
The clinical characteristics, treatments and prognosis of post-esophagectomy airway fistula: a multicenter cohort study
Introduction
Post-esophagectomy airway fistula (PEAF) is a serious complication after esophageal cancer resection. It can be divided into tracheobronchial fistula (TBF) and respiratory-digestive fistula (RDF) based on whether it is combined with digestive fistula. The former is typically caused by accidental injury or prolonged tracheal intubation (1,2), while the latter is often secondary to anastomotic fistula or thoracic avascular necrosis after esophagectomy (3). Digestive juice can contaminate the respiratory tract through the fistula, leading to severe lung infections in patients, with some patients experiencing respiratory failure and even death (4,5).
At present, the treatment of PEAF patients mainly includes non-surgical conservative treatment, surgical treatment, and stent implantation (6-9). The main principles of non-surgical treatment involve fasting, continuous gastrointestinal decompression, adequate drainage, active anti-infection treatment, jejunal nutrition tube placed through the nose or the abdomen for enteral nutrition support, as well as minimizing the aisle of the airway and the digestive tract. The role of surgical management is to remove the infected foci by surgical operation. Selecting the appropriate surgical procedure is based on the size and location of the fistula and the general condition of each patient. Based on its clear effect and low recurrence rate, surgical treatment has become the main method of PEAF treatment (10).
However, compared with conservative treatment and stent placement, surgical treatment is more traumatic, and some PEAF patients are generally in a poor condition and cannot tolerate surgical treatment (11). In recent years, many scholars have reported that stents have been successfully used in the treatment of PEAF (12,13). However, there are also reports about 20% of tracheal stents that are not tightly closed, and the effect is not satisfactory (14). Even with active comprehensive treatment, the current treatment effect of PEAF patients remains unsatisfactory. At the same time, due to the low incidence of PEAF, the number of existing relevant studies is relatively small, and the treatment options are various. Also, there are no guidelines and large-scale studies related to PEAF.
The most suitable treatment modality for the different types of PEAF patients remains inconclusive. In this retrospective study, we analyzed the clinical data of 26,608 patients undergoing esophageal cancer surgery from January 2010 to December 2020 in seven domestic esophageal cancer diagnosis and treatment units. Based on their anatomic characteristics, we divided the PEAF patients into different types, and then summarized the diagnostic methods and treatment experiences of each type, so as to provide a clinical reference for the diagnostic mode and treatment methods of PEAF patients. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-141/rc).
Methods
Patients
This is a retrospective cohort study involving consecutive patients that underwent esophagectomy for esophageal cancer in seven major Chinese esophageal cancer centers (including Peking University People’s Hospital, Shanghai Chest Hospital Shanghai Jiaotong University, Sun Yat-sen University Cancer Center, West China Hospital, Sichuan University, Sichuan Cancer Hospital, and Henan Cancer Hospital, Fujian Medical University Union Hospital) from January 2010 to December 2020. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee of Fujian Medical University Union Hospital (No. 2021KY021) and other hospitals. Individual consent for this retrospective analysis was waived. Through the follow-up databases of each hospital, we excluded patients with incomplete data, and found 26,608 patients with complete postoperative data. The PEAF patients were screened through postoperative medical records, bronchoscopy or computed tomography (CT) examination and treatment process. The discharged patients were followed up by telephone every 3-4 months after discharge and the results were recorded in the database. We ended follow up upon death and the last follow up date was September 15, 2021.
Based on the anatomic characteristics of PEAF patients, including whether they are combined with digestive tract fistula, we divided the PEAFs into the following two types (which we named ‘Union Types’) (Figure 1):
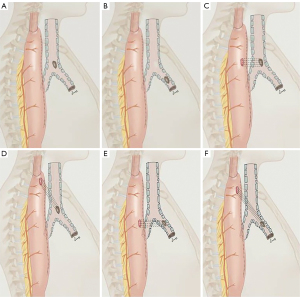
- Type I: TBF without digestive tract fistula.
- Type II: TBF combined with digestive tract fistula.
Next, we divided the two types of patients into the following subtypes based on whether the tracheal fistula was located in the trachea or the main bronchus, and whether the digestive tract fistula and the respiratory fistula were at the same horizontal plane:
- a: the airway fistula was located in the trachea.
