
Safety and efficacy of immune checkpoint inhibitors in non-small cell lung cancer patients with preexisting antinuclear antibodies: a retrospective cohort study
Introduction
Lung cancer, causing an estimated 1.6 million deaths each year, is the most common cause of cancer death globally (1,2). According to the histological type, lung cancer can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Adenocarcinoma comprises approximately 40–50% of NSCLC, and squamous cell carcinoma comprises 20–30%. In recent years, immunotherapy has brought new hope to patients with lung cancer (3-8). Immune checkpoint inhibitors (ICIs), monoclonal antibodies that bind to programmed cell death-1 (PD-1)/programmed death ligand-1 (PD-L1) and cytotoxic T-lymphocyte antigen 4 (CTLA-4), can boost anti-tumor immunity by blocking T-cell inhibition. However, an exaggerated T-cell response against normal tissues could generate high levels of proinflammatory cytokines, leading to unique toxicities named immune-related adverse events (irAEs).
Several potential risk factors for the development of irAEs from ICIs have been suggested, including interleukin (IL)-17, eosinophil counts, absolute lymphocyte count, preexisting autoimmune diseases (AIDs), and so on (9-14). Furthermore, previous studies indicated that preexisting anti-thyroglobulin (ATG) and anti-thyroid peroxidase (ATPO) were more common among patients receiving ICIs who subsequently developed thyroid dysfunction (15,16). Besides, Biomarkers including PD-L1 expression, density of tumor infiltrating lymphocyte (TIL), tumor mutational burden (TMB), and mismatch-repair (MMR) deficiency, gut microbiota, circulating biomarkers, and patient previous history are regarded as being associated with of efficacy of ICIs (17,18). Moreover, some researchers also attempted to discover the relationship between antinuclear antibodies (ANAs) and the safety and efficacy of ICIs but acquired different results (15,19-21).
ANAs, which identify nuclear macromolecules and their complexes, are a diverse group of autoantibodies (22,23). It is generally believed that ANAs are associated with various systemic rheumatic diseases, including systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), among others. However, ANAs are also present in a substantial proportion of healthy individuals, where they have been associated with demographic factors such as older age, female sex, and parity, among others (24). A previous study has shown that based on different cut-off values of serum dilution titer, the proportion of positive ANAs in the healthy population is quite different, including 31.7% at 1:40, 13.3% at 1:80, 5.0% at 1:160, and 3.3% at 1:320 (25).
Considering that individuals with positive ANAs might present susceptibility to AIDs which might compromise safety but boost efficacy of ICIs, it is reasonable to speculate that immunotherapy in NSCLC patients with positive ANAs may demonstrate different safety profiles and efficacy. However, clinical trial data on ICIs in these patients are not only sparse but also different from different studies (15,19-21). Moreover, the cut-off value of positive ANA titer varied among studies. Herein, our research investigates the safety and efficacy of ICIs in locally-advanced or metastatic NSCLC patients according to different ANA titers and briefly discusses the effects of ATG and ATPO on the safety of ICIs, in hopes of providing more information for the predictive biomarkers of ICIs. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-464/rc).
Methods
Patients
This cohort study was conducted retrospectively. Patients eligible for inclusion were collected under the following criteria: (I) All patients were confirmed to have NSCLC by a pathology assessment in Peking Union Medical College Hospital from January 1, 2015 to December 31, 2020; (II) TNM stages of tumors were III–IV; (III) All patients received ICIs (PD-1 or PD-L1 or CTLA-4 inhibition with or without chemotherapy). (IV) Peripheral blood samples were collected before the initiation of immunotherapy. The exclusion criteria included the followings: (I) patients suffering from any symptomatic autoimmune diseases; (II) critical medical records and imaging data were not available; (III) peripheral blood samples were not qualified for testing ANAs. Finally, 159 Chinese patients were included in our study. Patients were followed up every 2 months until December 31, 2020 or death or loss to follow-up and the number of follow-up times could be increased whenever they had new complaints of discomfort.
Data collection
The patients’ characteristics such as age, gender, Eastern Cooperative Oncology Group (ECOG) performance status score, smoking history, pathology, stage, coexisting driver gene mutations, treatment lines, and treatment strategies were retrieved from medical records. Missing values regarding characteristics and tumor response were ignored.
Evaluation of safety
The severity of irAEs was graded according to the Common Terminology Criteria for Adverse Events (CTCAE) 5.0.
Evaluation of efficacy
According to the solid tumor efficacy evaluation standard [Response Evaluation Criteria in Solid Tumors (RECIST 1.1)], the evaluation grade was divided into complete remission (complete response, CR), partial remission (partial response, PR), stable (stable disease, SD), and disease progression (progressive disease, PD). The objective response rate (ORR) was defined as the proportion of patients whose tumors shrunk to a certain amount and maintained for a certain period of time, and included the proportion of CR and PR. The disease control rate (DCR) was defined as the overall proportion of patients with remission or in a stable condition after treatment, including the proportion of CR, PR, and SD. Progression-free survival (PFS) and overall survival (OS) were defined as the time from the beginning of immunotherapy to tumor progression (PFS) or death (OS) from any cause.
