
Open-label, multi-center, phase II study of adjuvant pemetrexed plus cisplatin for completely resected stage IB to IIIA adenocarcinoma of the lung: APICAL trial
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. In South Korea, the crude mortality rates per 100,000 population in 2018 were 51.5 in men and 18.1 in women; lung cancer had the highest cancer death rate both in men and women (1-3). Since approximately 40% of patients with non-small cell lung cancer (NSCLC) were diagnosed with stage IV disease (4), the role of lung cancer screening and surgery has been increasingly important in improving the survival rate. According to previous studies of lung cancer registry in South Korea, patients with NSCLC underwent surgery as an initial treatment (37.6%) with (33.8%) or without adjuvant therapy (66.2%) (4,5).
Even for early-stage NSCLC resected with curative intent, nearly half of patients experienced relapse within 5 years after surgery. In a study of Japanese registry, the 5-year postoperative survival rates were reported to be 64.1% for stage IIA, 56.1% for stage IIB, and 47.9% for stage IIIA (6). Postoperative adjuvant chemotherapy using platinum doublet in patients with NSCLC showed a modest survival benefit with a hazard ratio of 0.89 [95% confidence interval (CI): 0.82–0.96] for death in a meta-analysis of 4,584 patients [the Lung Adjuvant Cisplatin Evaluation (LACE) trial] from phase III trials comparing cisplatin-based doublet chemotherapy and no chemotherapy (7). As the vinorelbine-platinum combination only showed a significant survival benefit in the subgroup analysis of the LACE trial, vinorelbine plus cisplatin was used as the standard adjuvant chemotherapy.
As the pemetrexed-platinum combination had been used as a standard regimen for metastatic non-squamous cell NSCLC (8), the Japanese group conducted a randomized phase III trial (JIPANG, a multi-center, open-label phase III trial) to evaluate the efficacy of adjuvant pemetrexed plus cisplatin (Pem-Cis) and that of vinorelbine plus cisplatin (Vin-Cis) in patients with stage II–IIIA non-squamous NSCLC (9). Although the superiority of Pem-Cis was not proven, Pem-Cis showed a better toxicity profile compared with Vin-Cis.
In South Korea, the pemetrexed-platinum combination regimen as postoperative adjuvant chemotherapy had not been reimbursed by the Korean Health Insurance Review and Assessment at the time of trial initiation in 2015 (which was later reimbursed in May 2021). To confirm the efficacy of Pem-Cis, a single-arm Pem-Cis adjuvant chemotherapy trial was conducted in patients with completely resected lung adenocarcinoma (LUAD). The primary endpoint was to confirm the superiority of a 2-year disease-free survival rate (DFSR) compared to historical control without adjuvant treatment (50%). We present the following article in accordance with the TREND reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-183/rc).
Methods
Study participants and procedures
An open-label, multi-center, prospective phase II study was conducted to evaluate the efficacy of postoperative adjuvant Pem-Cis. Between August 2015 and February 2018, patients from seven tertiary medical centers in South Korea were recruited. Those with completely resected pathologic stage IB–IIIA LUAD (Union for International Cancer Control TNM classification, seventh edition) were enrolled in this study. Figure 1 shows the flowchart of the patient enrollment process. The eligible patients were aged >20 years, had an Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0–2, had no history of receiving chemotherapy, and had adequate marrow, hepatic, and renal function. The other inclusion and exclusion criteria are described in Appendix 1.
The enrolled patients received intravenous infusions of pemetrexed (500 mg/m2) plus cisplatin (75 mg/m2) on day 1. Adjuvant treatments were initiated within 4 to 8 weeks after surgery and were administered every 3 weeks for 4 cycles. A daily dose of oral folic acid (1 mg per day) was administered a week before the initiation of pemetrexed treatment and maintained until at least 3 weeks after the final dose. In addition, an intramuscular injection of vitamin B12 (1 mg) was administered within 7 days after the first dose of pemetrexed and until 3 weeks after the last dose of pemetrexed was provided. Monitoring of toxicity, including physical examination and laboratory tests, were performed at every visit for administration and on days 8 to 15 at each cycle. In patients without recurrence, postoperative radiotherapy was not permitted. Follow-up assessment with chest computed tomography and bone scan was continued every 3 months for the first year and then every 4 months for the second year.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. This study was approved by the ethics review board of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2015-007) and also approved by each participating institution. All patients were required to provide written and informed consent before participating in the study. The trial was registered at clinicaltrial.gov (Identifier: NCT02498860).