- b: the airway fistula was located in the main bronchus.
- L1: both fistula orifices were located on the same horizontal plane.
- L2: two fistula orifices were located on different horizontal planes.
The number of cases on the area during the study period determined the sample size. The clinical characteristics, diagnoses, managements, and effects of the various types were retrospectively analyzed.
The clinical characteristics and diagnosis of PEAF
Most patients with PEAF have severe pneumonia. The clinical symptoms can manifest as repeated postoperative fever, cough, purulent sputum coughing, turbid fluid drained from chest tube, dyspnea, and even respiratory failure. Due to the large amount of gas entering the mediastinum and thorax through the airway fistula, patients with Union type I often present with sudden dyspnea, subcutaneous emphysema, and decline of saturation of pulse oxygen (SPO2). Chest computed tomography shows a large amount of pneumothorax or hydropneumothorax, and in severe cases, respiratory failure and other life-threatening complications such as sepsis may occur. For Union type II patients, the trachea fistula is often connected to the digestive tract fistula and gas can be discharged through the digestive tract fistula resulting in fewer pneumothorax-related complications. However, this type often stimulates the airway due to digestive tract pollution, resulting in more serious lung infection. Commonly, patients present with cough onset after swallowing and a failure to create negative pressure in the gastrointestinal decompression tube. Some patients can cough out digestive fluid, food residue, intraoperative anastomotic nail, and thoracogastric sutures through the mouth.
In this study, the method of PEAF diagnosis was mainly fiberoptic bronchoscopy, which can visually check the size and position of TBFs, and observe whether there is any infiltration of digestive juice; followed by gastroscopy, and esophagus swallow meglumine contrast and chest CT examination was also needed. Both bronchoscopy and gastroscopy can be considered at the same time (once tolerable by the patient) to determine whether there is gastrointestinal fistula and the size of fistula, and whether the fistulas are at the same level, and are connected. This would determine the type of PEAF which is the most important step in developing individualized management.
For patients with Union type I PEAF, bronchoscopy and chest CT examination are more important. For patients with suspected digestive tract fistula, esophageal barium swallowing is not recommended because the barium agent may enter the fistula cavity and cannot be absorbed, which would aggravate the airway infection; we recommend the use of diatrizoate meglumine for esophagography. The process of contrast agent entering the alimentary canal fistula and trachea fistula can be intuitively observed by esophagography swallowing diatrizoate meglumine, but there may be missed diagnosis. Chest CT examination can help to determine the severity of pneumonia as well as the presence of pneumothorax and chest cavity abscess, which provides a basis for determining the optimal management plan.
Treatment of PEAF patients
Patients with type I PEAF should be fasted temporarily when it is not clear whether the digestive tract fistula is combined. After confirming the absence of digestive tract fistula, it can be decided whether to resume oral diet based on the patient’s condition. If mediastinal or pulmonary infections exist, adequate drainage and antibiotics are the mainstay of therapy. For patients in this study cohort, the necessity of surgical treatment and stent implantation was evaluated after the infection was controlled.
Furthermore, once diagnosed, all type II patients should be strictly fasted and receive continuous gastrointestinal decompression. Antacids and antibiotics should also be administered, and jejunal nutrition tubes should be placed through the nose or the abdomen to support enteral nutrition. Sputum samples obtained during fiberoptic bronchoscopy were usually needed. In some patients, a fistula drainage tube was placed through the endoscope, and an external negative pressure device was used for continuous drainage. In this study, the surgical procedures were mainly for simple fistula repair and muscle flap transfer to repair the fistula, whereas endoscopic treatment included airway and esophageal stent implantations.
Statistical methods
Statistical analysis was performed using Statistical Product and Service Solutions (SPSS) 22.0 software (International Business Machines Corporation, IBM, America). Continuous variables were presented as mean ± standard deviation. Categorical variables were presented as numbers and percentages. Statistical significance was set at P<0.05 (two-sided). Survival times were analyzed with the Kaplan-Meier method.
