
Totality outcome of afatinib sequential treatment in patients with EGFR mutation-positive non-small cell lung cancer in South Korea (TOAST): Korean Cancer Study Group (KCSG) LU-19-22
IntroductionOther Section
Epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) are the standard of care for advanced EGFR M+ non-small cell lung cancer (NSCLC). Five major EGFR-TKIs—gefitinib, erlotinib, afatinib, dacomitinib, and osimertinib—have been approved for the treatment of patients with EGFR M+ NSCLC in South Korea since 2004. Gefitinib and erlotinib are first-generation (1G) EGFR-TKIs, which reversibly inhibit EGFR (HER1) kinase activity. Afatinib and dacomitinib, second-generation (2G) EGFR-TKIs, covalently and irreversibly bind to the intracellular tyrosine kinase domain and are selective blockers of members of the ErbB family (1,2). Osimertinib, a third-generation (3G) EGFR-TKI, covalently and irreversibly inhibits EGFR kinase activity with T790M, while sparing wild-type EGFR (3).
By inhibiting intracellular phosphorylation, downstream signaling is blocked and cell death results. In this manner, 1G and 2G EGFR-TKIs have demonstrated superior objective response rates (ORRs), and they significantly prolong progression-free survival (PFS) compared to platinum-based chemotherapy in randomized phase III studies (4-6). Interestingly, a combined analyses of LUX-Lung 3 and LUX-Lung 6 demonstrated that afatinib has a statistically significant benefit on OS for patients with EGFR exon 19 deletion (7). Furthermore, both afatinib (2G) and osimertinib (3G) have demonstrated superior PFS than 1G EGFR-TKIs in NSCLC patients harboring common EGFR mutations (Del19/L858R) in the LUX-Lung 7 and FLAURA studies, respectively (8,9). These results indicate that 2G or 3G EGFR-TKIs are preferable to 1G EGFR-TKIs as a first-line treatment. However, there is currently no prospective data directly comparing 2G to 3G EGFR-TKIs.
Regardless of which EGFR-TKI is used as the first-line treatment, acquired resistance to treatment inevitably occurs. Therefore, the availability of subsequent treatment options following disease progression is a key consideration when assessing therapeutic choices. The most frequent molecular resistance mechanism to 1G or 2G EGFR-TKIs is the T790M mutation in exon 20 of EGFR, which emerges in approximately 40–50% of tumors when resistance is acquired (10-12). The T790M mutation is highly sensitive to osimertinib, which has been approved for use following the failure of gefitinib, erlotinib, or afatinib. In contrast to 1G and 2G EGFR-TKIs, a predominant resistance mechanism to osimertinib when used as the first-line treatment has not been established (13-16); c-MET amplification only accounts for 15% of patients, and the emergence of the EGFR C797S mutation occurs in 7%, while over 60% of patients have no identified resistance mechanisms. As a result, targeted treatment options following first-line osimertinib failure remain limited. Thus, interest in the sequential administration of first-line 1G or 2G EGFR-TKIs followed by second line treatment including osimertinib in patients with EGFR M+ NSCLC has been growing.
GioTag was a real-world study demonstrating a total chemotherapy-free treatment duration of 27.7 months by sequential afatinib followed by osimertinib treatment for EGFR M+ NSCLC in patients acquiring the T790M mutation (16). However, half of the patients were ineligible for this treatment sequence because of T790M negative (T790M−) or unknown T790M status, and the total time on treatment (TOT) for patients who are T790M− is not well known. Therefore, in this retrospective study, we investigated the total TOT along with four treatment options starting from first-line afatinib treatment to various subsequent treatments, including osimertinib and cytotoxic chemotherapy. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-79/rc).
MethodsOther Section
Study subjects and data collection
This is a non-interventional, multicenter, multicohort retrospective study based on existing data from patients with EGFR M+ NSCLC treated with afatinib as the first-line treatment in fifteen university hospitals in Korea between October 1, 2013 and April 30, 2019. Osimertinib was approved for patients with T790M since May 19, 2016. Enrolled patients were categorized into four cohorts according to the type of second-line treatment, with biopsies taken before the start of second-line treatment (Figure S1). T790M+ patients who were treated with osimertinib were classified as cohort A; T790M− patients who were treated with chemotherapy or other treatments were classified as cohort B, and patients who had systemic treatment or supportive care due to unknown T790M status were classified as cohort C. Patients who were treated with afatinib only were classified as cohort D. Cohort D included patients who were undergoing afatinib treatment at the time of data collection, were dead, had discontinued treatment due to adverse events, or were lost to follow-up during the period of first-line afatinib treatment.