Outcomes and statistical analysis
The primary endpoint was to confirm whether adjuvant Pem-Cis in patients with completely resected LUAD is superior to historical control without adjuvant treatment (50%) in terms of a 2-year DFSR. The 2-year DFSR of the control group was calculated based on the median disease-free survival (DFS) reported in the ANITA trial, using a sample size calculation software, nQuery (2015, Statsols, Statistical Solutions Ltd., Cork, Ireland). The median DFS in the control and intervention groups were 20.7 months (95% CI: 16.1–28.6) and 36.3 months (95% CI: 28.0–52.1), respectively (10). Assuming that the DFS showed an exponential tendency, the 2-year DFSR was estimated to be 44.8% in the control group and 63.2% in the intervention group. In the present trial, we hypothesized that the 2-year DFSR of the historical control would be 50% because the survival rate of Asians is generally higher than that of Caucasians, and the 2-year DFSR of adjuvant Pem-Cis is at least 63%. Taken together with the expected follow-up time of 2 years, a significant level of 5% (two-sided), a power of 80%, and a drop-out rate of 10%, the required number of patients for enrollment was calculated as 105.
The secondary endpoints were overall survival (OS), toxicity profiles, ECOG PS score, and 4-cycle completion rate. According to the study protocol, DFS was measured from the first date of drug administration to the first date of objective recurrence or death from any cause and was censored at the date of last patient contact before the data lock point. OS was measured from the first date of drug administration to the date of death from any cause and was censored on the date of the last patient contact before the cut-off date. The adverse events were graded based on the severity, using the Common Terminology Criteria for Adverse Events (version 4.3). Efficacy analysis was performed for the intention-to-treat (ITT) population. For toxicity profiles, only patients who received at least one dose of Pem-Cis were included.
All data were expressed as mean ± standard deviation or as numbers and percentages. Intergroup comparisons were performed using t-test for continuous variables and Pearson’s χ2 test or Fisher’s exact test for categorical variables. Survival times were estimated using the Kaplan-Meier method. Statistical analysis was performed using IBM® SPSS® statistics version 25 (IBM Corp., Armonk, NY, USA) and R statistics (11), and a P value of <0.05 was considered significant.
Results
Patients’ characteristics
As shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram of Figure 1, 114 patients were recruited between August 2015 and February 2018. Nine patients were excluded due to squamous cell carcinoma (n=1), stage IV disease (n=1), and consent withdrawal (n=7). A total of 105 patients from seven institutions were enrolled in the study and designated as the ITT population.
The baseline characteristics of the 105 patients are shown in Table 1. The median age was 63 years (range, 38–78), and 54 (51.4%) patients were women. Thirty-three (31.4%) patients had pathologic stage IB, 34 (32.4%) had stage IIA, 12 (11.4%) had stage IIB, and 26 (24.8%) had stage IIIA determined based on the TNM staging 7th edition. When the 8th Edition of the TNM staging system was applied, six patients with stage IIIA were upstaged to stage IIIB. Most of the patients underwent lobectomy (n=98, 93.3%), three underwent sublobar resection (segmentectomy or wedge resection), and four underwent bilobectomy or pneumonectomy. The most frequent type of gene alteration was epidermal growth factor receptor (EGFR) mutation (37/90, 41.1%), followed by anaplastic lymphoma kinase (ALK) rearrangement (11/91, 12.1%). Four cycles of Pem-Cis were administered in 99 patients (94.3%), and dose reduction was performed in 38 patients (36.2%).
Table 1
| Characteristic | No. of patients (%) |
|---|---|
| Age, years, median [range] | 63.0 [38–78] |
| Sex: male/female | 51 (48.6)/54 (51.4) |
| Smoking: never/current/ex-smoker | 56 (53.3)/14 (33.3)/35 (13.3) |
| ECOG PS† : 0/1/2 | 60 (57.7)/44 (42.3)/0 (0.0) |
| Pathologic stage (TNM 7th) | |
| IB/IIA/IIB/IIIA | 33 (31.4)/34 (32.4)/12 (11.4)/26 (24.8) |
| pT1/pT2/pT3/pT4 | 22 (21.0)/69 (65.7)/13 (12.4)/1 (1.0) |
| pN0/pN1/pN2 | 49 (46.7)/32 (30.5)/24 (22.6) |
| Histology, adenocarcinoma | 105 (100.0) |
| Differentiation | |
| Well/moderate/poor/undifferentiated | 7 (6.7)/47 (44.8)/41 (39.0)/10 (9.5) |
| Surgery | |
| Sublobar resection‡ | 3 (2.9) |
| Lobectomy | 98 (93.3) |
| Bilobectomy/pneumonectomy | 3 (2.9)/1 (1.0) |
| TTF-1 IHC (n=73): positive/negative | 65(89.0)/8(11.0) |
| EGFR (n=90): mutant/wild | 37 (41.1)/53 (58.9) |
| ALK (n=91): mutant/wild | 11 (12.1)/80 (87.9) |
| ROS1 (n=28): mutant/wild | 0 (0.0)/28 (100.0) |
| Cycles of chemotherapy | |
| 1/2/3/4 | 3 (2.9)/2 (1.9)/1 (1.0)/99 (94.3) |
†, PS status data was missed in one patient; ‡, segmentectomy (n=2) or wedge resection (n=1). ECOG PS, Eastern Cooperative Oncology Group performance status; TTF-1, thyroid transcription factor-1; IHC, immunohistochemistry; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TNM, Tumor-Node-Metastasis.
