
Multimodal therapy of epithelioid pleural mesothelioma: improved survival by changing the surgical treatment approach
Introduction
Malignant pleural mesothelioma (MPM) is a rare and highly lethal cancer mainly associated with exposure to asbestos. Hereditary factors or radiotherapy might be responsible for development of MPM in some cases. Despite extensive research and current advances in systemic therapy, the prognosis is still very poor (1,2). Because of the laminar tumor growth on visceral and parietal pleural tissue, oncologic complete surgical resection is not feasible. Consequently, multimodal treatment is the standard of care for early-stage MPM, with the aim of improving postoperative local tumor control (3,4).
Since Sugarbaker and his team reported good outcomes after extrapleural pneumonectomy (EPP) in 1999, it has been performed in several high-volume thoracic surgery centers for early-stage MPM (5,6). In the 2000s, there was great hope that tumor control could even be further improved by a maximal radical approach flanking surgery using neoadjuvant chemotherapy and adjuvant radiation therapy (7). We performed trimodal EPP in our institution for MPM for more than a decade (8). However, this was changed to lung-sparing surgery following increasing evidence of similar survival rates, lower perioperative morbidity, and improved quality of life (9-11).
After an interim period between 2012 and 2013 in which both surgical concepts were performed, we completely changed our surgical approach. By the end of 2013, we performed only a combination of extended pleurectomy and decortication/hyperthermic intrathoracic chemoperfusion (EPD/HITOC), instead of trimodal EPP. Unlike the former trimodal concept with neoadjuvant chemotherapy followed by EPP and adjuvant radiotherapy, the new therapeutic concept is a combination of upfront cytoreductive surgery and HITOC, followed by four cycles of adjuvant cisplatin/pemetrexed chemotherapy.
The aim of this study was to analyze our 20-year single-institution experience of using multimodal treatment for patients with epithelioid MPM. We compared the period before and after the change of our surgical technique to the lung-sparing approach. Since there are only few studies comparing the results after surgical therapy with the survival of patients treated with palliative chemotherapy in the same institution, the outcomes of the two surgical treatment concepts were compared to the survival of patients treated with the standard of care, chemotherapy. We present this article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-199/rc).
Methods
Patients
Patients were retrospectively selected from a prospectively maintained database of patients who underwent surgery or chemotherapy for epithelioid MPM at Thoraxklinik Heidelberg from 2001 to August 2018. Patients with biphasic or sarcomatoid MPM were excluded from further analyses. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Heidelberg University Hospital’s institutional ethics committee approved the collection and analysis of data from the patients’ medical records (No. S-174/2019), and the requirement for individual informed patient consent for this retrospective study was waived.
Three different patient cohorts of epithelioid MPM were selected: (I) consecutive patients treated with trimodal EPP between February 2001 and June 2012 (EPP cohort), (II) consecutive patients treated with EPD, HITOC and adjuvant chemotherapy between January 2014 and August 2018 (EPD/HITOC cohort), and (III) consecutive patients treated with palliative systemic chemotherapy between February 2014 and December 2018 (CTx cohort). To minimize influence of therapeutic improvements on treatment, CTx cohort was selected according to the same time period of EPD/HITOC cohort. Patients who had localized disease with a maximum clinical stage of T4N1M0 according to the 8th edition of the Tumor-Node-Metastasis (TNM) classification were eligible for EPP or EPD/HITOC.
Patients who underwent surgery were followed-up routinely at Thoraxklinik Heidelberg with computed tomography of the chest every three months during the first two postoperative years and every six months thereafter.
Preoperative assessment
Multiple biopsies via single port thoracoscopy or computed tomography-guided punch of tumor tissue were used for histopathological and histological subtyping of MPM. Preoperative investigations were conducted at the time of diagnosis according to the guidelines of the European Respiratory Society and European Society of Thoracic Surgeons for the management of MPM (12). Positron emission tomography (PET) or bone scintigraphy and abdominal ultrasonography or computed tomography were used to exclude distant metastases. Functional respiratory and cardiac testing was performed for all the patients. Mediastinoscopy or endobronchial ultrasonography with transbronchial needle aspiration was performed to further evaluate suspicious mediastinal or hilar lymph nodes. Each patient was discussed in a multidisciplinary tumor board. Criteria for surgery have been defined as no relevant shrinkage of the affected hemithorax, good cardiopulmonary function test according to the guidelines mentioned above and potentially resectable tumor mass using a contrast-enhanced computed tomography. Contraindications were relevant cardiopulmonary comorbidities or reduced performance status.