Results
From January 2010 and December 2020, 85 patients (including eight females and 77 males) experienced PEAF, from a total of 26,608 patients who underwent esophagectomy at the seven centers. Thus, the incidence of PEAF was approximately 3.2%, while the incidence of Union type I and type II was 0.06% and 0.26%, respectively. Sixteen patients were diagnosed with type I, among which five (31.3%) patients were diagnosed with type Ia and 11 (68.7%) patients were diagnosed with type Ib. The other 69 patients were diagnosed with type II, among which 31 (44.9%) patients were diagnosed with type IIa and 38 (55.1%) patients were diagnosed with type IIb.
The characteristics of the patients including previous therapy, esophagectomy surgical procedure, reconstruction approach, replacement organ, and numbers of each type are shown in Table 1. The average age of the PEAF patients was 61.1±8.5 years (ranging from 43 to 82 years). The median diagnostic time of PEAF (postoperative day) was 13 days (ranging from 1 to 90 days).
Table 1
| Characteristics | Number |
|---|---|
| Sex (all) | 85 |
| Male/female | 77/8 |
| Age (average, years) | 61.1±8.5 |
| Previous therapy | 12 |
| Chemotherapy | 9 |
| Chemo-radiotherapy | 3 |
| Surgical procedure | |
| Sweet | 11 |
| Ivor Lewis | 15 |
| McKeown | 59 |
| Approach of reconstruction | |
| Posterior sternal approach | 17 |
| Posterior mediastinal pathway | 68 |
| Replacement organ | |
| Stomach | 84 |
| Colon | 1 |
| Types of fistulas | |
| Type I | 16 |
| Type I a | 5 |
| Type I b | 11 |
| Type II | 69 |
| Type IIa L1 | 25 |
| Type IIa L2 | 6 |
| Type IIb L1 | 31 |
| Type IIb L2 | 7 |
| Median diagnostic time (POD) | 13 |
PEAF, post-esophagectomy airway fistula; POD, postoperative day.
The management and the situation of the patients at discharge are shown in Table 2. All type Ia patients received conservative treatment. Among the 85 patients, the numbers of healings, non-healings, and deaths at discharge were 45 (52.9%), 20 (23.5%), and 20 (23.5%), respectively. Both surgical management and conservative treatment had healing rates of 50% at discharge, while the mortality rates at discharge were 37.5% and 26.9%, respectively. For patients who received stent implantation, the healing and mortality rates at discharge were 60% and 12%, respectively.
Table 2
| Managements and effects | Patients | Situation of patients at discharge, n (%) | ||
|---|---|---|---|---|
| Healing | Non-healing | Dead | ||
| All patients | 85 | 45 (52.9) | 20 (23.5) | 20 (23.5) |
| Surgical management | 8 | 4 (50.0) | 1 (12.5) | 3 (37.5) |
| Stent implantation | 25 | 15 (60.0) | 7 (28.0) | 3 (12.0) |
| Conservative treatment | 52 | 26 (50.0) | 12 (23.1) | 14 (26.9) |
| ICTG | 14 | 9 (64.3) | 4 (28.6) | 1 (7.1) |
| NICTG | 38 | 17 (44.7) | 8 (21.1) | 13 (34.2) |
| Type I | 16 | 10 (62.5) | – | 6 (37.5) |
| Surgical management | 3 | 1 (33.3) | – | 2 (66.7) |
| Stent placement | 4 | 4 (100.0) | – | – |
| Conservative treatment | 9 | 5 (55.6) | – | 4 (44.4) |
| Type Ia | 5 | 4 (80.0) | – | 1 (20.0) |
| Conservative treatment | 5 | 4 (80.0) | – | 1 (20.0) |
| Type Ib | 11 | 6 (54.5) | – | 5 (45.5) |
| Surgical management | 3 | 1 (33.3) | – | 2 (66.7) |
| Stent placement | 4 | 4 (100.0) | – | – |
| Conservative treatment | 4 | 1 (25.0) | – | 3 (75.0) |
| Type II | 69 | 35 (50.7) | 20 (29.0) | 14 (20.3) |
| Surgical management | 5 | 3 (60.0) | 1 (20.0) | 1 (20.0) |
| Stent placement | 21 | 11 (52.5) | 7 (33.3) | 3 (14.3) |