Survival outcomes
The survival outcomes of patients treated with Pem-Cis are shown in Figure 2. At a median follow-up of 57.7 months (95% CI: 55.3–58.7), the median DFS was not reached, while the 2-year DFSR was 78.1% (95% CI: 70.6–86.4, Figure 2A). In patients with pathologic stage IIA to IIIA, the median DFS was 60.0 months (95% CI: 35.6–not calculated, Figure S1A), while the 2-year DFSR was 73.6% (95% CI: 64.1–84.5%, Figure S1B). The OS was not yet mature (17 events, 16.2%).
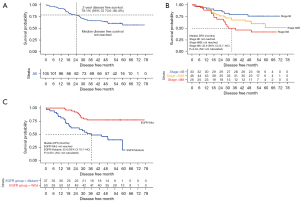
At the time of data cut-off (January 5, 2022), 43 patients (41.0%) experienced relapse (Figure 1). The characteristics of relapsed patients and subsequent treatment are described in Table 2. Seventeen patients (16.2%) experienced local relapse, while 23 patients (21.9%) showed distant metastasis. Distant metastasis commonly occurred in contralateral lung (9.5%, n=10), followed by the brain (7.6%, n=8) and bones (6.7%, n=7). With regard to the subsequent treatment after relapse (n=43), tyrosine kinase inhibitors (TKIs) for EGFR mutation or ALK rearrangement (51.2%, n=22) were frequently used, followed by cytotoxic chemotherapy (23.3%, n=10) and radiation therapy, including concurrent chemoradiation therapy (CCRT) (9.3%, n=4).
Table 2
| Characteristic, n (%) | Total (n=105) | EGFR mutation test (n=90) | EGFR wild-type (n=53) | EGFR mutant (n=37) | P |
|---|---|---|---|---|---|
| Progression status | |||||
| Disease-free | 59 (56.2) | 51 | 37 (69.8) | 14 (37.8) | 0.001 |
| Relapsed | 43 (41.0) | 36 | 13 (24.5) | 23 (62.2) | |
| Lost follow-up | 3 (2.9) | 3 | 3 (5.7) | 0 (0.0) | |
| Relapse pattern | |||||
| Local | 17 (16.2) | 14 | 4 (7.5) | 10 (27.0) | 0.452 |
| Distant metastasis | 23 (21.9) | 22 | 9 (17.0) | 13 (35.1) | |
| Contralateral lung | 10 (9.5) | 9 | 3 (33.3) | 6 (46.2) | – |
| Pleura | 5 (4.8) | 5 | 2 (22.2) | 3 (23.1) | – |
| Brain | 8 (7.6) | 8 | 4 (44.4) | 4 (30.8) | – |
| Bones | 7 (6.7) | 7 | 1 (11.1) | 6 (46.2) | – |
| Adrenal gland | 1 (1.0) | 1 | 0 (0.0) | 1 (7.7) | – |
| Unknown | 3 (2.9) | 0 | 0 (0.0) | 0 (0.0) | – |
| Subsequent treatment after relapse† | |||||
| Operation | 2 (1.9) | 2 | 0 (0.0) | 2 (8.7) | – |
| Radiation therapy | 4 (3.8) | 3 | 0 (0.0) | 3 (13.0) | – |
| Radical RT/CCRT | 1 (1.0)/3 (2.9) | 1/2 | 0 (0.0) | 1 (4.3)/2 (8.7) | |
| Cytotoxic chemotherapy | 10 (9.5) | 9 | 8 (61.5) | 1 (4.3) | – |
| Platinum doublet/monotherapy | 9 (8.6)/1 (1.0) | 8/1 | 7 (53.8)/1 (7.7) | 1 (4.3)/0 (0.0) | |
| Tyrosine kinase inhibitor | 22 (21.0) | 20 | 3 (23.1) | 17 (73.9) | – |
| EGFR/ALK | 20 (19.0)/2 (1.9) | 17/3 | 0 (0.0)/3 (23.1) | 17 (73.9)/0 (0.0) | |
| Immune checkpoint inhibitor | 1 (1.0) | 1 | 1 (7.7) | 0 (0.0) | – |
†, only the first treatment after relapse was described. RT, radiation therapy; CCRT, concurrent chemoradiation therapy; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase.
The Kaplan-Meier curves for DFS were clearly separated according to the pathologic stage from IB to IIIA (P=0.04; Figure 2B). The median DFS of stage IB and IIA–IIB disease was not yet reached, while that of stage IIIA disease was 32.6 months (95% CI: 25.7–not calculated). Although we did not investigate TTF-1 immunohistochemistry (IHC) prospectively, TTF-1 IHC was performed in 73 patients (69.5%), and a majority of patients showed positivity (n=65, 89.0%) (Table 1). There was no significant difference in DFS between TTF-1 IHC positive and negative patients (P=0.386). Patients with EGFR mutation showed a significantly higher recurrence rate (62.2% vs. 24.5%, P=0.001; Table 2) and shorter median DFS (35.6 months vs. not reached, P<0.001; Figure 2C) than patients with wild-type EGFR. In the multivariable analysis for DFS, pathologic stage IIIA disease and EGFR mutation were significant risk factors for DFS (Table 3).