EPP trimodality therapy
All the patients received neoadjuvant chemotherapy prior to thoracic surgery, consisting of standard treatment for mesothelioma using cisplatin/pemetrexed, carboplatin/pemetrexed, or cisplatin/gemcitabine. EPP, which included complete resection of the lung, parietal pleura, diaphragm, and pericardium, was performed after a minimum of 3 cycles of neoadjuvant chemotherapy. Anterolateral thoracotomy was performed by an S shaped line in the sixth intercostal space. Sites of prior open biopsy mainly thoracoscopy incisions and chest tube tracks were excised separately to avoid metastases in the thoracic wall. The diaphragm was replaced by a monofilament polypropylene mesh (Bard Mesh; Davol, Inc., Cranston, RI). The pericardium was reconstructed with a xenopericard patch (Supple Peri-Guard; Synovis Surgical Innovations, St Paul, MN). In all the patients who underwent EPP, mediastinal lymph node dissection was performed systematically. Adjuvant radiotherapy was performed either as step & shoot intensity-modulated radiotherapy (IMRT) or helical tomotherapy IMRT. The median target dose was 48–54 Gy in fractions of 2 Gy.
Cytoreduction by extended pleurectomy/decortication
After anterolateral thoracotomy or double thoracotomy, EPD of the parietal and visceral pleura with respect to the lobar fissures was performed. To achieve macroscopic complete resection with a tumor burden below 1 cm3, EPD with resection of the diaphragm and/or pericardium was performed according to the guidelines of the International Mesothelioma Interest Group if it was necessary and possible (13). Every effort was made to preserve diaphragmatic musculature. If full thickness diaphragm resections were necessary, either a direct suture or a reconstruction with a monofilament polypropylene mesh (Bard Mesh; Davol, Inc., Cranston, RI) was performed. The fibrous pericardium was resected, leaving the inner serous layer. If necessary, full thickness pericardial invasion was resected and reconstructed with a xenopericard patch (Supple Peri-Guard; Synovis Surgical Innovations, St Paul, MN). Systematic mediastinal lymph node dissection was performed. Patients with macroscopic tumor residuum above 1 cm3 were classified as macroscopically incomplete (R2). Within a multimodal treatment approach, patients received four cycles of adjuvant platin-based chemotherapy in combination with pemetrexed starting four to six weeks after cytoreductive surgery. There are no randomized data to guide us on this issue regarding optimal number of chemotherapy cycles as part of multimodality therapy for pleural mesothelioma. ASCO Guidelines 2018 recommends four to six cycles of pemetrexed/platin-based chemotherapy in context of multimodal treatment (14). Two currently recruiting randomized clinical trials in adjuvant setting (ClinicalTrials.gov Identifier: NCT04177953 and NCT04996017) allow four cycles of chemotherapy. Thus, our standard post-operative approach consists of four cycles of pemetrexed and cisplatin or carboplatin, also based on the NSCLC adjuvant chemotherapy paradigm.
Hyperthermic intrathoracic chemoperfusion
Four 28 Ch chest tubes were placed in the thoracic cavity before the anterolateral thoracotomy was closed. Hyperthermic perfusion was achieved at 42 ℃ using a RanD Performer HT (RanD S.r.l.; Medolla, Italy). Cisplatin (200 mg in 250 mL 0.9% saline solution) was added into the 4,750 mL 0.9% saline solution. HITOC was performed for 60 minutes at 42 ℃. The total perfusate volume was 5,000 mL. The final cisplatin concentration was 40 mg/L. The flow was set to 1,000 mL/min (15-17). By using the technique in a standardized scheme, HITOC can be implemented in other facilities as well. Within the EPD/HITOC cohort, all patients received HITOC.
Standard palliative chemotherapy
Cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) with folic acid and vitamin B12 supplements were administered as standard first line chemotherapy in the CTx cohort. In the responders or patients with stable disease, up to six cycles of chemotherapy were administered. In patients who could not tolerate cisplatin, carboplatin (AUC 5) was administered as a substitute for cisplatin. In patients with progressive disease, grade 3–4 toxicities, or cumulative toxic doses, the ongoing therapy was stopped or changed to second line. Bevazicumab was not administered during first-line therapy. Second line regimen at our institution included vinorelbine or gemcitabine monotherapy.
Statistical analysis
Results are expressed as mean ± standard deviation or median (interquartile range). Rates and proportions were analyzed using the chi-square or Fisher’s exact test, where appropriate. The Kaplan-Meier method was used to estimate overall survival (OS). The log-rank test was used to compare between subgroups. A multivariate analysis was performed using a Cox proportional hazard model. A P value <0.05 was considered significant. Survival was calculated from the start of treatment, including neoadjuvant chemotherapy. Patients with missing data concerning multimodal therapy regimes were excluded from analysis. In case of loss of follow-up, date of last documented contact and status were used for survival analysis. All statistical analyses were performed with IBM SPSS Statistics 26 or Graph Pad Prism Version 8.4.2. (GraphPad Software; San Diego, USA) software.
Results
Patient characteristics
The total cohort consisted of 182 patients; 69 were treated with trimodal EPP and 57 with EPD/HITOC and adjuvant chemotherapy. Fifty-six patients were treated with chemotherapy alone. The demographic and survival data of the cohorts are shown in Table 1. The age distribution is shown in Figure 1. Patients in both surgical cohorts were significantly younger than those treated with chemotherapy. In the EPD/HITOC cohort, patients were averagely 8 years older than those treated with EPP.