| Conservative treatment | 43 | 21 (48.8) | 12 (27.9) | 10 (23.3) |
| ICTG | 13 | 8 (61.5) | 4 (30.8) | 1 (7.7) |
| NICTG | 30 | 13 (43.3) | 8 (26.7) | 9 (30.0) |
| Type IIa L1 | 25 | 14 (56.0) | 6 (24.0) | 5 (20.0) |
| Surgical management | 1 | 1 (100.0) | – | – |
| Stent placement | 8 | 5 (62.5) | 2 (25.0) | 1 (12.5) |
| Conservative treatment | 16 | 8 (50.0) | 4 (25.0) | 4 (25.0) |
| Type IIa L2 | 6 | 1 (16.7) | 3 (50.0) | 2 (33.3) |
| Surgical management | 1 | – | 1 (100.0) | – |
| Stent placement | 1 | – | – | 1 (100.0) |
| Conservative treatment | 4 | 1 (25.0) | 2 (50.0) | 1 (25.0) |
| Type IIb L1 | 31 | 17 (54.8) | 9 (29.0) | 5 (16.1) |
| Surgical management | 3 | 2 (66.7) | – | 1 (33.3) |
| Stent placement | 11 | 5 (45.5) | 5 (45.5) | 1 (9.1) |
| Conservative treatment | 17 | 10 (58.8) | 4 (23.5) | 3 (17.6) |
| Type IIb L2 | 7 | 3 (42.9) | 2 (28.6) | 2 (28.6) |
| Stent placement | 1 | 1 (100.0) | – | – |
| Conservative treatment | 6 | 2 (33.3) | 2 (33.3) | 2 (33.3) |
PEAF, post-esophagectomy airway fistula; ICTG, interventional conservative treatment group; NICTG, non-interventional conservative treatment group.
The healing rates of type I and type II patients at discharge were 62.5% and 50.7%, respectively, and the mortality rates at discharge were 37.5% and 20.3%, respectively. For type I patients, the healing and mortality rates of the surgical group at discharge were 33.3% and 66.7%, respectively, while those of the conservative treatment group were 55.6% and 44.4%, respectively. All type I patients who received stent implantation were cured at discharge. For type II patients, the healing and mortality rates at discharge were 60% and 20% in surgical management group, 52.5% and 14.3% in the stent implantation group, and 48.8% and 23.3% in the conversation treatment group, respectively.
Of the 69 patients with type II, there were 56 (81.2%) patients with L1 and 13 (18.8%) patients with L2. The healing rates of type L1 and type L2 at discharge were 55.4% and 30.8%, and the mortality rates at discharge were 17.9% and 30.8%, respectively.
We divided the 52 patients who received conservative treatment into two groups: an interventional conservative treatment group (ICTG) which the fistula was closed during endoscopy or a fistula drainage tube was placed and a non-intervertional conservative treatment group (NICTG) with no endoscopy therapy or fistula drainage tube placement. There were 14 patients in the ICTG and 38 patients in NICTG; the healing rates at discharge of the two groups were 64.3% and 44.7%, while the mortality rates at discharge were 7.1% and 34.2%, respectively.
Follow-up
The longest follow-up time was 122.1 months and the average follow-up time was 19.6 months (ranging from 0 to 122.1 months). The 1-month survival rate for all patients was 82.4%, and the 2-month, 3-month, 6-month, 9-month, 1-year, 3-year, and 5-year survival rates were 80.0%, 74.1%, 60.7%, 55.6%, 53.1%, 29.0%, and 21.1%, respectively (Figure 2).
The longest follow-up time among types I and II patients was 49.2 and 122.1 months, respectively. The 1-month, 3-month, 6-month, 9-month, 1-year, 3-year, and 5-year survival rates of type I patients were 62.5%, 62.5%, 62.5%, 62.5%, 62.5%, 19.5%, and 0%, respectively, while the equivalent rates in type II patients were 87.0%, 76.8%, 60.2%, 54.0%, 50.9%, 31.0%, and 22.2%, respectively (Figure 3). However, there was no significant difference between the two groups [χ2=0.877; P=0.349, log-rank (Mantel-Cox) test] (Figure 3).