Primary outcomes were total TOT, TOT for first-line afatinib (TOT-1) and TOT for second-line treatments (TOT-2) in cohorts A, B, and C. Total TOT was defined as the duration from the first date of afatinib use till the last date of second-line treatment, resulting from any causes including toxicity, disease progression, or death. TOT-1 was defined as the duration from the first date of afatinib use till the last date, resulting from any causes including toxicity, disease progression, or death. TOT-2 was defined as the duration from the first date of second-line treatment till the last date, resulting from any causes including toxicity, disease progression, or death. Secondary outcomes were the acquisition rate of T790M mutation among patients in whom re-biopsy results were obtained, ORR for afatinib (ORR-1) and second-line treatments (ORR-2), overall survival (OS), central nervous system (CNS) response rate, and CNS-PFS.
Eligible patients were at least 18 years old with histologically confirmed EGFR M+ stage IIIB/IV NSCLC treated with first-line afatinib. Other eligibility criteria included that afatinib treatment was started 13 months prior to the data collection date to reduce premature censoring of patients, which was based on the median PFS of 13.6 months for EGFR M+ NSCLC patients reported in LUX-Lung 3 (7).
Ethical statement
This study was reviewed and approved by the Institutional Review Board at each institute (see the online file for detailed information) and was registered on ClinicalTrial.gov (identifier NCT04930133). The informed consent form was waived by each institutional review board due to the retrospective nature of this study. The study was conducted in accordance with the Helsinki Declaration (as revised in 2013).
Statistical analysis
The data cutoff date for the analyses was May 31, 2020. Descriptive statistics were used to analyze demographics, methods of repeat biopsies, and EGFR mutation subtypes. To compare the baseline characteristics between each group, the Chi-square test was used. Total TOT was estimated using the Kaplan-Meier method and the median along with the two-sided 95% confidence interval (CI). The OS rate was estimated using the Kaplan-Meier method. Patients undergoing afatinib treatment at the time of analysis were censored as of the last day of follow-up. The percentage of patients with ORR [complete response (CR) or partial response (PR)] and its 95% CI was calculated. The rates of successful repeat biopsy and T790M mutation were calculated.
The CNS-ORR was evaluated in patients who had brain metastasis at the time of initial diagnosis but no prior local treatment such as whole-brain radiotherapy (WBRT) and stereotactic radiosurgery (SRS). CNS-PFS was defined as the duration from the first date of afatinib treatment until CNS progression (i.e., new brain metastasis, leptomeningeal carcinomatosis, or increase of preexisting CNS lesions) or death resulting from any cause. Patients with no CNS progression or death at the time of analysis were censored as of the last date of administration of afatinib. CNS-PFS was only assessed in patients with brain metastases at afatinib initiation. CNS-PFS is presented as the median value with the two-sided 95% CI. A competing risk analysis estimating the cumulative incidence for CNS failure in the presence of two competing risk events (non-CNS progression and death) was performed using the semiparametric Fine-Gray regression model. Event time was defined as either the earliest occurrence of three events or the time of their last assessment.
All P values were two-sided, and a P value of <0.05 was considered statistically significant. Data were analyzed using SAS (Statistical Analysis System) version 9.4.