Measurement of ANAs, ATG and ATPO
Blood samples were collected before the initiation of immunotherapy and archival samples were allowed. ANAs were detected by the indirect immunofluorescence assay, and the detection reagent which used Hep-2 cells and monkey liver as the antigen matrix was provided by EUROIMMUN Medizinische Labordiagnostika AG (Germany). ANA titer ≥1:80 is commonly regarded as positive. In our study, in order to explore the effect of different ANA titers on the efficacy and safety of immunotherapy, we used 1:80, 1:160, and 1:320 as the cut-off values for negative (less than the cut-off value) or positive (greater than or equal to the cut-off value) ANA. ATG and ATPO were measured by the electrochemiluminescence immunoassay, ECLIA (Cobas; Roche, Basel, Switzerland). The reference intervals were 0 to 115 IU/mL for ATG and 0 to 34 IU/mL for ATPO.
Statistical analysis
All data were analyzed and processed by SPSS 22.0. The χ2 test or Fisher’s exact test was used to compare categorical variables. Student’s t and Kruskal-Wallis H tests were used to compare continuous variables. Survival outcomes were estimated with the Kaplan-Meier method and compared between groups using the log-rank test. For multivariate analysis, logistic regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs). All statistical tests were two-sided, and P<0.05 indicated a statistical difference. Figures were created with GraphPad Prism (San Diego, CA, USA).
Ethical approval
This study was performed in accordance with the principles for Good Clinical Practice and the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital on September 1, 2021 (No. S-K1744) and individual consent for this retrospective analysis was waived.
Results
Baseline characteristics
A total of 159 NSCLC patients treated with ICIs were enrolled in this study. The baseline characteristics are shown in Table 1. Whether 1:80, 1:160, or 1:320 were used as the cut-off values, baseline characteristics including age, gender, ECOG performance status score, smoking history, pathology, stage, coexisting driver gene mutations, treatment lines and strategies showed no significant differences between the negative and positive groups.
Table 1
| Characteristics | ANA <1:80 (N=73) | ANA ≥1:80 (N=86) | P | ANA <1:160 (N=103) | ANA ≥1:160 (N=56) | P | ANA <1:320 (N=135) | ANA ≥1:320 (N=24) | P |
|---|---|---|---|---|---|---|---|---|---|
| Age | 0.484 | 0.889 | 0.242 | ||||||
| ≤60 years | 25 (34.2%) | 25 (29.2%) | 32 (31.1%) | 18 (32.1%) | 40 (29.6%) | 10 (41.7%) | |||
| >60 years | 48 (65.8%) | 61 (70.9%) | 71 (68.9%) | 38 (67.9%) | 95 (70.4%) | 14 (58.3%) | |||
| Gender | 0.864 | 0.324 | 0.442 | ||||||
| Male | 51 (69.9%) | 59 (68.6%) | 74 (71.8%) | 36 (64.3%) | 95 (70.4%) | 15 (62.5%) | |||
| Female | 22 (30.1%) | 27 (31.4%) | 29 (28.2%) | 20 (35.7%) | 40 (29.6%) | 9 (37.5%) | |||
| ECOG performance status | 0.591 | 1.000 | 0.235 | ||||||
| 0–1 | 67 (91.8%) | 79 (95.2%) | 95 (93.1%) | 51 (94.4%) | 125 (94.0%) | 21 (91.3%) | |||
| ≥2 | 6 (8.2%) | 4 (4.8%) | 7 (6.9%) | 3 (5.6%) | 8 (6.0%) | 2 (8.8%) | |||
| Smoking history | 0.632 | 0.544 | 0.656 | ||||||
| No | 27 (37.0%) | 28 (33.3%) | 34 (33.3%) | 21 (38.2%) | 46 (34.3%) | 9 (39.1%) | |||
| Yes | 46 (63.0%) | 56 (66.7%) | 68 (66.7%) | 34 (61.8%) | 88 (66.7%) | 14 (60.9%) | |||
| Pathology | 0.437 | 0.166 | 0.581 | ||||||
| Adenocarcinoma | 44 (60.3%) | 47 (54.7%) | 58 (56.3%) | 33 (58.9%) | 78 (57.8%) | 13 (54.2%) | |||
| Squamous cell carcinoma | 25 (34.2%) | 35 (40.7%) | 40 (38.8%) | 20 (35.7%) | 49 (36.3%) | 11 (45.8%) | |||
| Adenosquamous carcinoma | 1 (1.4%) | 3 (3.5%) | 1 (1.0%) | 3 (5.4%) | 4 (3.0%) | 0 | |||
| Large cell carcinoma | 3 (4.1%) | 1 (1.2%) | 4 (3.9%) | 0 | 4 (3.0%) | 0 | |||