The longest follow-up time among types II L1 and II L2 was 122.1 and 40.2 months, respectively. The 1-month, 3-month, 6-month, 9-month, 1-year, 3-year, and 5-year survival rates in type II L1 patients were 87.5%, 80.4%, 65.4%, 59.6%, 55.8%, 34.3%, and 24.3%, respectively, while the equivalent rates in type II L2 patients were 84.6%, 61.5%, 38.5%, 30.8%, 30.8%, 15.4%, and 0%, respectively. The overall survival of type II L2 patients was shorter than that of type II L1 patients [χ2=3.276; P=0.070, log-rank (Mantel-Cox) test] (Figure 4).
Discussion
To our knowledge, this is the first time that PEAF has been divided into two types based on the anatomic characteristics of fistulas. Type I patients had simple respiratory fistula without digestive fistula, including tracheal fistula and main bronchial fistula, whereas type II patients had TBF combined with digestive tract fistula. We also further divided the two types of patients into subtypes based on whether the tracheal fistula was located in the trachea or the main bronchus, and whether the digestive tract and respiratory fistulas were at the same horizontal plane: Ia, Ib, IIa L1, IIa L2, IIb L1, and IIb L2. Wang et al. (3) classified airway-gastric fistulas into two types: type I patients have a fistula orifice in the digestive tract higher than in the airway, while type II patients have a the fistula orifices in digestive tract and the airway are in the same horizontal plane. In their study, the incidence of aerodigestive fistula was 0.4 percent which is closer to what we reported in our study. There was significant difference in mortality between both groups with type I and II reported mortality of 16.7% versus 64.3% respectively (P=0.014).
The incidence of type I PEAF has rarely been reported in the literature. In our study, there were 16 cases of type I PEAF, with an incidence of approximately 0.06%, among which five (31.3%) patients were diagnosed with type Ia and 11 (68.7%) patients were diagnosed with type Ib. This indicates that type Ib was common in type I. We believe that the reasons for this are as follows: (I) sweeping the subcarinal lymph nodes during esophagectomy may cause thermal damage to the main bronchus; and (II) esophageal squamous cell carcinoma is mostly located in the middle third of the esophagus; locally advanced esophageal mid-thoracic cancer usually has multiple adhesions to the main bronchus, which is the most difficult part of surgery as freeing the tumor may damage the main bronchus.
According to previous reports, the incidence of type II PEAF ranges from 0.3% to 1.5% (5,15,16); in this study, the incidence was approximately 0.26%. Type II PEAF was often secondary to anastomotic fistula or thoracic avascular necrosis after esophagectomy (3). We found that among the 69 patients, there were 31 (44.9%) patients with type IIa and 38 (55.1%) patients with IIb, and there was no difference in proportions of type IIa and IIb. Type II L1 was common in type II; there were 56 (81.2%) patients with type II L1 and 13 (18.8%) patients with type II L2, which indicated that most patients with type II PEAF had both fistulas located on the same horizontal plane.
Among the 16 patients with type I, three patients received surgical management, four patients were treated with airway stent implantation, while the other nine patients received conservative treatment. All type I patients treated with stent implantation were healed at discharge, which indicates that tracheal stent placement may have the potential to improve efficacy for type I patients, compared with surgical management and conservative treatment. However, more data is needed to support this hypothesis.
As previously reported, surgical management plays an important role in the treatment of type II PEAF patients, which can effectively restore normal nutrition and reduce airway aspiration with a very low risk of recurrence. Surgical management is relatively successful, and includes esophageal exclusion and fistula repaired with a strap muscle (8), intercostal muscle (5,17), latissimus dorsi myocutaneous flap (18,19), pedicled pericardial flap (15), pectoralis major muscle flap (20), and omental and pleural patch (5), as well as reconstruction by the interposition of colon (5) and jejunum (21).