Table 1
| Characteristics | CTx | EPP | EPD/HITOC | P value |
|---|---|---|---|---|
| Patients, n | 56 | 69 | 57 | – |
| Treatment period | 2014–2018 | 2001–2012 | 2014–2018 | – |
| Age, years, median [IQR] | 75 [68–77] | 59 [54–65] | 67 [61–72] | <0.0001a; 0.0004b |
| Gender, n [%] | NS | |||
| Female | 9 [16] | 11 [16] | 8 [14] | |
| Male | 47 [84] | 58 [84] | 49 [86] | |
| ECOG, n [%] | <0.0001c | |||
| 0 | 13 [23] | 27 [39] | 45 [79] | |
| 1 | 43 [77] | 42 [61] | 12 [21] | |
| Side, n [%] | NS | |||
| Left | 20 [36] | 28 [41] | 19 [33] | |
| Right | 36 [64] | 41 [59] | 38 [67] | |
| Neoadjuvant CTx, n [%] | n/a | 69 [100] | 5 [9] | – |
| Adjuvant CTx, n [%] | n/a | n/a | 43 [75] | – |
| Adjuvant RT, n [%] | n/a | 57 [83] | n/a | – |
| Overall survival data | ||||
| MOST, month (95% CI) | 15.5 (11.8–19.1) | 24.0 (16.9–31.1) | 38.1 (30.3–45.9) | <0.0001 |
| Survival rate ± SE, % | – | |||
| 1 year | 59±7 | 80±5 | 96±2 | |
| 2 years | 26±6 | 49±6 | 71±6 | |
| 3 years | 15±5 | 34±6 | 52±7 |
Applied statistical tests are as follows. age: ordinary one-way ANOVA; gender: Eastern Cooperative Oncology Group (ECOG); side: chi-square test; median survival: log-rank test. a, CTx vs. EPP & EPP vs. EPD; b, CTx vs. EPD. c, EPD vs. EPP & EPD vs. CTx. NS, non-significant; n/a, not available; CTx, chemotherapy; RT, radiotherapy; EPP, extrapleural pneumonectomy; EPD/HITOC, extended pleurectomy and decortication/hyperthermic intrathoracic chemoperfusion; MOST, median overall survival time; ECOG, Eastern Cooperative Oncology Group; SE, standard error of the mean; CI, confidence interval; IQR, interquartile range.
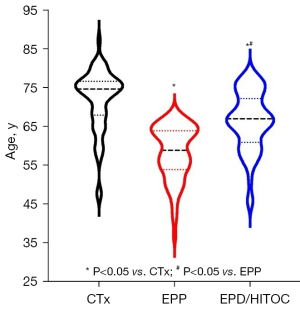
There were no significant differences in gender or side between all the cohorts. The difference in performance status before surgery or before chemotherapy initiation was significant: the proportion of patients with an Eastern Cooperative Oncology Group (ECOG) status of 1 was higher in the EPP and CTx group than in the EPD/HITOC group.
The distribution of the tumor TNM stage is provided in Table 2. More than 60% of patients suffered from prior exposure to asbestos. The proportion of T3/T4 tumors was higher in the EPP and EPD/HITOC groups than in the CTx cohort. There was no significant difference in the proportion of the T stages between the EPD/HITOC and EPP groups (P=0.072, Fisher’s exact test). Within the proportion of T3/T4 tumors in the surgical cohorts, most of the patients showed tumor infiltration of the mediastinal fat or the pericardium or solitary nodules of tumor invading the thoracic wall. These structures have been resected and pericardium was replaced if necessary. Concerning the proportion of T1/T2 tumors in the chemotherapy cohort, patients did not undergo surgery due to comorbidities, high local tumor burden on computed tomography scan, patient refusal, and grade 3–4 toxicity during chemotherapy within a neoadjuvant treatment concept. T4 tumors in the EPP group showed unexpected infiltration of the esophagus in one case and transmural infiltration of the pericardium in all other cases.