Table 3
| Variables, n (%) | Total (n=105) | Univariable | Multivariable | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |||
| Age | 0.799 | – | ||||
| <65 years | 60 (57.1) | 1 | – | |||
| ≥65 years | 45 (42.9) | 1.081 (0.592–1.975) | – | |||
| Sex | 0.643 | – | ||||
| Female | 54 (51.4) | 1 | – | |||
| Male | 51 (48.6) | 0.867 (0.475–1.584) | – | |||
| Smoking | 0.767 | – | ||||
| Never | 56 (53.3) | 1 | – | |||
| Ever | 49 (46.7) | 1.095 (0.601–1.994) | – | |||
| ECOG PS (n=104) | 0.378 | – | ||||
| 0 | 60 (57.7) | 1 | – | |||
| 1 | 44 (42.3) | 1.309 (0.719–2.385) | – | |||
| Pathologic stage (TNM 7th) | ||||||
| IB | 33 (31.4) | 1 | 0.028 | 1 | 0.008 | |
| IIA–IIB | 46 (43.8) | 1.978 (0.865–4.522) | 0.106 | 2.125 (0.776–5.815) | 0.142 | |
| IIIA | 26 (24.8) | 3.172 (1.355–7.428) | 0.008 | 4.741 (1.672–13.440) | 0.006 | |
| Pathologic T stage | 0.151 | – | ||||
| pT1–2 | 91 (86.7) | 1 | – | |||
| pT3–4 | 14 (13.3) | 1.757 (0.815–3.789) | – | |||
| Pathologic N stage | 0.071 | – | ||||
| pN0–1 | 81 (77.1) | 1 | – | |||
| pN2 | 24 (22.9) | 1.800 (0.950–3.409) | – | |||
| Differentiation | 0.215 | – | ||||
| Well or moderate | 54 (51.4) | 1 | – | |||
| Poor or Undifferentiated | 51 (48.6) | 0.681 (0.371–1.250) | – | |||
| Surgery | 0.917 | – | ||||
| Lobectomy | 98 (93.3) | 1 | – | |||
| Others† | 7 (6.7) | 0.939 (0.290–3.039) | – | |||
| EGFR mutation (n=90) | <0.001 | <0.001 | ||||
| Negative | 53 (58.9) | 1 | 1 | |||
| Positive | 37 (41.1) | 3.548 (1.789–7.039) | 4.178 (2.055–8.492) | |||
| ALK rearrangement (n=91) | 0.457 | – | ||||
| Negative | 80 (87.9) | 1 | – | |||
| Positive | 11 (12.1) | 0.674 (0.239–1.904) | – | |||
| Cycles of chemotherapy | 0.508 | – | ||||
| Complete (4 cycles) | 99 (94.3) | 1 | – | |||
| Incomplete (<4 cycles) | 6 (5.7) | 1.486 (0.460–4.808) | – | |||
†, segmentectomy (n=2), wedge resection (n=1), bilobectomy (n=3) or pneumonectomy (n=1). HR, hazard ratio; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ALK, anaplastic lymphoma kinase; TNM, Tumor-Node-Metastasis.
Toxicity and safety
The adverse events of 101 patients are summarized in Tables S1,S2. Fatigue, cough, and gastrointestinal symptoms, including anorexia, nausea, and vomiting, were common treatment-related adverse events of Pem-Cis. None of the patients developed grade 4 adverse events. A total of 21 serious adverse events were reported, including pneumonia and pulmonary thromboembolism, in each of the three patients (2.9%) (Table 4). Grade 3 adverse events occurred in 10 patients (9.5%) with leukopenia as the most common adverse event (n=3, 2.9%). No treatment-related death was reported.
Table 4
| Adverse events | No. of patients (%) |
|---|---|
| Pneumonia | 3 (2.9) |
| Pulmonary thromboembolism | 3 (2.9) |
| Nausea | 3 (2.9) |
| Vomiting | 2 (1.9) |
| Liver enzyme increased | 2 (1.9) |
| Abdominal pain | 1 (1.0) |
| Colitis | 1 (1.0) |
| Fever | 1 (1.0) |
| Hyponatremia | 1 (1.0) |
| Leukocytosis | 1 (1.0) |
| White blood cell decreased | 1 (1.0) |
| Other infections† | 1 (1.0) |
| Pleural effusion | 1 (1.0) |
†, acute phase reactant elevation.
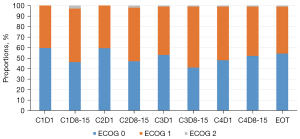
Monitoring of toxicity, including ECOG PS score, physical examination, and laboratory tests, was performed at every visit for administration and on days 8–15 at each cycle. The proportions of patients with ECOG PS score 1 or 2 increased on days 8–15 at cycles 1–3, while the proportion of patients whose ECOG PS score was obtained on cycle 4 and end-of-treatment (EOT) visit remained unchanged (Figure 3).