| Stage | 0.260 | 0.182 | 0.493 | ||||||
| III | 19 (26.0%) | 16 (18.6%) | 26 (25.2%) | 9 (16.1%) | 31 (23.0%) | 4 (16.7%) | |||
| IV | 54 (74.0%) | 70 (81.4%) | 77 (74.8%) | 47 (83.9%) | 104 (77.0%) | 20 (83.3%) | |||
| Driver gene mutations | 0.742 | 0.602 | 0.266 | ||||||
| Positive | |||||||||
| EGFR | 19 (26.0%) | 18 (20.9%) | 25 (24.3%) | 12 (21.4%) | 30 (22.2%) | 7 (29.2%) | |||
| KRAS | 6 (8.2%) | 5 (5.8%) | 9 (8.7%) | 2 (3.6%) | 11 (8.1%) | 0 | |||
| ROS-1 | 4 (5.5%) | 4 (4.7%) | 5 (4.9%) | 3 (5.4%) | 8 (5.9%) | 0 | |||
| Negative | 44 (60.3%) | 61 (70.9%) | 64 (62.1%) | 39 (69.6%) | 86 (63.7%) | 17 (70.8%) | |||
| Treatment lines | 0.395 | 0.983 | 0.726 | ||||||
| First line | 32 (43.8%) | 45 (52.3%) | 49 (47.6%) | 28 (50.0%) | 66 (48.9%) | 11 (45.8%) | |||
| Second line | 22 (30.1%) | 27 (31.4%) | 32 (31.1%) | 17 (30.4%) | 43 (31.9%) | 6 (25.0%) | |||
| Third line | 12 (16.4%) | 7 (8.1%) | 13 (12.6%) | 6 (10.7%) | 15 (11.1%) | 4 (16.7%) | |||
| ≥ Fourth line | 7 (9.6%) | 7 (8.1%) | 9 (8.7%) | 5 (8.9%) | 11 (8.1.%) | 3 (12.5%) | |||
| Treatment received | 0.763 | 0.413 | 0.354 | ||||||
| ICI monotherapy [anti-PD-(L)-1] | 25 (34.2%) | 33 (38.4%) | 36 (35.0%) | 22 (39.3%) | 47 (34.8%) | 11 (45.8%) | |||
| Combined immunotherapy [anti-PD-(L)1 & anti-CTLA4] | 1 (1.4%) | 2 (2.3%) | 1 (1.0%) | 2 (3.6%) | 2 (1.5%) | 1 (4.2%) | |||
| Chemoimmunotherapy* | 47 (64.4%) | 51 (59.3%) | 66 (64.1%) | 32 (57.1%) | 86 (63.7%) | 12 (50.0%) | |||
| Median duration of ICIs, months | 6.5 | 6.7 | 0.946 | 6.3 | 7.5 | 0.386 | 6.5 | 6.6 | 0.817 |
*, chemoimmunotherapy means immunotherapy [anti-PD-(L)1 ± anti-CTLA4] combined with chemotherapy (platinum doublet or single drug chemotherapy including paclitaxel, docetaxel, pemetrexed and gemcitabine). ANA, antinuclear antibodies; ECOG, Eastern Cooperative Oncology Group; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; CTLA-4, cytotoxic T-lymphocyte antigen 4; ICI, immune checkpoint inhibitor.
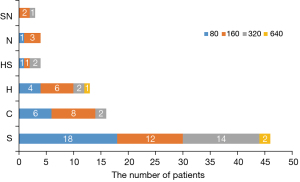
The positive rate of ANA in patients with NSCLC when using 1:80 as the cut-off value was 54.1%, significantly higher than 13.3% in healthy controls (25). As shown in Figure 1, the staining pattern included speckled type (S, 53.5%), cytoplasmic type (C, 18.6%), homogeneous type (H, 15.1%), homogeneous-speckled type (HS, 4.7%), nucleolar type (N, 4.7%), and speckled-nucleolar type (SN, 3.5%).
Safety and efficacy
A total of 28.9% (46/159) of the patients developed 64 irAEs, and 5.7% (9/159) developed 11 grade 3–5 irAEs (Table 2). These adverse reactions manifested as pruritus, rash, scalp ulcer, hypothyroidism, hyperthyroidism, elevated alanine aminotransferase, hepatitis, interstitial pneumonia, renal failure, and nephrotic syndrome, among others. Most adverse reactions were mild, without the need for glucocorticoids and ICI treatment interruption.
Table 2
| Adverse events | N (%)* | Grade ≥3 irAEs, N |
|---|---|---|
| Cutaneous | 21 (32.8) | |
| Rash | 18 (28.1) | 3 |
| Pruritus | 2 (3.1) | |
| Scalp ulcer | 1 (1.6) | |
| Endocrine disorders | 20 (31.3) | |
| Hypothyroidism | 9 (14.1) | |
| Hyperthyroidism | 8 (12.5) | |
| Hypophysitis | 1 (1.6) | 1 |
| Adrenocortical dysfunction | 1 (1.6) | |
| Hyperglycemia | 1 (1.6) | |
| Gastrointestinal | 10 (15.6) | |
| Alanine aminotransferase/bilirubin increase | 8 (12.5) | 1 |
| Pancreatitis or lipase/amylase increase | 2 (3.1) | 1 |
| Cardiovascular | 5 (7.8) | |
| Myocarditis/cardiac troponin I increase | 4 (6.3) | |
| Heart failure | 1 (1.6) | 1 |
| Pneumonitis | 4 (6.3) | 1 |
| Renal | 2 (3.1) | |
| Elevated creatinine | 1 (1.6) | |
| Membranous nephropathy | 1 (1.6) | 1 |
| Fever | 1 (1.6) | |
| Nervous system | 1 (1.6) |
*, total =64 events in 46 patients. irAEs, immune-related adverse events.