ResultsOther Section
Patient characteristics
A total of 737 patients received afatinib as the first-line treatment during the study period. Table 1 shows the baseline characteristics in each cohort. Of the total, 116 (15.7%), 143 (19.4%), 111 (15.1%), and 367 (49.8%) were included in cohorts A, B, C, and D, respectively; 391 patients (53.1%) were female and the median age was 62 years (range, 22–90 years). Most patients (80.2%) had good performance with an ECOG 0–1. More than half (59.3%) were never smokers. Pathological confirmation was performed via percutaneous needle biopsy (42.7%), bronchoscopic biopsy (29.2%), endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) (25.4%), video-assisted thoracoscopic surgery (13.7%), and excision and mediastinoscopic biopsy. The subtypes of EGFR mutations were as follows: deletion 19 (Del19) (57.5%), L858R (31.5%), uncommon mutations (7.3%), and compound mutations (3.7%). At the beginning of afatinib treatment, most of the patients (93.6%) had distant metastases, including in the bone (41.5%), brain (38.9%), pleura (33.9%), lung to lung (27.3%), liver (9.1%), and adrenal gland (5.2%). The incidence of distant metastases increased at the beginning of second-line treatment as follows: bone (50.3%), brain (48.9%), lung to lung (35.8%), pleura (33.8%), liver (14.2%), and adrenal gland (9.7%) in cohorts A + B + C. In cohort D, preexisting brain metastasis was less frequent (34.3%) than for cohorts A + B + C (43.5%); however, the distribution of the EGFR Del19 and L858R mutations did not differ from cohorts A + B + C (56.7% and 31.3%) compared to cohort D (58.4% and 30.0%), respectively.
Table 1
| Characteristics | Category | Cohort A (n=116) | Cohort B (n=143) | Cohort C (n=111) | Cohort D (n=367) | Total (n=737) | P |
|---|---|---|---|---|---|---|---|
| Sex, n (%) | Male | 58 (50.0) | 74 (51.8) | 56 (50.5) | 158 (43.1) | 346 (46.9) | 0.21 |
| Female | 58 (50.0) | 69 (48.2) | 55 (49.5) | 209 (56.9) | 391 (53.1) | ||
| Age* (years) | Median (range) | 60.5 (22.0–87.0) | 59.0 (37.0–84.0) | 62.0 (31.0–84.0) | 63.0 (33.0–90.0) | 62.0 (22.0–90.0) | <0.001 |
| ECOG, n (%) | 0–1 | 95 (81.9) | 123 (86.0) | 89 (80.2) | 284 (77.4) | 591 (80.2) | 0.22 |
| ≥2 | 5 (4.3) | 6 (4.2) | 10 (9.0) | 35 (9.5) | 56 (7.6) | ||
| Unknown | 16 (13.8) | 14 (9.8) | 12 (10.8) | 48 (13.1) | 90 (12.2) | ||
| Smoking, n (%) | Never smoker | 66 (56.9) | 84 (58.7) | 64 (57.7) | 223 (60.8) | 437 (59.3) | 0.34 |
| Ex-smoker** | 32 (27.6) | 32 (22.4) | 20 (18.0) | 86 (23.4) | 170 (23.1) | ||
| Current smoker | 15 (12.9) | 26 (18.2) | 23 (20.7) | 47 (12.8) | 111 (15.1) | ||
| Unknown | 3 (2.6) | 1 (0.7) | 4 (3.6) | 11 (3.0) | 19 (2.6) | ||
| Pack/year | Median (range) | 20.0 (1.5–70.5) | 27.5 (6.0–80.0) | 25.0 (0.5–60.0) | 26.0 (0.5–80.0) | 25.0 (0.5–80.0) | 0.45 |
| Subtype of EGFR mutation, n (%) | Del19 | 85 (73.3) | 71 (49.7) | 60 (54.1) | 208 (56.7) | 424 (57.5) | 0.003 |
| L858R | 27 (23.3) | 49 (34.3) | 35 (31.5) | 121 (33.0) | 232 (31.5) | ||
| Uncommon | 1 (0.9) | 13 (9.1) | 13 (11.7) | 27 (7.4) | 54 (7.3) | ||
| Compound | 3 (2.6) | 10 (7.0) | 3 (2.7) | 11 (3.0) | 27 (3.7) | ||
| Distant metastasis***, n (%) | 112 (96.6) | 134 (93.7) | 104 (93.7) | 340 (92.6) | 690 (93.6) | ||
| Lung | 50 (43.1) | 38 (26.6) | 19 (17.1) | 94 (25.6) | 201 (27.3) | <0.001 | |
| Brain | 54 (46.6) | 61 (42.7) | 46 (41.4) | 126 (34.3) | 287 (38.9) | 0.07 | |
| Pleural | 44 (37.9) | 44 (30.8) | 39 (35.1) | 123 (33.5) | 250 (33.9) | 0.67 | |