Discussion
This open-label, multi-center, prospective phase II trial showed the favorable efficacy of Pem-Cis as postoperative adjuvant chemotherapy for LUAD compared with historical control without adjuvant treatment. The 2-year DFSR as the primary endpoint was 78.1% (95% CI: 70.6–86.4), while the median DFS was not reached. Although we cannot compare DFS directly with prior trials, the 2-year DFSR was numerically longer than historical control (50%) and chemotherapy group of ANITA trial (63.2%). Compared with previous trials on Pem-Cis as adjuvant therapy (Table 5), the median follow-up duration of the present trial is the longest, which is close to 5 years (57.7 months), and the survival rate is outstanding (9,10,12-15). Although the proportion of patients with stage IB disease was relatively high, the median DFS of the present trial was superior even if it was evaluated only in patients with stage IIA–IIIA disease (60.0 months). The relatively low percentage of stage IIIA patients in this trial might have contributed to a longer DFS compared with the ANITA trial. The proportion of pathologic stage IIIA patients (24.8%) in the present trial was numerically lower compared with that in the control arm of ANITA trial (36.7%). However, these two studies applied different editions of TNM classification: the 7th edition in the present trial and the 1986 version in the ANITA trial. The T descriptors have changed with version updates, while the N descriptors have remained consistent. The proportions of pathologic N stage (N0, N1, N2) between two trials were similar: 46.7%, 30.5%, 22.6% in the present trial, and 43.4%, 31.4%, 24.5% in the control arm of ANITA trial.
Table 5
| Characteristics, n (%) | ANITA (10) - control | Zhang, et al. (12) | TREAT (13,14) | JIPANG (9) | Tachihara, et al. (15) | APICAL - Present trial |
|---|---|---|---|---|---|---|
| Nationality | Europe (14 countries) | China | Germany and Belgium | Japan | Japan | South Korea |
| Ethnicity | Caucasian | Asian | Caucasian | Asian | Asian | Asian |
| No. of patients | 433 | 82 | 67 | 389 | 21 | 105 |
| Age, mean or median | 59 | 58 | 58 | 64 | 66 | 63 |
| Sex: female/male | 13%/87% | 32%/68% | 28%/72% | 42%/58% | 43%/57% | 51%/49% |
| Stage | IB–IIIA (TNM 1986) | II–IIIA (TNM 7th) | IB–IIIA(T3N1) (TNM 6th) | II–IIIA (TNM 7th) | II–IIIA (TNM 7th) | IB–IIIA (TNM 7th) |
| Histology | NSCLC | Non-squamous NSCLC | NSCLC | Non-squamous NSCLC | Non-squamous NSCLC | Adenocarcinoma |
| EGFR mutation | NA | NA | NA | 24.9% | 19.0% | 41.1% (37/90) |
| Regimen | Observation | Pemetrexed-Carboplatin | Pemetrexed-Cisplatin | Pemetrexed-Cisplatin | Pemetrexed-Cisplatin | Pemetrexed-Cisplatin |
| Planned cycle | NA | 4 | 4 | 4 | 4 | 4 |
| Completion rate | NA | 85.4% | 77.6% | 87.9% | 81.0% | 94.3% |
| Median follow-up, months (range) | 77 (43–116) | 33 (9–53) | 34.1 (1.2–58.3) | 45.2 (34.7–57.1) | 20.7 (7.6–55.9) | 57.7 (95% CI: 55.3–58.7) |
| Median DFS, months (95% CI) | 20.7 (16.1–28.6) | Stage II, 38.0 (28.1–47.9); IIIA, 21.0 (13.7–28.3) | NR | 38.9 (28.7–55.3) | 25.8 (19.6–NR) | Stage IB–IIIA, NR; IIA–IIIA, 60.0 (95% CI: 35.6–NR) |
| 2-year DFSR, % (95% CI) | 44.8% (estimation) | Stage II, 70.5%; IIIA, 45.9% | 59% (3 years) | 58.3% (53.2–63.0%) (2 years); 51.1% (45.8–56.0%) (3 years) | 57.3% (32.2–76.1%) | Stage IB–IIIA, 78.1% (95% CI: 70.6–86.4%); IIA–IIIA, 73.6% (64.1–84.5%) |
| Median OS, months (95% CI) | 43.7 (35.7–52.3) | Stage II, NR; IIIA, 36 (25.9–46.1) | NR | NR; 3-year OS rate, 87.2% | NA | NR |
| Grade 3 or 4 neutropenia | <1% | 13.4% | 9% | 19.4% | 0% | 2.9% (leukopenia) |
NSCLC, non-small cell lung cancer; NA, not applicable; DFS, disease-free survival; CI, confidence interval; NR, not reached; DFSR, disease-free survival rate; OS, overall survival; EGFR, epidermal growth factor receptor; TNM, Tumor-Node-Metastasis.