Table 2
| Characteristics | CTx (n=56) | EPP (n=69) | EPD/HITOC (n=57) | χ2, P value |
|---|---|---|---|---|
| T-stage, n [%] | <0.0001 | |||
| T1 | 1 [2] | 1 [1] | 1 [2] | |
| T2 | 31 [55] | 5 [7] | 11 [19] | |
| T3 | 13 [23] | 58 [84] | 44 [77] | |
| T4 | 11 [20] | 5 [7] | 1 [2] | |
| T1+T2 | 32 [57] | 6 [9] | 12 [21] | <0.0001 |
| T3+T4 | 24 [43] | 63 [91] | 45 [79] | |
| N-stage, n [%] | – | |||
| N0 | 35 [63] | 59 [86] | 45 [79] | |
| N1 | 17 [30] | 10 [14] | 12 [21] | |
| N2 | 4 [7] | 0 [0] | 0 [0] | |
| N1+N2 | 21 [37] | 10 [14] | 12 [21] | 0.0092 |
| M-stage, n [%] | NS | |||
| M0 | 55 [98] | 69 [100] | 56 [98] | |
| M1 | 1 [2] | 0 [0] | 1 [2] | |
| TNM stage, n [%] | – | |||
| IA/B | 31 [55] | 55 [80] | 43 [75] | |
| II | 7 [13] | 9 [13] | 1 [2] | |
| IIIA/B | 17 [30] | 5 [7] | 12 [21] | |
| IV | 1 [2] | 0 [0] | 1 [2] | |
| I+II | 38 [68] | 64 [93] | 44 [77] | 0.0019 |
| III+IV | 18 [32] | 5 [7] | 13 [23] |
T, N, M descriptors and stage classified according to the 8th edition of the TNM classification. The c stage is provided for the CTx cohort and the p-stage for the EPP and EPD/HITOC cohorts. NS, non-significant; CTx, chemotherapy; EPP, extrapleural pneumonectomy; EPD/HITOC, extended pleurectomy and decortication/hyperthermic intrathoracic chemoperfusion; TNM, Tumor-Node-Metastasis.
A higher proportion of lymph node metastasis was found in the CTx cohort than in the EPP and EPD/HITOC cohorts. Regarding tumor stage, a higher proportion of patients with stage III/IV tumors were observed in the EPD/HITOC and CTx groups than in the EPP group (P=0.0198 Fisher’s Exact test), whereas there was no significant difference in tumor stage between the CTx and EPD/HITOC cohorts (P=0.2973, Fischer’s exact test). This shows that indication for surgery by EPD and HITOC within a multimodal treatment concept is feasible for older patients and for locally advanced tumor stages. The one M1 patient in the EPD/HITOC group suffered from very small bipulmonary nodules, unfortunately lung metastases of mesothelioma. In the EPD/HITOC group macroscopic complete resection could be achieved for 51 (89%) patients. Macroscopically incomplete resection had to be accepted for 6 (11%) patients (Table 3). This resulted in an incomplete resection ratio of 1:8.5.
Table 3
| Characteristics | EPP (n=69) | EPD/HITOC (n=57) | P value |
|---|---|---|---|
| Extent of resection, n [%] | NS | ||
| Macroscopic complete (R1) | 66 [96] | 51 [89] | |
| Macroscopic incomplete (R2) | 3 [4] | 6 [11] | |
| 30-day mortality, n [%] | 2 [2.9] | 0 [0] | NS |
| 90-day mortality, n [%] | 4 [5.8] | 0 [0] | NS |
| Perioperative morbidity, n [%] | 25 [36.2] | 11 [18.0] | 0.048 |
| Prolonged air leakage, n [%] | 0 [0] | 3 [4.9] | NS |
| Cardiac complications, n [%] | 6 [8.7] | 1 [1.6] | NS |
| Pulmonary embolism, n [%] | 1 [1.4] | 0 [0] | NS |
| Pleural empyema, n [%] | 3 [4.3] | 1 [1.6] | NS |
| Respiratory insufficiency, n [%] | 2 [2.9] | 0 [0] | NS |
| Chylothorax, n [%] | 1 [1.4] | 1 [1.6] | NS |
| Bleeding, n [%] | 7 [10.1] | 2 [3.3] | NS |
| Reoperation, n [%] | 18 [26.1] | 4 [6.5] | 0.005 |
Differences in rates and proportions were statistically analyzed using the Fisher’s exact test. NS, non-significant; EPP, extrapleural pneumonectomy; EPD/HITOC, extended pleurectomy and decortication/hyperthermic intrathoracic chemoperfusion.
Perioperative morbidity and mortality in surgical cohorts
The perioperative morbidity of the EPD/HITOC group was 18% (11 patients). Three patients (4.9%) had prolonged air leakage, while one patient (1.6%) had atrial fibrillation requiring oral medication. Seven patients (11.5%) developed pneumonia requiring antibiotics; however, there was no patient with respiratory insufficiency requiring reintubation. Surgical intervention of complications was necessary in four patients (6.5%): chylothorax, n=1; bleeding, n=2; and pericardial effusion, n=1. No patient in the EPD/HITOC cohort died within 30 or 90 days.