However, many PEAF patients are generally in a poor condition after esophagectomy and cannot tolerate surgical management (11). For these patients, other treatments need to be considered. In recent years, with the development of stent implantation, tracheal and esophageal stents have been increasingly used in clinical practice, which can effectively and quickly seal the fistula and rapidly control inflammation. Through stent implantation, some patients can achieve healing or sealing of the fistula, the oral diet can be restored, and the quality of life is significantly improved. Therefore, for patients with aero-digestive fistula who are not suitable for surgery or have high postoperative risk, stent implantation therapy maybe a suitable palliative treatment and in some patients, can be the definitive and curative therapy (12,22,23). Huang et al. (13) reported retrievable covered metallic segmented Y airway stent in six Union type II patients, and the fistulas were all cured after stent removal. Coincidentally, Han et al. (24) also reported that individualized airway-covered stent implantation therapy may be a suitable palliative treatment. Sun et al.’s 2008 study (25) concluded that stenting of a gastric tube was not a suitable option, owing to poor fixation of the stent and the high risk of migration of the gastric stent. However, Okamoto et al. (9) performed esophageal stent placement in three cases of gastrointestinal-airway fistula after esophagectomy. All patients were successfully managed and the stents were removed after endoscopic confirmation of fistula closure on days 8, 23, and 71. Only one patient with a long-term indwelling stent developed a manageable secondary gastrobronchial fistula as a procedure-related complication. Also, esophageal stent placement was shown to be a less-invasive and effective therapeutic modality for the treatment of RDF.
For type II patients, the healing and mortality rates at discharge in the three treatment groups showed no significant differences, which indicated that for type II patients, all the three treatment methods could be applied. However, we found that the healing rate of type II L1 was higher than that of type II L2 (55.4% vs. 30.8%), while type II L1 had a lower mortality rate at discharge (17.9% vs. 30.8%). Through the survival curves shown in Figure 4, we can also see that the long-term survival rates of patients with type II L1 were higher than those of type L2. We believe that the reason for this may be that the two fistulas of type II L1 patients were connected, and the path of digestive juice passing through the mediastinum was relatively short. Some digestive juice can be coughed up through the trachea, which may reduce the pollution of the mediastinum. However, the digestive juice of type II L2 patients corroded the mediastinum and trachea through a longer path, which is difficult to drain in the mediastinum. This may lead to more serious and uncontrollable mediastinal inflammation, resulting in a poor treatment effect for patients.
In addition, in recent years, there have been reports of successful endoscopic embolization of refractory esophageal bronchiolar fistula after esophagectomy with silica gel. Patients receiving this treatment reportedly returned to an oral diet and were discharged 2 weeks later, without fistula recurrence in the following 3 years (26). At the same time, another study has shown that endoscopy provides a minimally invasive and safe option for the intervention of esophagobronchial fistula, which may improve the quality of life of patients despite the overall clinical success and survival rates (27).
In this study, we divided the 52 patients who received conservative treatment into the ICTG and NICTG. The healing rate at discharge of the ICTG was higher than that of the NICTG, while the mortality rate was lower in the ICTG (7.1% vs. 34.2%). Therefore, we believe that even if conservative treatment is adopted, various methods should be actively adopted to reduce digestive juice damage to the respiratory tract, including the placement of a fistula drainage tube under the endoscope and fistula closure under endoscopy, which can effectively improve the healing rates and reduce the mortality rates in these groups of patients.
All patients in this study were followed up in this study. The 1-, 3- and 5-year survival rates were 53.1%, 29.0%, and 21.1%, respectively (Figure 2), which was significantly lower than that reported in the literature (82.6%, 61.6%, and 52.9% for resectable esophageal cancer, respectively) (28). We also constructed survival curves of type I and type II patients, and found that there was no significant statistical difference between the two groups. This indicated that both type I and type II PEAF will seriously reduce the survival of patients after esophageal cancer surgery.
This study has some limitations that should be noted. Firstly, this is a retrospective study that covered a period of 11 years, where the treatment approaches evolved progressively and often mirrored methods reported in the literature. Secondary, due to the low incidence of PEAF, the total number of cases was only 85 cases, and therefore, we did not perform further stratification according to the patients’ characteristics. Thirdly, we did not re-type according to the time sequence of tracheal fistula or gastrointestinal fistula, because we found that most type II patients had the gastrointestinal fistula appear first, followed by the airway fistula. Fourthly, we found that Kaplan-Meier analysis was not adequate for the data, and we also performed univariate and multivariate analysis between the subgroups, including sex, age, previous therapy, surgical procedure, approach of reconstruction, replacement organ, types of fistulas and median diagnostic time, etc. However, due to the number of cases in each subgroup was still relatively small, the bias caused by the small sample size was large, it was difficult for us to reasonably explain the analysis results. Therefore, we did not list the univariate and multivariate analysis tables between each subgroup in the paper. We hope to conduct univariate and multivariate analysis by collecting more data.