The present trial had no control arm comparable to adjuvant Pem-Cis. To date, two representative randomized trials comparing the efficacy of adjuvant Pem-Cis and Vin-Cis have been reported (9,13). The TREAT trial was conducted in Caucasian patients with stage IB–IIIA (TNM 6th) NSCLC (13), while the JIPANG trial was performed in Asian patients with stage II–IIIA (TNM 7th) non-squamous NSCLC (9). These two trials reported that Pem-Cis had better feasibility with less toxicity compared with Vin-Cis; however, they failed to prove the superiority of Pem-Cis in terms of survival rates (9,14). Although the outcomes between the two previous studies and the present trial should be carefully compared, the DFS rates and DFS of Pem-Cis in the present trial were better compared with that of Vin-Cis in the two previous studies (Table 5). The high proportion of women, high rates of completion of four treatment cycles, low frequency of severe adverse events, and advances in surgical techniques and perioperative management might improve the survival of patients in the present trial. Therefore, Pem-Cis could be a favorable adjuvant chemotherapy for non-squamous NSCLC.
The high completion rate of this trial might be associated with the well-known tolerability of the Pem-Cis regimen and advances in supportive care during chemotherapy. Figure 3 depicts the serial changes in ECOG PS score; in this figure, the patients showed generally favorable PS during chemotherapy. A temporary decrease in PS score was observed during the first three cycles of Pem-Cis, while no changes were observed in the fourth cycle and at the EOT visit. Cisplatin is a typical antineoplastic agent with high emetic risk; several guidelines recommend the use of a 3- or 4-antiemetic drug combination, such as an NK1 receptor antagonist, a serotonin (5-HT3) receptor antagonist, dexamethasone, and/or olanzapine in cisplatin-containing regimens (16-18). The optimal management of chemotherapy-induced nausea and vomiting using guideline-driven antiemetics has been associated with improved quality of life, longer duration of anticancer treatment, and decreased utilization of emergency care (19). In South Korea, a 3-drug combination with NK1 receptor antagonist, 5-HT3 receptor antagonist, and dexamethasone has been reimbursed, and this regimen was used in all participating institutions.
Previous adjuvant trials demonstrated the superiority in safety profiles of Pem-Cis over Vin-Cis, especially in terms of hematologic toxicity such as neutropenia. In TREAT and JIPANG trials, the incidence of > grade 3 neutropenia was significantly lower in Pem-Cis compared with that in Vin-Cis, 9% versus 69% in TREAT trial (13) and 22.7% versus 81.2% in JIPANG trial, respectively (9). In the present trial, grade 3 leukopenia was reported in only three patients (2.9%). Although the possibility that it has been underreported could not be ruled out, only one case of leukopenia was reported, and grade 3 adverse events occurred only in 10 patients (9.5%). Therefore, Pem-Cis could be an alternative to Vin-Cis due to its safety and efficacy.
The efficacy of the first-line Pem-Cis treatment in patients with advanced non-squamous NSCLC was superior compared with that of other platinum doublet regimens, especially in East Asian patients (20). In addition, the use of a pemetrexed-containing regimen improved the progression-free survival (PFS) as a first-line treatment or a sequential option following TKI failure in advanced NSCLC patients with targetable driver mutations, such as EGFR, ALK, or ROS1 (21,22). However, in the present trial, patients who harbored EGFR mutations showed a worse DFS compared with those with wild-type EGFR. In the JIPANG trial, the subgroup analysis of patients who harbored EGFR mutations showed that recurrence-free survival tended to be worse in patients treated with Pem-Cis compared with those treated with Vin-Cis (9). However, in patients without EGFR mutations, recurrence-free survival tended to be better in patients treated with Pem-Cis compared with those treated with Vin-Cis. Hence, EGFR mutation status could influence the action of pemetrexed, which is a multi-target antifolate agent, and affect the efficacy of chemotherapy. This hypothesis was based on the result of a previous study, which indicated that EGFR-mutant cells were less sensitive to fluorouracil compared with EGFR wild-type cells in an in vitro experiment; moreover, the adjuvant chemotherapy with uracil-tegafur (an antimetabolite), which combines fluorouracil prodrug and uracil, did not prolong the survival of patients with resected EGFR-mutant LUAD (23).
Besides the molecular mechanism mentioned above, EGFR mutations have been associated with a higher risk of systemic recurrence and a worse PFS after definitive treatment in patients with stage I to III NSCLC (24,25). In a Korean retrospective study, patients with EGFR mutation showed shorter PFS compared with those without EGFR mutation, and the brain was the most common site of distant metastasis in patients with stage III non-squamous NSCLC who underwent CCRT (24). In another retrospective study, the metastatic recurrence rate was significantly higher in NSCLC patients with EGFR mutation than those with wild-type EGFR, especially in stage I patients who underwent definitive surgery (25). In the present trial, compared with wild-type EGFR, EGFR mutation was significantly associated with a higher recurrence rate and shorter median DFS. In the ADAURA study, osimertinib, a third-generation EGFR-TKI, as adjuvant therapy, was associated with a significantly longer DFS in stage IB to IIIA EGFR-mutated NSCLC compared with placebo, regardless of prior adjuvant cytotoxic chemotherapy (26). Thus, EGFR-TKI, especially osimertinib, can be an effective adjuvant regimen for this patient group, while the OS results of the ADAURA trial were immature; the impact of osimertinib with or without adjuvant chemotherapy on the cure rate still needs to be evaluated further. In a real-world study of Chinese patients with resected stage I to III LUAD, adjuvant chemotherapy did not improve the DFS and OS of patients with EGFR mutation (27). In the present trial, the median OS of patients with an EGFR mutation was not statistically different. The majority of patients who harbored EGFR mutation and developed recurrence after adjuvant Pem-Cis treatment received EGFR-TKI therapy (73.9%, 17/23), while the remaining 6 patients were treated with radiation, surgery or chemotherapy for local relapses (Table 2).