The perioperative overall morbidity of the EPP group was significantly higher than that of the EPD/HITOC cohort (36.2% vs. 18%; P=0.0475). Six patients (8.7%) had cardiac arrhythmia requiring oral medication. One patient (1.4%) had pulmonary embolism. Two patients (2.9%) developed respiratory insufficiency requiring non-invasive ventilation. Reoperation was necessary in 18 patients (26.1%) due to chylothorax (n=1), hemothorax (n=7, 10.1%), and pleural empyema (n=3, 4.3%). The diaphragmatic membrane tore in one patient. One patient had a dislocation of the pericardial membrane, and five patients suffered from recurrent, massive pleural effusion with compression of the contralateral lung (7.2%). The 30-day mortality was 2.9% due to cardiac decompensation and pulmonary embolism. The 90-day mortality was 5.8% due to two additional deaths from rapid tumor progression in one patient and radiation pneumonitis with suspected cardiac decompensation in another.
Survival
The median follow-up time for all the patients was 22.6 [95% confidence interval (CI): 19.4–25.5] months; 147 (81%) patients died within this period. The median OS of all the patients was 22.3 months (Figure 2A). The 1-, 2-, and 3-year survival rates were 79%±3%, 49%±4%, and 34%±4%, respectively.
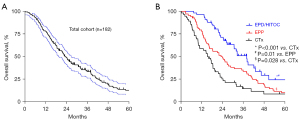
Stratification of the treatment cohorts showed a higher OS in both surgical cohorts than in the CTx cohort (Figure 2B). EPP improved the survival of the patients better than CTx, from 15.8 to 24 months (P=0.028). The OS for the patients who completed trimodal EPP with adjuvant radiation was 26.8 months, while it was 14.6 months for patients who did not receive adjuvant radiotherapy (P=0.119). EPD/HITOC was significantly associated with a prolonged survival (median overall survival of 38.1 months), compared to EPP and CTx (P=0.01 and P<0.001). Patients in the EPD/HITOC group showed better survival with adjuvant chemotherapy than patients without; there was no statistically significant difference.
Besides the treatment modality, better survival was significantly associated with an ECOG 0 performance status (Figure 3A), age below 70 years (Figure 3B), and negative lymph node metastasis (Figure 3C).

In the multivariate cox regression analysis, ECOG, age, nodal status, gender, stage, and therapeutic modality were relevant covariates for OS (Table 4). EPP reduced the relative risk compared to chemotherapy by 20%. EPD/HITOC reduced the relative risk of death significantly by 57%. After multivariate analysis, only EPD/HITOC and not EPP had significant impact on OS.
Table 4
| Factors | HR | 95% CI | P value |
|---|---|---|---|
| ECOG 1 | 1.091 | 0.746–1.597 | 0.654 |
| Age >70 years | 1.325 | 0.802–2.189 | 0.272 |
| N status (N0 ref.) | |||
| N1 | 1.296 | 0.704–2.385 | 0.405 |
| N2 | 2.226 | 0.685–7.227 | 0.183 |
| Female sex | 0.778 | 0.485–1.246 | 0.296 |
| Stages III & IV | 1.116 | 0.620–2.010 | 0.715 |
| Therapy (CTx ref.) EPP | 0.806 | 0.467–1.393 | 0.440 |
| EPD/HITOC | 0.428 | 0.245–0.748 | 0.003 |
Data of potential prognostic factors are expressed in HR and CI [entire cohort (n=182)]. HR, hazard ratio; CI, confidence interval; CTx, chemotherapy; EPP, extrapleural pneumonectomy; EPD/HITOC, extended pleurectomy and decortication/hyperthermic intrathoracic chemoperfusion; ECOG, Eastern Cooperative Oncology Group; N status, node status.
Discussion
The role of surgery and the surgical option for the treatment of MPM is one of the most controversial topics in thoracic surgery and thoracic oncology (4,14,18). Lung-sparing cytoreductive surgery is the most suitable surgical technique for localized MPM to ensure good oncological outcomes and improved quality of life (4,19).
Due to the absence of prospective randomized studies, this recommendation is based on few retrospective cohort studies and meta-analyses, of which many lack a direct comparison between surgery and non-surgical therapy (11,20,21).
To add further evidence, we thoroughly analyzed our 20-year single-center experience in epithelioid mesothelioma surgery including data on trimodal EPP, lung-sparing EPD/HITOC, and chemotherapy alone. OS was significantly improved in the EPD/HITOC with adjuvant chemotherapy than in the EPP and CTx groups.
The median OS was 24 months in the EPP cohort; this is similar to the median OS of studies conducted in other high-volume centers: the median OS was 23 months in the study by Kostron et al. (22) and 20 months in the part 2 SAKK 17/04 trial (23). Recently Cho et al. reported remarkable improved survival after neoadjuvant IMRT and EPP (24). In the post-hoc analysis of the SMART trial, the median OS was 42.8 months in patients with epithelioid MPM and 24.4 months in the overall cohort with a 28% biphasic histology.
The median survival of the EPD/HITOC group in this study was 38 months; this was slightly shorter than that in the SMART trial, but it is still one of the longest reported survival durations following lung-sparing surgery in epithelioid mesothelioma. In the Swiss propensity-matched cohort with a 94% epithelioid histology, the median survival time was 32 months (22). Patients with epithelioid tumors had a median OS of 23 months after lung- and diaphragm-sparing surgery combined with HITOC in the study by Ambrogi et al. (25).