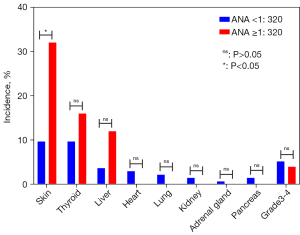
The median follow-up time was 12.2 months (95% CI: 10.5 to 13.9). Among the 46 patients who suffered from irAEs, 19 (26.0%) patients in the negative group (ANA <1:80) and 27 (31.4%) in the positive group (ANA ≥1:80) developed irAEs (P=0.457). Similarly, when using 1:160 as the cut-off value, the incidence of irAEs was 26.2% vs. 33.9% (P=0.305) in both groups. However, with the cut-off value of 1:320, the incidence of irAEs in the negative (ANA titer <1:320) and positive (ANA titer ≥1:320) groups was 25.9% vs. 45.8% (P=0.047). In multivariate models (Table 3), ANA titer ≥1:320 was also associated with irAEs (OR =4.9, 95% CI: 1.45–16.52, P=0.01). Further analysis showed a significant difference in the incidence of adverse skin reactions between the 2 groups (9.7% vs. 32%, P=0.003). Adverse reactions involving other systems, such as the thyroid, liver, heart, lung, and kidney, showed no significant differences between the 2 groups (Figure 2), and no significant differences were observed regarding grade 3/4 irAEs.
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Age | |||||
| ≤60 years | 1 (reference) | 1 (reference) | |||
| >60 years | 0.61 (0.3–1.26) | 0.18 | 0.79 (0.35–1.77) | 0.56 | |
| Gender | |||||
| Male | 1 (reference) | 1 (reference) | |||
| Female | 0.98 (0.46–2.05) | 0.95 | |||
| ECOG performance status | 1.63 (0.49–5.44) | 0.42 | |||
| 0–1 | 1 (reference) | 1 (reference) | |||
| ≥2 | 0.25 (0.03–2.03) | 0.19 | 0.17 (0.02–1.67) | 0.13 | |
| Smoking history | |||||
| No | 1 (reference) | 1 (reference) | |||
| Yes | 1.02 (0.49–2.09) | 0.97 | 0.96 (0.3–3.02) | 0.94 | |
| Pathology | |||||
| Adenocarcinoma | 1 (reference) | 1 (reference) | |||
| Squamous cell carcinoma | 1.15 (0.56–2.37) | 0.71 | 0.87 (0.33–2.3) | 0.77 | |
| Adenosquamous carcinoma | 2.83 (0.37–21.55) | 0.31 | 0.81 (0.06–10.17) | 0.87 | |
| Large cell carcinoma | 0.72 (0.07-7.99) | 0.79 | 3.96 (0.35–44.21) | 0.26 | |
| Stage | |||||
| III | 1 (reference) | 1 (reference) | |||
| IV | 0.72 (0.32–1.61) | 0.43 | 0.72 (0.27–1.89) | 0.50 | |
| Driver gene mutations | |||||
| Negative | 1 (reference) | 1 (reference) | |||
| Positive | 0.80 (0.38–1.67) | 0.55 | 0.74 (0.23–2.46) | 0.63 | |
| Treatment lines | |||||
| First line | 1 (reference) | 1 (reference) | |||
| Second line | 0.78 (0.36–1.71) | 0.54 | 1.29 (0.38–4.4) | 0.68 | |
| Third line | 0.37 (0.1–1.38) | 0.14 | 0.41 (0.08–2.13) | 0.29 | |
| ≥ Fourth line | 0.53 (0.14–2.09) | 0.37 | 1 (0.15–6.52) | 1.00 | |
| Treatment received | |||||
| ICI monotherapy [anti-PD-(L)-1] | 1 (reference) | 1 (reference) | |||
| Combined immunotherapy [anti-PD-(L)1 & anti-CTLA4] | 1.93 (0.14–25.95) | 0.62 | 1.45 (0.06–32.97) | 0.82 | |
| Chemoimmunotherapy* | 1.50 (0.71–3.16) | 0.29 | 1.51 (0.48–4.79) | 0.49 | |
| ANA titers | |||||
| <1:320 | 1 (reference) | 1 (reference) | |||
| ≥1:320 | 2.42 (0.99–5.89) | 0.05 | 4.9 (1.45–16.52) | 0.01 | |
| ANA karyotypes | |||||
| None | 1 (reference) | 1 (reference) | |||
| S | 1.20 (0.53–2.72) | 0.67 | 0.78 (0.28–2.17) | 0.64 | |
| C | 1.64 (0.52–5.14) | 0.39 | 0.9 (0.25–3.31) | 0.88 | |
| H | 0.50 (0.10–2.45) | 0.39 | 0.22 (0.03–1.39) | 0.11 | |
| SN | 0 (0–0) | 1.00 | 0 | 1.00 | |
| HS | 2.74 (0.36–20.82) | 0.33 | 1 (0.09–11.17) | 1.00 | |
| N | 8.21 (0.8–83.83) | 0.08 | 7 (0.6–81.77) | 0.12 | |
*, chemoimmunotherapy means immunotherapy [anti-PD-(L)1 ± anti-CTLA4] combined with chemotherapy (platinum doublet or single drug chemotherapy including paclitaxel, docetaxel, pemetrexed and gemcitabine). irAEs, immune-related adverse events; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibitor; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; ANA, antinuclear antibody; S, speckled type; C, cytoplasmic type; H, homogeneous type; SN, speckled-nucleolar type; HS, homogeneous-speckled type; N, nucleolar type; OR, odds ratio; CI, confidence interval.