In the last few years, immune checkpoint inhibitor (ICI) therapy with or without chemotherapy is a well-established adjuvant or neoadjuvant treatment for NSCLC. The IMpower 010 study demonstrated significantly improved the DFS of stage II–IIIA NSCLC patients treated with adjuvant atezolizumab following surgery and adjuvant chemotherapy, particularly those with tumor PD-L1 of ≥1% (28). Moving forward, neoadjuvant immunotherapy has been in the spotlight owing to the fact that ICI therapy prior to surgery resulted in an increase in diverse T-cell responses compared with adjuvant therapy; moreover, a systemic antitumor response from previously activated T-cells still occurred even after the surgical resection of tumors (29). In the CheckMate 816 study, neoadjuvant platinum-doublet chemotherapy plus nivolumab produced significant improvements in pathologic complete response compared with chemotherapy alone (30). Therefore, ICI therapy combined with cytotoxic chemotherapy as adjuvant and neoadjuvant treatments could have a synergistic influence on the survival benefit of early-stage high-risk NSCLC patients; at the same time, a more personalized approach with reliable biomarkers should be developed for optimal treatment.
This trial has several limitations. First, OS was not yet mature, and no control arm was used to compare the effect of Pem-Cis in patients who relapsed. Hence, a longer follow-up should be performed, and other parameters for predicting the survival should be considered in order to assess whether the DFS benefit can safely be presumed as an OS benefit. Second, a biomarker study to select the appropriate patients who would have benefitted from adjuvant Pem-Cis therapy was not performed. However, the significantly poor survival of EGFR mutants suggests the need to further study the role of adjuvant chemotherapy and/or target treatments in patients with driver mutations. Lastly, we had no plan to proceed to a phase III trial. When we planned this trial, adjuvant Pem-Cis was not reimbursed by national health insurance in South Korea. Thus, the purpose of this trial was to support Pem-Cis as one of the adjuvant chemotherapy regimen.
In conclusion, this study proved the superiority of adjuvant Pem-Cis in terms of 2-year DFSR compared with historical control without adjuvant treatment; meanwhile, the proportion of patients with stage IB disease and driver mutations were relatively higher compared with that of patients included in previous trials (Table 5) (9,10,12-15). The Pem-Cis regimen also showed favorable efficacy in stage IIA to IIIA LUAD; moreover, patients with EGFR mutation might consider EGFR-TKI therapy as an adjuvant or subsequent treatment. In addition, Pem-Cis showed favorable tolerability as an adjuvant chemotherapy.
Acknowledgments
This study was presented at e-poster session of the 2021 Korean Association for Lung Cancer International Conference.
Funding: This study was supported by Shin Poong Pharm. Co., Ltd.
Footnote
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-183/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-183/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-183/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-183/coif). YCK reports a company funding from Shin Poong Pharm. Co., Ltd. for this study; grants from AstraZeneca and Boehringer Ingelheim; payments from AstraZeneca, MSD, Yuhan, Roche, Boehringer Ingelheim, Ono, BMS and Amgen. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and Good Clinical Practice guidelines. This study was approved by the ethics review board of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2015-007) and also approved by each participating institution. All patients were required to provide written and informed consent before participating in the study. The trial was registered at clinicaltrial.gov (Identifier: NCT02498860).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Park S, Choi CM, Hwang SS, et al. Lung Cancer in Korea. J Thorac Oncol 2021;16:1988-93. [Crossref] [PubMed]
- Hong S, Won YJ, Lee JJ, et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res Treat 2021;53:301-15. [Crossref] [PubMed]
- Kweon SS. Updates on Cancer Epidemiology in Korea, 2018. Chonnam Med J 2018;54:90-100. [Crossref] [PubMed]
- Choi CM, Kim HC, Jung CY, et al. Report of the Korean Association of Lung Cancer Registry (KALC-R), 2014. Cancer Res Treat 2019;51:1400-10. [Crossref] [PubMed]
- Lee JG, Kim HC, Choi CM. Recent Trends of Lung Cancer in Korea. Tuberc Respir Dis (Seoul) 2021;84:89-95. [Crossref] [PubMed]
- Okami J, Shintani Y, Okumura M, et al. Demographics, Safety and Quality, and Prognostic Information in Both the Seventh and Eighth Editions of the TNM Classification in 18,973 Surgical Cases of the Japanese Joint Committee of Lung Cancer Registry Database in 2010. J Thorac Oncol 2019;14:212-22.