| Lymph node | 49 (42.2) | 65 (45.5) | 58 (52.3) | 165 (45.0) | 337 (45.7) | 0.46 | |
| Adrenal | 6 (5.2) | 9 (6.3) | 10 (9.0) | 13 (3.5) | 38 (5.2) | 0.13 | |
| Bone | 52 (44.8) | 66 (46.2) | 54 (48.6) | 134 (36.5) | 306 (41.5) | 0.05 | |
| Liver | 13 (11.2) | 17 (11.8) | 7 (6.3) | 30 (8.2) | 67 (9.1) | 0.33 | |
| Mediastinum | 11 (9.4) | 12 (8.4) | 11 (9.9) | 44 (12.0) | 78 (10.6) | 0.64 | |
| Leptomeninges | 2 (1.7) | 1 (0.7) | 1 (0.9) | 3 (0.8) | 7 (0.9) | 0.82 | |
| Other | 6 (5.2) | 14 (9.8) | 11 (9.9) | 30 (8.2) | 61 (8.3) | 0.51 | |
| Initial brain metastasis, n (%) | Number of metastases | 54 (46.6) | 61 (42.7) | 46 (41.4) | 126 (34.3) | 287 (38.9) | 0.07 |
| Single | 8 (14.8) | 13 (21.3) | 8 (17.4) | 30 (23.8) | 59 (20.5) | 0.79 | |
| Oligo [2–4] | 9 (16.7) | 10 (16.4) | 9 (19.6) | 25 (19.8) | 53 (18.5) | ||
| Multiple [≥5] | 37 (68.5) | 38 (62.3) | 29 (63.0) | 71 (56.3) | 175 (61.0) | ||
| Neurologic symptom | |||||||
| Symptomatic | 12 (22.2) | 21 (34.4) | 9 (19.6) | 30 (23.8) | 72 (25.1) | 0.27 | |
| Asymptomatic | 42 (77.8) | 40 (65.6) | 37 (80.4) | 96 (76.2) | 215 (74.9) | ||
| Local therapy**** before starting TKIs | 26 | 39 | 20 | 71 | 156 | 0.25 | |
| SRS | 8 (30.8) | 16 (41.0) | 3 (15.0) | 25 (35.2) | 52 (33.3) | ||
| WBRT | 11 (42.3) | 9 (23.1) | 9 (45.0) | 29 (40.8) | 58 (37.2) | ||
| Surgery | 4 (15.4) | 9 (23.1) | 4 (20.0) | 13 (18.3) | 30 (19.2) | ||
| SRS and WBRT | 0 (0.0) | 1 (2.6) | 0 (0.0) | 2 (2.8) | 3 (1.9) | ||
| Surgery and WBRT | 2 (7.7) | 3 (7.7) | 1 (5.0) | 1 (1.4) | 7 (4.5) | ||
| Surgery and SRS | 1 (3.8) | 1 (2.6) | 3 (15.0) | 1 (1.4) | 6 (3.8) |
*, age defined at the time of starting afatinib; **, ex-smoker defined as stopping smoking at least 6 months before start of afatinib treatment; ***, distant metastasis at the time of starting afatinib; ****, local therapy included SRS, WBRT, and surgery. ECOG, European Cooperative Oncology Group; EGFR, epidermal growth factor receptor; TKI, tyrosine kinase inhibitor; SRS, stereotactic radiosurgery; WBRT, whole-brain radiotherapy.
Time on treatment and ORR
The median follow-up duration was 21.30 months (range, 0.10–75.66 months). In all study groups (cohorts A, B, C, and D), the median total TOT was 23.40 months (95% CI: 20.66–25.99 months). The median total TOTs for cohorts A, B, C, and D were 35.10 months (95% CI: 30.09–43.53 months), 18.80 months (95% CI: 16.92–20.20 months), 12.00 months (95% CI: 10.22–14.98 months), and 42.60 months (95% CI: 30.95–59.23 months), respectively (P<0.0001) (Figure 1A). The median total TOT in patients who had preexisting brain metastasis before the start of afatinib treatment was 14.50 months (95% CI: 12.45–16.39 months). Among these patients, there was no difference in the total TOT between patients with and without prior local treatment (14.3 vs. 14.6 months, P=0.622). In all study groups, the median TOT-1 was 16.60 months (95% CI: 15.01–17.81 months). The median TOT-1 for cohorts A, B, C, and D were 17.40 months (95% CI: 15.64–20.04 months), 14.20 months (95% CI: 12.48–14.95 months), 7.10 months (95% CI: 5.26–9.53 months), and 42.60 months (95% CI: 30.95–59.23 months), respectively (P<0.001) (Figure 1B). In cohorts A + B + C, the median TOT-2 was 4.00 months (95% CI: 3.48–4.40 months). The median TOT-2 for cohorts A, B, and C separately were 11.00 months (95% CI: 7.10–14.13 months), 3.30 months (95% CI: 2.69–3.71 months), and 2.40 months (95% CI: 1.74–3.81 months), respectively (P<0.001) (Figure 1C).