As a limitation, the retrospective nature of the study imposed related bias from selection of the patients for the different cohorts during the long study period. Since patients with comorbidities and high tumor burden were excluded by physicians from surgical resection, the three treatment groups might be affected as the patients are not evenly distributed.
Compared to a EPD/HITOC cohort from Munich (26), the OS improved markedly in the EPD/HITOC cohort from Heidelberg. This might be due to the strict criteria that were used to select patients before cytoreductive surgery: lung-sparing cytoreductive surgery was performed only in patients with epithelioid tumors, and the lack of relevant shrinkage of the affected hemithorax diagnosed on preoperative imaging was used as a predictive factor of a successful macroscopic complete resection by EPD. As a result, the proportion of macroscopic complete resection was higher in our cohort (89%) than in the former Munich cohort (75%) (26). Another relevant factor might be the concept of additive postoperative chemotherapy within the multimodal treatment approach to further target postoperative locoregional microscopic minimal residual disease. Within the EPD/HITOC cohort, there was a trend towards better survival in combination with adjuvant chemotherapy. Possibly due to the small number of patients without adjuvant treatment, there was no significant overall survival benefit for patients as seen by Lapidot et al. (27).
In most cases, the extension of tumor infiltration was diagnosed during surgery and not preoperatively. Preoperative radiologic staging is often misleading in mesothelioma patients by “understaging” mesothelioma patients as shown by Gill et al. with more than 60% of patients having discordant c- and p-stage (28). The amount of tumor tissue and potential infiltration into surrounding structures can only be reliably diagnosed during surgery.
There are still two main treatment paradigms for chemotherapy combined with cytoreductive surgery: while induction chemotherapy followed by surgery is used in some centers, upfront surgery followed by postoperative adjuvant chemotherapy is preferred in others, similarly to what was done in our EPD/HITOC cohort. To find an evidence-based recommendation for this highly controversial issue, the European Organization for Research and Treatment of Cancer is conducting a randomized phase II trial (NCT02436733) aimed at comparing the effect of neoadjuvant and adjuvant chemotherapy in combination with cytoreductive surgery in all mesothelioma subtypes (29). So far, data from this prospective trial are not available, and recommendations can only be drawn from a large retrospective propensity-matched score analysis by Verma et al. (30). They analyzed data from the United States’ national cancer database, which showed (I) a decreasing trend of inductive chemotherapy over time and (II) similar survival outcomes but worse postoperative outcomes after inductive chemotherapy than upfront surgery followed by adjuvant chemotherapy which was also seen by Voigt et al. (31). In accordance with these results, we support a combination of cytoreductive surgery and adjuvant chemotherapy for two reasons. First, in many tumor entities, especially lung cancer, the concept of neoadjuvant chemotherapy is to tackle locoregional or distant microscopic metastasis at an early timepoint and herby to improve overall survival. In contrast to lung cancer in which locoregional and distant microscopic metastasis is frequently observed at time of diagnosis, mesothelioma is characterized by a long-lasting local tumor growth and very late distant metastasis. The concept of early treatment of distant microscopic metastasis is therefore not applicable for mesothelioma. Second, changing EPP to PD is associated with a significantly higher probability of intraoperative tumor cell spilling, as decortication is carried out in direct contact with the tumor tissue without any safety margins. Adjuvant chemotherapy targets these residual tumor cells and may therefore contribute to a better local tumor control and improved OS.
In addition to adjuvant chemotherapy, HITOC was performed in our EPD cohort, with the aim to improve the radicality of our surgical resection by the local effect of chemotherapy and hyperthermia on residual tumor cells. We could not assess the impact of the HITOC procedure on OS in the EPD group, since there was no control group. So far, the efficacy of HITOC has not been proven in prospective trials and an improvement of recurrence-free survival and OS has been observed in only one retrospective single-center cohort analysis (17).
Beside OS, therapy-associated morbidity and quality of life need to be assessed when choosing the optimal treatment modality. Several studies report a significantly reduced postoperative morbidity for patients following lung-sparing surgery compared to EPP (13,21,32,33). Perioperative morbidity was significantly lower in the present EPD/HITOC cohort compared to our EPP cohort and also compared to a rather high number (49%) of grade 3 or 4 adverse events reported in the SMART trial (24). Therefore, our data supports the hypothesis of improved postoperative quality of life following lung-sparing surgery due to a lower postoperative complication rate, as shown in the meta-analysis by van Gerwen et al. (19). The comparable low postoperative morbidity in our EPD/HITOC cohort suggests that the standardized HITOC procedure did not increase the postoperative morbidity and mortality more than EPD alone.