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008;26:3543-51. [Crossref] [PubMed]
- Kenmotsu H, Yamamoto N, Yamanaka T, et al. Randomized Phase III Study of Pemetrexed Plus Cisplatin Versus Vinorelbine Plus Cisplatin for Completely Resected Stage II to IIIA Nonsquamous Non-Small-Cell Lung Cancer. J Clin Oncol 2020;38:2187-96. [Crossref] [PubMed]
- Douillard JY, Rosell R, De Lena M, et al. Adjuvant vinorelbine plus cisplatin versus observation in patients with completely resected stage IB-IIIA non-small-cell lung cancer (Adjuvant Navelbine International Trialist Association [ANITA]): a randomised controlled trial. Lancet Oncol 2006;7:719-27. [Crossref] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ 2021.
- Zhang L, Ou W, Liu Q, et al. Pemetrexed plus carboplatin as adjuvant chemotherapy in patients with curative resected non-squamous non-small cell lung cancer. Thorac Cancer 2014;5:50-6. [Crossref] [PubMed]
- Kreuter M, Vansteenkiste J, Fischer JR, et al. Randomized phase 2 trial on refinement of early-stage NSCLC adjuvant chemotherapy with cisplatin and pemetrexed versus cisplatin and vinorelbine: the TREAT study. Ann Oncol 2013;24:986-92. [Crossref] [PubMed]
- Kreuter M, Vansteenkiste J, Fischer JR, et al. Three-Year Follow-Up of a Randomized Phase II Trial on Refinement of Early-Stage NSCLC Adjuvant Chemotherapy with Cisplatin and Pemetrexed versus Cisplatin and Vinorelbine (the TREAT Study). J Thorac Oncol 2016;11:85-93. [Crossref] [PubMed]
- Tachihara M, Dokuni R, Okuno K, et al. Phase II study of adjuvant chemotherapy with pemetrexed and cisplatin with a short hydration method for completely resected nonsquamous non-small cell lung cancer. Thorac Cancer 2020;11:2536-41. [Crossref] [PubMed]
- Hesketh PJ, Kris MG, Basch E, et al. Antiemetics: ASCO Guideline Update. J Clin Oncol 2020;38:2782-97. [Crossref] [PubMed]
- Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol 2016;27:v119-33. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Antiemesis, Version 1. 2022.
- Natale JJ. Overview of the prevention and management of CINV. Am J Manag Care 2018;24:S391-7. [PubMed]
- Yang CH, Simms L, Park K, et al. Efficacy and safety of cisplatin/pemetrexed versus cisplatin/gemcitabine as first-line treatment in East Asian patients with advanced non-small cell lung cancer: results of an exploratory subgroup analysis of a phase III trial. J Thorac Oncol 2010;5:688-95. [Crossref] [PubMed]
- Liang Y, Wakelee HA, Neal JW. Relationship of Driver Oncogenes to Long-Term Pemetrexed Response in Non-Small-Cell Lung Cancer. Clin Lung Cancer 2015;16:366-73. [Crossref] [PubMed]
- Shih JY, Inoue A, Cheng R, et al. Does Pemetrexed Work in Targetable, Nonsquamous Non-Small-Cell Lung Cancer? A Narrative Review. Cancers (Basel) 2020;12:2658. [Crossref] [PubMed]
- Suehisa H, Toyooka S, Hotta K, et al. Epidermal growth factor receptor mutation status and adjuvant chemotherapy with uracil-tegafur for adenocarcinoma of the lung. J Clin Oncol 2007;25:3952-7. [Crossref] [PubMed]
- Park SE, Noh JM, Kim YJ, et al. EGFR Mutation Is Associated with Short Progression-Free Survival in Patients with Stage III Non-squamous Cell Lung Cancer Treated with Concurrent Chemoradiotherapy. Cancer Res Treat 2019;51:493-501. [Crossref] [PubMed]
- Galvez C, Jacob S, Finkelman BS, et al. The role of EGFR mutations in predicting recurrence in early and locally advanced lung adenocarcinoma following definitive therapy. Oncotarget 2020;11:1953-60. [Crossref] [PubMed]
- Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020;383:1711-23. [Crossref] [PubMed]
- Yang XN, Yan HH, Wang J, et al. Real-World Survival Outcomes Based on EGFR Mutation Status in Chinese Patients With Lung Adenocarcinoma After Complete Resection: Results From the ICAN Study. JTO Clin Res Rep 2021;3:100257. [Crossref] [PubMed]
- Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet 2021;398:1344-57. [Crossref] [PubMed]
- Versluis JM, Long GV, Blank CU. Learning from clinical trials of neoadjuvant checkpoint blockade. Nat Med 2020;26:475-84. [Crossref] [PubMed]
- Spicer J, Wang C, Tanaka F, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol 2021;39:8503. [Crossref]



