoracostomy tube removal and the need for subsequent invasive pleural drainage. Am J Surg 2020;220:476-81. [Crossref] [PubMed]
- Yan S, Wang X, Wang Y, et al. Intermittent chest tube clamping may shorten chest tube drainage and postoperative hospital stay after lung cancer surgery: a propensity score matching analysis. J Thorac Dis 2017;9:5061-7. [Crossref] [PubMed]
- Refai M, Brunelli A, Salati M, et al. The impact of chest tube removal on pain and pulmonary function after pulmonary resection. Eur J Cardiothorac Surg 2012;41:820-2; discussion 823. [Crossref] [PubMed]
- Göttgens KW, Siebenga J, Belgers EH, et al. Early removal of the chest tube after complete video-assisted thoracoscopic lobectomies. Eur J Cardiothorac Surg 2011;39:575-8. [Crossref] [PubMed]
- Pfeuty K, Lenot B. Early postoperative day 0 chest tube removal using a digital drainage device protocol after thoracoscopic major pulmonary resection. Interact Cardiovasc Thorac Surg 2020;31:657-63. [Crossref] [PubMed]
- Gocyk W, Kużdżał J, Włodarczyk J, et al. Comparison of Suction Versus Nonsuction Drainage After Lung Resections: A Prospective Randomized Trial. Ann Thorac Surg 2016;102:1119-24. [Crossref] [PubMed]
- Brunelli A, Monteverde M, Borri A, et al. Comparison of water seal and suction after pulmonary lobectomy: a prospective, randomized trial. Ann Thorac Surg 2004;77:1932-7; discussion 1937. [Crossref] [PubMed]
- Grodzki T. Prospective algorithm to remove chest tubes after pulmonary resection with high output--is it valid everywhere? J Thorac Cardiovasc Surg 2008;136:536-author reply 536-7. [Crossref] [PubMed]
- Negrini D, Ballard ST, Benoit JN. Contribution of lymphatic myogenic activity and respiratory movements to pleural lymph flow. J Appl Physiol (1985) 1994;76:2267-74. [PubMed]
- Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J 1997;10:219-25. [Crossref] [PubMed]
- Miserocchi G, Negrini D. Contribution of Starling and lymphatic flows to pleural liquid exchanges in anesthetized rabbits. J Appl Physiol (1985) 1986;61:325-30. [PubMed]
- Negrini D, Pistolesi M, Miniati M, et al. Regional protein absorption rates from the pleural cavity in dogs. J Appl Physiol (1985) 1985;58:2062-7. [Crossref] [PubMed]