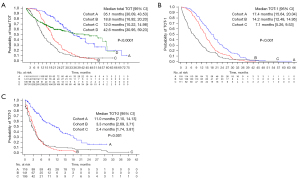
Regarding the subtype of EGFR mutations, the median total TOTs were 26.40 months (95% CI: 24.08–29.34 months), 20.60 months (95% CI: 19.91–26.45 months), 11.40 months (95% CI: 8.61–16.33 months), and 15.40 months (95% CI: 5.22–18.00 months) for Del19, L858R, uncommon mutations, and compound mutations, respectively (P<0.001).
Table 2 shows the ORR for each cohort. Among the 700 patients who could be evaluated, the ORR was 75.7%. The ORR-1 was similar in cohort A (86.0%), cohort B (82.5%), and cohort D (74.6%). Meanwhile, the ORR-2 in cohort A (56.0%) was superior to cohort B (29.1%) and cohort C (21.9%) (P<0.001). Regarding the subtype of EGFR mutation, the ORR-1 were 76.7%, 70.3%, 50.0%, and 55.6% for patients with Del19, L858R, uncommon mutations, and compound mutations, respectively.
Table 2
| ORR | Cohort A (n=116) | Cohort B (n=143) | Cohort C (n=111) | Cohort D (n=367) | Total (n=737) | P* |
|---|---|---|---|---|---|---|
| ORR-1, n (%) | 114 (100.0) | 143 (100.0) | 104 (100.0) | 339 (100.0) | 700 (100.0) | <0.001 |
| CR or PR | 98 (86.0) | 118 (82.5) | 61 (58.7) | 253 (74.6) | 530 (75.7) | |
| SD | 16 (14.0) | 23 (16.1) | 27 (26.0) | 81 (23.9) | 147 (21.0) | |
| PD | 0 (0.00) | 2 (1.40) | 16 (15.4) | 5 (1.5) | 23 (3.3) | |
| ORR-2, n (%) | 116 (100.0) | 141 (100.0) | 105 (100.0) | – | 362 (100.0) | <0.001 |
| CR or PR | 65 (56.0) | 41 (29.1) | 23 (21.9) | – | 129 (35.6) | |
| SD | 44 (37.9) | 79 (56.0) | 58 (55.2) | – | 181 (50.0) | |
| PD | 7 (6.0) | 21 (14.9) | 24 (22.9) | – | 52 (14.4) |
*, chi-square test. ORR, objective response rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
The rate of successful repeat biopsy and detection rate of T790M mutation
Among 370 patients who progressed during the period of afatinib treatment, re-biopsy was performed for 262 patients (70.8%). Tumor tissue biopsies made up 80.5% and liquid biopsies, including blood, cerebrospinal fluid, and ascites, made up 19.5% of the total re-biopsies. Common sites for tumor biopsies were lung (62.1%), lymph nodes (17.1%), and liver (4.3%). The detection rate for T790M was 44.3% in the patients having re-biopsy.
OS
The median OS was not yet reached within 21.3 months of median follow-up in the total study population. The 36-month OS rates in the total study population (cohorts A, B, C, and D) and in patients with brain metastases at the beginning of afatinib treatment were 67.3% and 65.2%, respectively. The 36-month OS rates for cohorts A, B, C, and D were 84.0%, 53.2%, 51.2%, and 73.7%, respectively (Figure 2 and Table S1). As for each EGFR mutation, the 36-month OS rates were 71.8%, 63.8%, 57.4%, and 30.1% for Del19, L858R, uncommon mutations, and compound mutations, respectively (P=0.001).
CNS efficacy
Among 287 patients with brain metastases at the beginning of afatinib treatment, 20.5% had single metastasis, 18.5% had oligo-metastases, and 61.0% had multiple metastases. Many of the patients with brain metastases were asymptomatic (74.9%). Before the beginning of afatinib treatment, 156 patients (54.4%) received local treatment: 52 patients had SRS, 58 patients had WBRT, and 3 patients underwent craniotomy and tumor removal.