In conclusion, PEAF is an infrequent and life-threatening complication after esophagectomy, with a low cure rate and an extremely high mortality rate. Patients with different types of PEAF often have different inducements. For type I patients, stent implantation is expected to improve survival and reduce mortality. Meanwhile, for type II patients, the effects of conservative, endoscopic including stenting and surgical treatment were equivalent; each treatment option has its own advantages and supplements other treatments. Prospective studies comparing outcomes between treatment modalities is warranted but are challenging to conduct due to the rarity of this condition. If the fistula does not close after 4–6 weeks of conservative treatment, other treatments should be considered (5). At the same time, conservative treatment and stent implantation can become a bridge for surgical management, and therefore, it is necessary to formulate an individualized diagnosis and treatment plan according to the patient’s condition (Figure 5).
Acknowledgments
The authors appreciate the academic support from the AME Thoracic Surgery Collaborative Group.
Funding: The study was supported by Climbing project of Science and Technology Department of Fujian Province (No. 2018Y9058) and Fujian Provincial Joint Research Project of Health Care and Education (No. WKJ2016-2-09).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-141/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-141/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-141/coif). All authors report that the study was supported by Climbing project of Science and Technology Department of Fujian Province (No. 2018Y9058) and Fujian Provincial Joint Research Project of Health Care and Education (No. WKJ2016-2-09). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional committee of Fujian Medical University Union Hospital (No. 2021KY021) and other hospitals. Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paraschiv M. Tracheoesophageal fistula--a complication of prolonged tracheal intubation. J Med Life 2014;7:516-21. [PubMed]
- Reed MF, Mathisen DJ. Tracheoesophageal fistula. Chest Surg Clin N Am 2003;13:271-89. [Crossref] [PubMed]
- Wang C, Li C, Yang X, et al. The classification and treatment strategies of post-esophagectomy airway-gastric fistula. J Thorac Dis 2020;12:3602-10. [Crossref] [PubMed]
- Maruyama K, Motoyama S, Sato Y, et al. Tracheobronchial lesions following esophagectomy: erosions, ulcers, and fistulae, and the predictive value of lymph node-related factors. World J Surg 2009;33:778-84. [Crossref] [PubMed]
- Buskens CJ, Hulscher JB, Fockens P, et al. Benign tracheo-neo-esophageal fistulas after subtotal esophagectomy. Ann Thorac Surg 2001;72:221-4. [Crossref] [PubMed]
- Li Y, Wang Y, Chen J, et al. Management of thoracogastric airway fistula after esophagectomy for esophageal cancer: A systematic literature review. J Int Med Res 2020;48:300060520926025. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical management of acquired nonmalignant tracheoesophageal and bronchoesophageal fistulae. Ann Thorac Surg 2010;90:914-8; discussion 919. [Crossref] [PubMed]
- Balakrishnan A, Tapias L, Wright CD, et al. Surgical Management of Post-Esophagectomy Tracheo-Bronchial-Esophageal Fistula. Ann Thorac Surg 2018;106:1640-6. [Crossref] [PubMed]
- Okamoto K, Ninomiya I, Fujiwara Y, et al. Use of esophageal stent for the treatment of postoperative gastrointestinal-airway fistula after esophagectomy. Esophagus 2019;16:413-7. [Crossref] [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg 2000;119:268-76. [Crossref] [PubMed]