The ORR and DCR of the ANA negative and positive groups with the cut-off value of 1:80 were 37.1% vs. 43.9% (P=0.398) and 88.6% vs. 82.9% (P=0.324), respectively. As shown in the Kaplan-Meier curves of PFS (Figure 3), the median progression-free survival (mPFS) was 17.7 vs. 10 months (P=0.603). When using 1:160 as the cut-off value, the ORR, DCR and mPFS of the ANA negative and positive groups were 35.7% vs. 50.0% (P=0.086), 87.8% vs. 81.5% (P=0.293), 11.9 vs. 10.6 months (P=0.957) respectively (Figure 4). What is more, the ORR, DCR and mPFS of the ANA negative (ANA <1:320) and positive groups (ANA ≥1:320) were 40.3% vs. 43.5% (P=0.450), 87.6% vs. 73.9% (P=0.086) and 11.9 vs. 7.7 months (P=0.471), respectively (Figure 5).



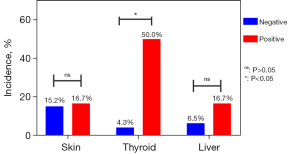
Moreover, 52 out of 159 patients were tested for ATG and ATPO. Of these, 46 (88.5%) patients were negative and 6 (11.5%) were positive. A total of 34.6% (18/52) of the patients developed 27 irAEs, mainly including 8 skin adverse events (rash: 7; pruritus: 1), 5 thyroid disfunction (hypothyroidism: 3, hyperthyroidism: 2), 4 liver adverse events (elevated alanine aminotransferase), 2 pulmonary adverse events (interstitial pneumonia) and 2 renal adverse events (nephrotic syndrome: 1; elevated serum creatinine: 1) and some more scarce ones. 7.7% (4/52) of patients developed 6 grade 3–5 irAEs. Because of limited data, further analysis was carried out on 3 mostly affected systems. As shown in Figure 6, the incidence of thyroid dysfunction was 4.3% vs. 50% (P=0.005) between the negative and positive groups. Adverse reactions involving the skin and liver showed no significant differences.
Discussion
The introduction of ICIs into the treatment of NSCLC has transformed the therapeutic landscape. A large number of studies have shown that the application of immunotherapy for specific NSCLC patients can produce profound benefits (26-29). However, patients with preexisting AIDs are typically excluded from clinical trials of ICIs given concerns of exacerbating the underlying AIDs and leading to irAEs. Immune-related toxicities include the more commonly seen events such as hypothyroidism or skin rash and the more scarce and severe diseases like colitis, pneumonitis, autoimmune hepatitis, and encephalitis (30-34). A total of 7–13% of NSCLC patients treated with PD-1 inhibitors experienced grade 3 or higher toxicities, and up to 2% of patients receiving ICIs have died from therapy-related toxicities (35-38). Fortunately, corticosteroids and immunosuppressants can manage most irAEs successfully. Most adverse reactions in our study were mild, without the need to stop ICIs and initiate corticosteroids and immunosuppressants.
Some researchers in recent years have been exploring the influence of preexisting AIDs on immunotherapy. In a study that enrolled 751 patients who suffered from malignant tumors and received ICI monotherapy, 85 patients (11.3%) had preexisting AIDs, including clinically active state (17.6%) and inactive state (82.4%) (39). Among the patients with preexisting AIDs, the incidence of irAEs of any grade was significantly higher than those without AIDs (65.9% vs. 39.9%, P<0.0001). However, no significant differences were observed regarding grade 3/4 irAEs. Regarding ORR, PFS, and OS, there were also no significant differences between the 2 groups. In a single-arm international study named SAUL (NCT02928406), 997 patients were enrolled and received atezolizumab, among which 35 patients had AIDs at baseline. Median OS was 8.2 months (95% CI: 6.5–11.7 months) and 8.8 months (95% CI: 7.6–9.9 months) in patients with or without pre-existing AIDs, respectively, which meant efficacy was similar between 2 groups (40). These findings indicate that AIDs have no significant effects on the effectiveness of immunotherapy, but will compromise safety (40-43). However, more data is needed in this population.
ANAs, observed in patients with a wide range of AIDs, are regarded as critical biomarkers when assessing systemic rheumatic diseases. ANAs may also be involved in the pathogenesis of cancer as well as other premalignant diseases (44). Meanwhile, ANA testing has come into common use in clinical practice. Growing evidence indicates that autoantibodies occur several years earlier than symptomatic AIDs. Because of this, clinicians are cautious when administering immunotherapy to these patients. Nevertheless, the prevalence of ANAs in the US has increased from 11.0% to 15.9% in the recent 2 decades (45). Therefore, there is an urgent need to clarify the effects of ANAs on the efficacy and safety of immunotherapy in tumor patients.