The proportion of patients with brain metastases increased to 48.9% (n=172) at the time of second-line treatment: 12.8% had single metastasis, 19.2% had oligo-metastases, and 68.0% had multiple metastases. Before the start of second-line treatment, 64 patients (37.2%) received local treatment as follows: 31 patients had SRS, 23 patients had WBRT, and 10 patients underwent craniotomy and tumor removal.
CNS response rate and PFS
Among 287 patients with brain metastases at the beginning of afatinib treatment, 131 patients with no previous local therapy were classified into a CNS response cohort. In 106 patients in whom CNS response was assessed, the CNS-ORR was 66.98%. The median CNS-PFS in 287 patients with brain metastases at afatinib initiation was 24.70 months (95% CI: 19.84–33.15 months) (Figure 3A).
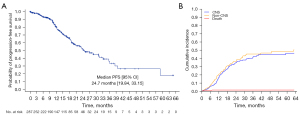
Cumulative incidence of CNS failure in patients with preexisting brain metastases
As the existence of initial brain metastasis may influence the CNS efficacy of afatinib, we performed subgroup analysis on the cumulative incidence of CNS failure for patients with preexisting brain metastasis. In 287 patients with brain metastasis before afatinib treatment, the 12-, 24-, and 36-month cumulative incidences of CNS failure were 16.5%, 34.8%, and 41.2%, respectively (Figure 3B and Table S2).
DiscussionOther Section
Debate remains regarding whether a sequential approach with 1G or 2G EGFR-TKIs or upfront treatment with a 3G EGFR-TKI is preferable in patients with EGFR M+ NSCLC. However, in clinical practice, there is no detailed data on the various clinical situation including second line treatment that has been administered after afatinib treatment. To the best of our knowledge, this is the largest retrospective study of afatinib followed by various second-line therapies, with the study design reflecting real clinical conditions with the results of re-biopsies.
With a median follow-up of 21.3 months, 50.2% of the total cohort discontinued afatinib treatment. Among 370 patients experiencing progression during the period of afatinib treatment, re-biopsy was done in 70.8% and the acquisition rate of T790M was 44.3%. The GioTag study provided the first real-world evidence that the total chemotherapy-free treatment duration was 27.7 months (37.1 months for an Asian subpopulation) using sequential afatinib followed by osimertinib treatment for EGFR M+ NSCLC patients acquiring the T790M mutation (16). In a pooled analysis of the LUX-Lung 3, 6, and 7 studies, 71% of total patients received further treatments following discontinuation of afatinib, with 28% of patients having four lines of subsequent treatment. Among them, 37% received subsequent osimertinib after treatment failure to afatinib. The median PFS of afatinib and osimertinib were 21.9 and 20.2 months, respectively (17). In cohort A, the median total TOT was 35.1 months, which is comparable to that of the GioTag study and the pooled analysis of the LUX-Lung 3, 6, and 7 studies. The median TOT-1 was 17.4 months with afatinib, and TOT-2 was 11.0 months with osimertinib. Although a direct comparison of the median TOT-2 in cohort A and the median PFS (10.1 months) in the AURA3 study requires caution, the results of the two studies are quite similar (18). In this study, only 117 out of 370 patients (32%) received osimertinib after afatinib failure. At the data cutoff, about 50% of patients were still on afatinib treatment. Because these patients whose tumors were controlled by afatinib for a long time were excluded from cohort A, the TOT in cohort A could be shorter in this study. The follow-up time to conduct the final analysis should be extended in the future.
Meanwhile, the number of patients who still showed T790M− in the re-biopsy results was 38.7% of the total study population. These patients received cytotoxic chemotherapy or other systemic therapy after treatment failure with afatinib (cohort B). The median TOT-1 was 14.2 months, but TOT-2 was 3.3 months even with systemic treatments including cytotoxic chemotherapy, which was slightly shorter than the median PFS (4.2 months) of subjects treated with pemetrexed plus carboplatin as the control arm in AURA3 (18). One possible explanation is that in cohort B of our study, a significant number of patients received pemetrexed monotherapy without platinum. Because pemetrexed plus platinum doublet after the treatment failure of the first-line EGFR-TKIs has not been reimbursed in South Korea.