There is still no clear evidence on the impact of surgery on survival in patients with pleural mesothelioma compared to chemotherapy. There is only one randomized study, the MARS I trial, that showed no advantage of EPP over chemotherapy alone (7). However, the MARS trial has several sources of bias from the study design and data analysis/interpretation. The aim of the MARS 2 trial is to compare the extent of survival improvement by EPD with that by non-surgical therapy (34). Recruitment for this trial is ongoing since 2015, and therefore reliable data are missing so far. A large retrospective multicenter analysis investigating the impact of surgery on the outcome of patients with malignant mesothelioma did not show a significant improvement of OS after EPP or EPD, unlike chemotherapy alone, in patients below 70 years and with epithelioid tumors (35). In contrast to these results, the multivariate analysis in our overall cohort revealed a significant survival benefit in patients with epithelioid tumors who were treated with EPD/HITOC. Thus, the role of EPP for mesothelioma should be questioned (36). In the framework of a multimodal treatment, EPD/HITOC can achieve a promising OS enabling a reasonable quality of life due to lung-sparing surgery (37,38). As a limitation, we cannot rule out that the observed differences of performance status between the three groups might have influenced overall survival. OS of patients receiving chemotherapy has improved over time possibly due to patient selection, earlier diagnosis, better supportive care as well as improved surgical techniques. Furthermore, potential improvements concerning perioperative management and recent treatment advancements for tumor recurrence might have influenced overall survival of the EPD/HITOC cohort.
The median survival duration in the CTx group was 15.5 months, which was comparable to that in the CTx groups in the MAPS (OS, 16.1 months) (2) or CheckMate 743 study (1); in this study, the OS was 16.5 months for patients with epithelioid tumors who received cisplatin/pemetrexed chemotherapy. The CheckMate 743 study showed that first line immunotherapy improved the OS more than chemotherapy with platinum plus pemetrexed in unresectable malignant mesothelioma. Although there was no significant benefit for the subgroup of epithelioid mesothelioma, this encourages the inclusion of new systemic therapy modalities such as immunotherapy by checkpoint inhibition in multimodal surgical treatment to further improve OS. Following this, we developed a new post-cytoreductive surgery adjuvant treatment schedule, which involves adding immunotherapy to our existing standard treatment of adjuvant chemotherapy. The NICITA IIT trial, a prospective, 1:1 randomized, open-label, multi-center phase II study (NCT04177953) is investigating the impact of adjuvant chemotherapy with or without the immune checkpoint inhibitor, nivolumab, on local tumor control following cytoreductive lung-sparing surgery (39).
Another limitation of our study is the comparison of clinical tumor stages in the CTx cohort to pathological tumor stages in the surgical cohorts; this may have led to an understaging of the CTx cohort compared to the surgical cohorts.
In conclusion, the OS of patients with epithelioid pleural mesothelioma was improved after surgery, which changed from maximal radical surgery to moderate lung-sparing surgery in combination with local HITOC and adjuvant chemotherapy. Our results show the greater benefit of cytoreductive surgery in patients with resectable epithelial mesothelioma than non-surgical therapy. Therefore, lung-sparing surgery should be performed in patients selected carefully after a multidisciplinary decision.
Acknowledgments
Funding: This study was funded by the German Center for Lung Research (DZL), Germany and the Thoraxklinik Heidelberg foundation, Germany.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-199/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-199/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-199/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-199/coif). RS has received honoraria for lectures from Roche and AstraZeneca and an institutional grant from BMS outside the submitted work. FE received consulting fees from the Roche Pharma AG outside the submitted work. CG received consulting fees from Bristol Myers Squibb and speakers honoraria from Astra Zeneca, all outside the submitted work. PC has received research funding from AstraZeneca, Novartis, Roche, and Takeda, speaker’s honoraria from AstraZeneca, Novartis, Roche, Takeda, support for attending meetings from AstraZeneca, Eli Lilly, Gilead, Novartis, Takeda, and personal fees for participating to advisory boards from Boehringer Ingelheim, Chugai, Pfizer and Roche, all outside the submitted work. MT received institutional grants from Astra Zeneca, Bristol-Myers Squibb, Merck, Roche, and Takeda, speakers honoraria from AbbVie, AstraZeneca, Beigene, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Daiichi Sankyo, GlaxoSmithKline, Janssen