Our study analyzed a total of 159 Chinese patients diagnosed with locally-advanced or metastatic NSCLC and given immunotherapy (PD-1 or PD-L1 or CTLA-4 inhibition with or without chemotherapy). All patients were tested for the status of ANAs. The influence of different cut-off values of ANA titers including 1:80, 1:160, and 1:320 on the safety and efficacy of immunotherapy were also discussed. When using 1:80 or 1:160 as the cut-off values of negative or positive ANA, irAEs of any grade, ORR, DCR, mPFS and median overall survival (mOS) of ICIs between groups showed no significant difference. However, with the cut-off value of 1:320, the incidence rates of irAEs were 25.9% vs. 45.8% (P=0.047) for patients in the negative or positive group. In multivariate models, ANA titer ≥1:320 was also associated with irAEs (OR =4.9, 95% CI: 1.45–16.52, P=0.01). Nevertheless, the probability of grade 3–4 adverse reactions did not increase significantly in the positive group. Further analysis showed a significant difference in the incidence of adverse skin reactions between the 2 groups (9.7% vs. 32%, P=0.003). The ORR, DCR and mPFS between the ANA negative (ANA <1:320) and positive groups (ANA ≥1:320) showed no significant difference. Besides, our study suggested patients with ATG or ATPO were more likely to suffer from thyroid dysfunction when receiving immunotherapy.
Meanwhile, we retrieved 4 relevant articles by searching the keywords “Immunotherapy”, “Immune Checkpoint Inhibitors”, “Neoplasms” and “Antinuclear Antibodies” on PubMed (Figure 7 and Table 4). Morimoto et al. conducted a retrospective study of 77 patients with advanced NSCLC treated with ICIs and chemotherapy, focusing on the effects of preexisting ANAs on the safety and efficacy of immunotherapy (20). Using ANA=1:160 as a cut-off value, patients were divided into the negative group (ANA <1:160) and positive group (ANA ≥1:160). The incidence of irAEs of any grade in patients with or without ANAs was 62.5% vs. 49.3% (P=0.71). Whereas, the incidence of discontinuation of all treatment components due to severe adverse events was higher in the ANA-positive group (50% vs. 15.9%, P=0.042). The ANA-positive group had a shorter PFS and OS than the ANA-negative group [hazard ratio (HR) =2.11, 95% CI: 0.88–5.07, P=0.093; and HR =3.11, 95% CI: 1.14–8.49, P=0.027, respectively].
Table 4
| Author | Toi (15) | Yoneshima (21) | Sakakida (19) | Morimoto (20) |
|---|---|---|---|---|
| Year | 2019 | 2019 | 2020 | 2020 |
| Cases | 137 | 83 | 191 | 77 |
| Treatment | PD-1 monotherapy | PD-1 monotherapy | PD-1/PD-L1 | ICI + chemotherapy |
| ANA cut-off value | 1:40 | 1:40 | 1:160 | 1:160 |
| Overall response rate (PG/NG) | 38%/28%, P=0.35 | NA | 12.50%/26.50%, P=0.38 | NA |
| Disease control rate (PG/NG) | 77%/66%, P=0.26 | NA | 37.50%/67.50%, P=0.08 | NA |
| Median progression-free survival (PG/NG) (months) | NA, P=0.67 | 2.9/3.8, P=0.03 | NA | 6.2/10.2, P=0.09 |
| Median overall survival (PG/NG) (months) | NA | 11.6/15.8, P=0.03 | NA | 7/not reached, P=0.02 |
| Development of irAE (PG/NG) | 60%/42%, P=0.05 | 33.30%/32.30%, P=1.00 | 44.40%/37.90%, P=0.69 | 62.50%/49.30%, P=0.71 |
| Development of irAE ≥ grade 3 (PG/NG) | 8%/6%, P=0.80 | 11.10%/6.20%, P=0.64 | NA | 37.50%/14.50%, P=0.13 |
NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death ligand 1; ICI, immune checkpoint inhibitor; ANA, antinuclear antibodies; PG, positive group, with preexisting ANAs; NG, negative group, without preexisting ANAs; NA, not applicable; irAE, immune-related adverse events.
However, according to the research performed by Sakakida et al. (19) which used the same cut-off value, the incidence of irAEs of any grade in both groups did not show a significant difference despite the fact that patients with positive ANAs were more likely to develop colitis (2/9 vs. 3/182, P=0.0002). Similarly, ORR and DCR also showed no significant differences between the 2 groups.
Moreover, using another cut-off value, some researchers obtained utterly different results. In 2019, Yoneshima et al. published an article using 1:40 as the cut-off value for negative or positive ANA (21). A total of 83 patients were analyzed in the study, and 18 (21.7%) were positive for ANA (ANA ≥1:40). Negative and positive groups did not differ in terms of the incidence of irAEs (32.3% vs. 33.3%), but PFS (3.8 vs. 2.9 months, P=0.03) and OS (15.8 vs. 11.6 months, P=0.03) were significantly longer in the negative group.
In another retrospective study involving 137 patients (15), the presence of preexisting antibodies including rheumatoid factor, ANAs, ATG, and ATPO was independently associated with any grade of irAEs (OR =3.25, 95% CI: 1.59–6.65, P=0.001). The median PFS for patients with or without any preexisting antibodies was 6.5 months (95% CI: 4.4–12.9) vs. 3.5 months (95% CI: 2.4–4.1) (HR =0.53, 95% CI: 0.36–0.79, P=0.002). However, in the subgroup analysis of patients with ANAs at pretreatment, there were no significant differences in the increase of the incidence of irAEs of any grade and benefits of PFS, ORR, DCR compared with those without ANAs.