Cohort C represented the patient population in whom re-biopsy was not possible due to various clinical situations including rapid disease progression, an inaccessible biopsy site, or contraindications to biopsy. In this cohort, second-line treatment was chosen without knowing the T790M mutation status, hence, the very short TOT was in line with our expectations. In addition, re-biopsy was not carried out in 29.2% of patients showing progression during the period of afatinib treatment, and liquid biopsy was available only for 19.5% of those who were tested. During the study period, EGFR detection tests using liquid biopsies have become possible and have been available since February 2017. Since liquid biopsy may become routine clinical practice, the number of patients such as those in cohort C is expected to decrease in the future.
In cohort D, the median TOT was 42.6 months, and 49.8% of patients continued with afatinib treatment after completing the collection data. This group is a unique population demonstrating a long-term duration of response with clinically controllable disease. In the clinical characteristics of long-term responders, except for less frequent brain metastases before initiation of afatinib treatment in cohort D (34.3%) vs. cohorts A + B + C (43.5%), no differences were found between the groups. Additional research including a more detailed molecular profile of co-existing genetic alterations (e.g., TP53) as well as EGFR mutations in long-term responders are warranted in the future.
In this study, the median OS was not reached at the time of data closure. The 24- and 36-month survival rates in the total study population were 80.3% and 67.3%, respectively. The 24-month survival rate was comparable that of GioTag study. In the GioTag study, the 24-month survival rate for sequential afatinib followed by osimertinib was 80%, and the median OS in the total population was 37.6 months, with 44.8 months for the Asian subpopulation. In the UpSwinG study, the median time to treatment failure was 27.7 months and the median OS was 36.5 months (19). Meanwhile, in our study, the 36-month survival rates for cohorts A, B, C, and D were 84.0%, 53.2%, 51.2%, and 73.7%, respectively. The 36-month survival rate in cohort D was lower than that of cohort A, which may be attributed to the fact that cohort D included not only patients continuing with afatinib treatment comprising those with progression, but also those who died or discontinued due to adverse drug events or were lost to follow-up. In the analysis of OS in cohort D, 164 death events (60 early-death events occurred within 36 months) occurred during the study period. Additional OS analysis with afatinib followed by sequential treatments and long-term follow-up data in cohort D are warranted. There is a lot of controversy about first-line treatment strategy for the advanced EGFR M+ NSCLC: sequential approach with afatinib vs. osimertinib upfront. Even cautious interpretation is required, it would be interesting to indirectly compare our results with those obtained from FLAURA study. In that study, the median PFS and OS were 18.9 and 38.6 months in the osimertinib group, respectively. Among the patients, 61.6% received subsequent therapy, but 28.2% died without subsequent therapy after discontinuation of osimertinib. In our study, the median OS was not reached, and median total TOT was 23.4 months, which is comparable to that of FLAURA study.
Another aim of this study was to investigate the CNS efficacy induced by afatinib-only treatment in the CNS response cohort. In the present study, the frequency of brain metastasis at the beginning of afatinib treatment was 38.9%. The most compelling reason why initial brain metastasis was much more frequent compared to historical data of 20−30% of incidence of brain metastasis is that high-resolution brain MRI scans are now performed for nearly all lung cancer patients in South Korea, so the detection rate has improved (20). The CNS response rate was 67.0% and the median CNS-PFS was 24.7 months in patients with brain metastasis prior to the initiation of afatinib treatment and no previous local therapy. In the FLAURA study, the CNS response rate was 91% in patients having measurable CNS lesions and 66% in patients having measurable and/or non-measurable lesions (21). In a previous study on CNS efficacy by afatinib at a single institute, the CNS-ORR for measurable CNS lesions was 72.9% and the CNS-PFS was 23.3 months (22). To interpret retrospective studies including our study, we must consider that cross-trial comparisons should be done with caution because MRIs were not performed at regular intervals.
Finally, we analyzed the antitumor efficacy of afatinib depending on dose adjustments. When compared with the group treated with a constant dose, the dose-adjustment group showed a similar ORR-1 and a longer TOT-1. Our results indicate that active dose adjustment due to adverse drug events did not have a negative influence on the antitumor efficacy of afatinib.