- Jha PK, Deiraniya AK, Keeling-Roberts CS, et al. Gastrobronchial fistula--a recent series. Interact Cardiovasc Thorac Surg 2003;2:6-8. [Crossref] [PubMed]
- Bennie MJ, Sabharwal T, Dussek J, et al. Bronchogastric fistula successfully treated with the insertion of a covered bronchial stent. Eur Radiol 2003;13:2222-5. [Crossref] [PubMed]
- Huang W, Shan Q, Wu Z, et al. Retrievable covered metallic segmented Y airway stent for gastrorespiratory fistula of carina or main bronchi. J Thorac Cardiovasc Surg 2021;161:1664-1671.e2. [Crossref] [PubMed]
- Shin JH, Song HY, Ko GY, et al. Treatment of tracheobronchial obstruction with a polytetrafluoroethylene-covered retrievable expandable nitinol stent. J Vasc Interv Radiol 2006;17:657-63. [Crossref] [PubMed]
- Song SW, Lee HS, Kim MS, et al. Repair of gastrotracheal fistula with a pedicled pericardial flap after Ivor Lewis esophagogastrectomy for esophageal cancer. J Thorac Cardiovasc Surg 2006;132:716-7. [Crossref] [PubMed]
- Yasuda T, Sugimura K, Yamasaki M, et al. Ten cases of gastro-tracheobronchial fistula: a serious complication after esophagectomy and reconstruction using posterior mediastinal gastric tube. Dis Esophagus 2012;25:687-93. [Crossref] [PubMed]
- Nardella JE, Van Raemdonck D, Piessevaux H, et al. Gastro-tracheal fistula--unusual and life threatening complication after esophagectomy for cancer: a case report. J Cardiothorac Surg 2009;4:69. [Crossref] [PubMed]
- Hayashi K, Ando N, Ozawa S, et al. Gastric tube-to-tracheal fistula closed with a latissimus dorsi myocutaneous flap. Ann Thorac Surg 1999;68:561-2. [Crossref] [PubMed]
- Miyata K, Fukaya M, Nagino M. Repair of gastro-tracheobronchial fistula after esophagectomy for esophageal cancer using intercostal muscle and latissimus dorsi muscle flaps: a case report. Surg Case Rep 2020;6:172. [Crossref] [PubMed]
- Silberhumer GR, Györi G, Burghuber C, et al. The value of protecting the longitudinal staple line with invaginating sutures during esophageal reconstruction by gastric tube pull-up. Dig Surg 2009;26:337-41. [Crossref] [PubMed]
- Reames BN, Lin J. Repair of a complex bronchogastric fistula after esophagectomy with biologic mesh. Ann Thorac Surg 2013;95:1096-7. [Crossref] [PubMed]
- Wang H, Tao M, Zhang N, et al. Single application of airway stents in thoracogastric-airway fistula: results and prognostic factors for its healing. Ther Adv Respir Dis 2019;13:1753466619871523. [Crossref] [PubMed]
- Chaddha U, Hogarth DK, Murgu S. Perspective on airway stenting in inoperable patients with tracheoesophageal fistula after curative-intent treatment for esophageal cancer. J Thorac Dis 2019;11:2165-74. [Crossref] [PubMed]
- Han X, Li L, Zhao Y, et al. Individualized airway-covered stent implantation therapy for thoracogastric airway fistula after esophagectomy. Surg Endosc 2017;31:1713-8. [Crossref] [PubMed]
- Sun JS, Park KJ, Choi JH, et al. Benign bronchogastric fistula as a late complication after transhiatal oesophagogastrectomy: evaluation with multidetector row CT. Br J Radiol 2008;81:e255-8. [Crossref] [PubMed]
- Uesato M, Kono T, Akutsu Y, et al. Endoscopic occlusion with silicone spigots for the closure of refractory esophago-bronchiole fistula after esophagectomy. World J Gastroenterol 2017;23:5253-6. [Crossref] [PubMed]
- Silon B, Siddiqui AA, Taylor LJ, et al. Endoscopic Management of Esophagorespiratory Fistulas: A Multicenter Retrospective Study of Techniques and Outcomes. Dig Dis Sci 2017;62:424-31. [Crossref] [PubMed]
- Mao YS, Gao SG, Wang Q, et al. Epidemiological characteristic and current status of surgical treatment for esophageal cancer by analysis of national registry database. Zhonghua Zhong Liu Za Zhi 2020;42:228-33. [PubMed]