It can be seen from the above studies that different research has shown quite different effects of preexisting ANAs on the safety and efficacy of immunotherapy. In terms of security (the incidence of irAEs), the negative group was not inferior to the positive group. However, in terms of efficacy (ORR or DCR or mPFS or mOS), several studies have drawn contrasting results. Our study suggested that with the cut-off value of 1:320, patients with preexisting ANAs were more likely to suffer from irAEs, especially in terms of skin adverse events. But no significant differences were observed regarding grade 3/4 irAEs. Besides, the status of ANAs might have no correlation with the clinical benefit of immunotherapy.
However, our study has some limitations. Firstly, its retrospective nature and limited number of patients, especially those with positive ANA (titer ≥1:320) or ATG/ATPO, may have introduced case selection bias and restricted the generalizability of the results. Moreover, the expression levels of PD-L1 were not routinely evaluated in China. Therefore, we were unable to assess the baseline expression levels of PD-L1 between groups. We believe further prospective investigations with a large sample size are urgently needed.
Conclusions
Our study suggested preexisting ANAs might not be associated with the clinical benefit of ICI treatment in patients with NSCLC. However, patients with ANA titer ≥1:320 have a greater risk of suffering from skin adverse reactions. Additionally, patients with positive ATG or ATPO had the potential to develop abnormal thyroid function. In summary, for NSCLC patients with ANAs at baseline, we believe that it may be acceptable to use immunotherapy under close monitoring for adverse reactions, especially for those with significantly elevated ANA titers. Further studies are still needed to confirm these findings.
Acknowledgments
The authors appreciate the academic support from the AME Lung Cancer Collaborative Group.
Funding: This study was supported by the Youth Program of the National Natural Science Foundation of China (to YX) (No. 82003309), CAMS Innovation Fund for Medical Sciences (CIFMS) (to YX) (No. 2021-I2M-C&T-B-014), and by the CAPTRA-Lung Research Funds (No. CAPTRALung2021005).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-464/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-464/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-464/coif). LK receives honoraria from AMGEN. TH has received personal fees from Chugai Pharmaceutical outside the submitted work. MP received grants and consulting fees from BMS, MSD, Lilly, AZ and Takeda; received support for attending meetings from MSD and AZ; received payment honoraria for lectures from BMS, MSD, AZ and Takeda. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was performed in accordance with the principles for Good Clinical Practice and the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Peking Union Medical College Hospital on September 1, 2021 (No. S-K1744) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389:299-311. [Crossref] [PubMed]
- Neal RD, Sun F, Emery JD, et al. Lung cancer. BMJ 2019;365:l1725. [Crossref] [PubMed]
- Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res 2019;25:4592-602. [Crossref] [PubMed]
- Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc 2019;94:1623-40. [Crossref] [PubMed]
- Buddharaju LNR, Ganti AK. Immunotherapy in lung cancer: the chemotherapy conundrum. Chin Clin Oncol 2020;9:59. [Crossref] [PubMed]
- Khozin S, Miksad RA, Adami J, et al. Real-world progression, treatment, and survival outcomes during rapid adoption of immunotherapy for advanced non-small cell lung cancer. Cancer 2019;125:4019-32. [Crossref] [PubMed]
- Proto C, Ferrara R, Signorelli D, et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev 2019;75:39-51. [Crossref] [PubMed]
- Steven A, Fisher SA, Robinson BW. Immunotherapy for lung cancer. Respirology 2016;21:821-33. [Crossref] [PubMed]
- Gowen MF, Giles KM, Simpson D, et al. Baseline antibody profiles predict toxicity in melanoma patients treated with immune checkpoint inhibitors. J Transl Med 2018;16:82. [Crossref] [PubMed]
- Johnson DB, Balko JM. Biomarkers for Immunotherapy Toxicity: Are Cytokines the Answer? Clin Cancer Res 2019;25:1452-4. [Crossref] [PubMed]
- Lim SY, Lee JH, Gide TN, et al. Circulating Cytokines Predict Immune-Related Toxicity in Melanoma Patients Receiving Anti-PD-1-Based Immunotherapy. Clin Cancer Res 2019;25:1557-63. [Crossref] [PubMed]
- Nakamura Y, Tanaka R, Maruyama H, et al. Correlation between blood cell count and outcome of melanoma patients treated with anti-PD-1 antibodies. Jpn J Clin Oncol 2019;49:431-7. [Crossref] [PubMed]
- Shi Y, Liu X, Liu J, et al. Correlations between peripheral blood biomarkers and clinical outcomes in advanced non-small cell lung cancer patients who received immunotherapy-based treatments. Transl Lung Cancer Res 2021;10:4477-93. [Crossref] [PubMed]