This retrospective study provides valuable insights into the treatment regimens of patients with EGFR M+ NSCLC in everyday clinical practice. Given that this study was a retrospective analysis based on electronic medical records, it has several limitations. First, a low percentage of liquid biopsies was performed for patients in whom tissue re-biopsy was not feasible. Second, there are some limitations for interpretation of CNS efficacy: the measurable CNS lesion was not defined, and a follow-up on the brain MRI was not preplanned. We were able to analyze CNS-PFS and cumulative incidence of CNS metastasis only for patients with initial brain metastasis since there was insufficient data with irregular follow-up of brain MRI scans for the brain metastasis-free patients. Last, physicians tended to choose afatinib rather than 1G EGFR-TKIs for younger patients or those with good performance status. Accordingly, the patients who were enrolled in our study may not represent all EGFR M+ NSCLC patients receiving EGFR-TKIs. However, our study reflects real-world practice and provides useful guidance for treatment decision-making.
ConclusionsOther Section
In conclusion, this study demonstrates the efficacy of first-line afatinib and subsequent treatment for advanced EGFR M+ NSCLC. A sequential approach of first-line afatinib followed by various subsequent treatments is a feasible and appropriate treatment option for patients with EGFR M+ NSCLC.
AcknowledgmentsOther Section
We thank Sunyoung Lee who performed statistical data analysis and Jimi Kim who performed data curation. Thanks to Ms. Na-rae Kim for playing important roles in writing research synopsis with coordination.
Funding: This was an independent, investigator-initiated study supported by Böehringer Ingelheim (BI). BI had no role in the design, analysis or interpretation of the results in this study; BI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BI substances, as well as intellectual property considerations. This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (No. 1720150).
FootnoteOther Section
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-79/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-79/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-79/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-79/coif). JHK has acted as an advisor for Merck Sharp & Dohme Corp. (MSD), Ono Pharmaceutical/Bristol Myers Squibb (BMS), AstraZeneca, Yuhan, Genexin, and NeoimmuneTech; has received research funds from AstraZeneca, Böehringer Ingelheim, Daichi Sankyo, and Yuhan; has acted as a speaker for Roche, Böehringer Ingelheim, and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Institutional Review Board at each institute (see the online file for detailed information) and was registered on ClinicalTrial.gov (identifier NCT04930133). The informed consent form was waived by each institutional review board due to the retrospective nature of this study. The study was conducted in accordance with the Helsinki Declaration (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
ReferencesOther Section
- Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342-50. [Crossref] [PubMed]
- Gonzales AJ, Hook KE, Althaus IW, et al. Antitumor activity and pharmacokinetic properties of PF-00299804, a second-generation irreversible pan-erbB receptor tyrosine kinase inhibitor. Mol Cancer Ther 2008;7:1880-9. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [Crossref] [PubMed]
- Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol 2016;17:577-89. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to EGFR inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid-based assay. Clin Cancer Res 2011;17:1169-80. [Crossref] [PubMed]
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Yang Z, Yang N, Ou Q, et al. Investigating Novel Resistance Mechanisms to Third-Generation EGFR Tyrosine Kinase Inhibitor Osimertinib in Non-Small Cell Lung Cancer Patients. Clin Cancer Res 2018;24:3097-107. [Crossref] [PubMed]
- Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol 2018;4:1527-34. [Crossref] [PubMed]
- Ramalingam SS, Yang JC, Lee CK, et al. Osimertinib As First-Line Treatment of EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:841-9. [Crossref] [PubMed]
- Hochmair MJ, Morabito A, Hao D, et al. Sequential treatment with afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: an observational study. Future Oncol 2018;14:2861-74. [Crossref] [PubMed]
- Park K, Bennouna J, Boyer M, et al. Sequencing of therapy following first-line afatinib in patients with EGFR mutation-positive non-small cell lung cancer. Lung Cancer 2019;132:126-31. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Popat S, Jung HA, Lee SY, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG). Lung Cancer 2021;162:9-15. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Itakura M, et al. Frequency of brain metastases in non-small-cell lung cancer, and their association with epidermal growth factor receptor mutations. Int J Clin Oncol 2015;20:674-9. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Jung HA, Woo SY, Lee SH, et al. The different central nervous system efficacy among gefitinib, erlotinib and afatinib in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer. Transl Lung Cancer Res 2020;9:1749-58. [Crossref] [PubMed]