- Tarhini AA, Zahoor H, Lin Y, et al. Baseline circulating IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother Cancer 2015;3:39. [Crossref] [PubMed]
- Toi Y, Sugawara S, Sugisaka J, et al. Profiling Preexisting Antibodies in Patients Treated With Anti-PD-1 Therapy for Advanced Non-Small Cell Lung Cancer. JAMA Oncol 2019;5:376-83. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [Crossref] [PubMed]
- Qu J, Mei Q, Liu L, et al. The progress and challenge of anti-PD-1/PD-L1 immunotherapy in treating non-small cell lung cancer. Ther Adv Med Oncol 2021;13:1758835921992968. [Crossref] [PubMed]
- Yi M, Jiao D, Xu H, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer 2018;17:129. [Crossref] [PubMed]
- Sakakida T, Ishikawa T, Chihara Y, et al. Safety and efficacy of PD-1/PD-L1 blockade in patients with preexisting antinuclear antibodies. Clin Transl Oncol 2020;22:919-27. [Crossref] [PubMed]
- Morimoto K, Yamada T, Nakamura R, et al. Impact of preexisting antinuclear antibodies on combined immunotherapy and chemotherapy in advanced non-small cell lung cancer patients. Med Oncol 2020;37:111. [Crossref] [PubMed]
- Yoneshima Y, Tanaka K, Shiraishi Y, et al. Safety and efficacy of PD-1 inhibitors in non-small cell lung cancer patients positive for antinuclear antibodies. Lung Cancer 2019;130:5-9. [Crossref] [PubMed]
- Bossuyt X, De Langhe E, Borghi MO, et al. Understanding and interpreting antinuclear antibody tests in systemic rheumatic diseases. Nat Rev Rheumatol 2020;16:715-26. [Crossref] [PubMed]
- Pisetsky DS, Lipsky PE. New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 2020;16:565-79. [Crossref] [PubMed]
- Pisetsky DS. Antinuclear antibody testing - misunderstood or misbegotten? Nat Rev Rheumatol 2017;13:495-502. [Crossref] [PubMed]
- Tan EM, Feltkamp TE, Smolen JS, et al. Range of antinuclear antibodies in "healthy" individuals. Arthritis Rheum 1997;40:1601-11. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. First-Line Nivolumab Plus Ipilimumab in Advanced NSCLC: 4-Year Outcomes From the Randomized, Open-Label, Phase 3 CheckMate 227 Part 1 Trial. J Thorac Oncol 2022;17:289-308. [Crossref] [PubMed]
- Rogers JE, Ajani JA. The role of ramucirumab and pembrolizumab combination in patients with advanced non-small cell lung cancer, gastroesophageal adenocarcinoma, or urothelial carcinoma. Chin Clin Oncol 2021;10:30. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Gutierrez C, McEvoy C, Munshi L, et al. Critical Care Management of Toxicities Associated With Targeted Agents and Immunotherapies for Cancer. Crit Care Med 2020;48:10-21. [Crossref] [PubMed]
- Reid PD, Cifu AS, Bass AR. Management of Immunotherapy-Related Toxicities in Patients Treated With Immune Checkpoint Inhibitor Therapy. JAMA 2021;325:482-483. [Crossref] [PubMed]
- Spiers L, Coupe N, Payne M. Toxicities associated with checkpoint inhibitors-an overview. Rheumatology (Oxford) 2019;58:vii7-16. [Crossref] [PubMed]
- Fujiwara Y, Horita N, Harrington M, et al. Incidence of hepatotoxicity associated with addition of immune checkpoint blockade to systemic solid tumor therapy: a meta-analysis of phase 3 randomized controlled trials. Cancer Immunol Immunother 2022; Epub ahead of print. [Crossref] [PubMed]
- Fujiwara Y, Horita N, Namkoong H, et al. The effect of adding immune checkpoint inhibitors on the risk of pneumonitis for solid tumours: a meta-analysis of phase III randomised controlled trials. Eur J Cancer 2021;150:168-78. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:700-12. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Cortellini A, Buti S, Santini D, et al. Clinical Outcomes of Patients with Advanced Cancer and Pre-Existing Autoimmune Diseases Treated with Anti-Programmed Death-1 Immunotherapy: A Real-World Transverse Study. Oncologist 2019;24:e327-37. [Crossref] [PubMed]
- Loriot Y, Sternberg CN, Castellano D, et al. Safety and efficacy of atezolizumab in patients with autoimmune disease: Subgroup analysis of the SAUL study in locally advanced/metastatic urinary tract carcinoma. Eur J Cancer 2020;138:202-11. [Crossref] [PubMed]
- Danlos FX, Voisin AL, Dyevre V, et al. Safety and efficacy of anti-programmed death 1 antibodies in patients with cancer and pre-existing autoimmune or inflammatory disease. Eur J Cancer 2018;91:21-9. [Crossref] [PubMed]
- Kehl KL, Yang S, Awad MM, et al. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother 2019;68:917-26. [Crossref] [PubMed]
- Martinez Chanza N, Xie W, Issa M, et al. Safety and efficacy of immune checkpoint inhibitors in advanced urological cancers with pre-existing autoimmune disorders: a retrospective international multicenter study. J Immunother Cancer 2020;8:e000538. [Crossref] [PubMed]
- Vlagea A, Falagan S, Gutiérrez-Gutiérrez G, et al. Antinuclear antibodies and cancer: A literature review. Crit Rev Oncol Hematol 2018;127:42-9. [Crossref] [PubMed]
- Dinse GE, Parks CG, Weinberg CR, et al. Increasing Prevalence of Antinuclear Antibodies in the United States. Arthritis Rheumatol 2020;72:1026-35. [Crossref] [PubMed]