Oncology, Lilly, MSD, Novartis, Pfizer, Sanofi, Roche, and Takeda as well as support for attendance of meetings from AstraZeneca, Bristol-Myers Squibb, Janssen Oncology, MSD, Pfizer, Roche, and Takeda. For participation in the advisory board, MT received honoraria from AbbVie, AstraZeneca, Beigene, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Chugai, Daiichi Sankyo, GlaxoSmithKline, Janssen Oncology, Lilly, MSD, Novartis, Pfizer, Sanofi, Roche, and Takeda, all outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Heidelberg University Hospital’s institutional ethics committee approved the collection and analysis of data from the patients’ medical records (No. S-174/2019), and the requirement for individual informed patient consent for this retrospective study was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375-86. [Crossref] [PubMed]
- Zalcman G, Mazieres J, Margery J, et al. Bevacizumab for newly diagnosed pleural mesothelioma in the Mesothelioma Avastin Cisplatin Pemetrexed Study (MAPS): a randomised, controlled, open-label, phase 3 trial. Lancet 2016;387:1405-14. [Crossref] [PubMed]
- Song KJ, Flores RM, Wolf AS. Taken Together: Effective Multimodal Approaches for Malignant Pleural Mesothelioma. Thorac Surg Clin 2020;30:481-7. [Crossref] [PubMed]
- Scherpereel A, Opitz I, Berghmans T, et al. ERS/ESTS/EACTS/ESTRO guidelines for the management of malignant pleural mesothelioma. Eur Respir J 2020;55:1900953. [Crossref] [PubMed]
- Sugarbaker DJ, Flores RM, Jaklitsch MT, et al. Resection margins, extrapleural nodal status, and cell type determine postoperative long-term survival in trimodality therapy of malignant pleural mesothelioma: results in 183 patients. J Thorac Cardiovasc Surg 1999;117:54-63; discussion 63-5. [Crossref] [PubMed]
- Grondin SC, Sugarbaker DJ. Pleuropneumonectomy in the treatment of malignant pleural mesothelioma. Chest 1999;116:450S-4S. [Crossref] [PubMed]
- Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol 2011;12:763-72. [Crossref] [PubMed]
- Thieke C, Nicolay NH, Sterzing F, et al. Long-term results in malignant pleural mesothelioma treated with neoadjuvant chemotherapy, extrapleural pneumonectomy and intensity-modulated radiotherapy. Radiat Oncol 2015;10:267. [Crossref] [PubMed]
- Rusch V, Baldini EH, Bueno R, et al. The role of surgical cytoreduction in the treatment of malignant pleural mesothelioma: meeting summary of the International Mesothelioma Interest Group Congress, September 11-14, 2012, Boston, Mass. J Thorac Cardiovasc Surg 2013;145:909-10.
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [Crossref] [PubMed]
- Flores RM, Pass HI, Seshan VE, et al. Extrapleural pneumonectomy versus pleurectomy/decortication in the surgical management of malignant pleural mesothelioma: results in 663 patients. J Thorac Cardiovasc Surg 2008;135:620-6, 626.e1-3.
- Scherpereel A, Astoul P, Baas P, et al. Guidelines of the European Respiratory Society and the European Society of Thoracic Surgeons for the management of malignant pleural mesothelioma. Eur Respir J 2010;35:479-95. [Crossref] [PubMed]
- Cao C, Tian D, Park J, et al. A systematic review and meta-analysis of surgical treatments for malignant pleural mesothelioma. Lung Cancer 2014;83:240-5. [Crossref] [PubMed]
- Kindler HL, Ismaila N, Armato SG 3rd, et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2018;36:1343-73. [Crossref] [PubMed]
- Klotz LV, Gruenewald C, Bulut EL, et al. Cytoreductive Thoracic Surgery Combined with Hyperthermic Chemoperfusion for Pleural Malignancies: A Single-Center Experience. Respiration 2021;100:1165-73. [Crossref] [PubMed]
- Ried M, Kovács J, Markowiak T, et al. Hyperthermic Intrathoracic Chemotherapy (HITOC) after Cytoreductive Surgery for Pleural Malignancies-A Retrospective, Multicentre Study. Cancers (Basel) 2021;13:4580. [Crossref] [PubMed]
- Sugarbaker DJ, Gill RR, Yeap BY, et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 2013;145:955-63. [Crossref] [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Malignant Pleural Mesothelioma, Version 3.2016. J Natl Compr Canc Netw 2016;14:825-36. [Crossref] [PubMed]
- van Gerwen M, Wolf A, Liu B, et al. Short-term outcomes of pleurectomy decortication and extrapleural pneumonectomy in mesothelioma. J Surg Oncol 2018;118:1178-87. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. National Cancer Database Report on Pneumonectomy Versus Lung-Sparing Surgery for Malignant Pleural Mesothelioma. J Thorac Oncol 2017;12:1704-14. [Crossref] [PubMed]
- Taioli E, Wolf AS, Flores RM. Meta-analysis of survival after pleurectomy decortication versus extrapleural pneumonectomy in mesothelioma. Ann Thorac Surg 2015;99:472-80. [Crossref] [PubMed]
- Kostron A, Friess M, Inci I, et al. Propensity matched comparison of extrapleural pneumonectomy and pleurectomy/decortication for mesothelioma patients. Interact Cardiovasc Thorac Surg 2017;24:740-6. [Crossref] [PubMed]
- Stahel RA, Riesterer O, Xyrafas A, et al. Neoadjuvant chemotherapy and extrapleural pneumonectomy of malignant pleural mesothelioma with or without hemithoracic radiotherapy (SAKK 17/04): a randomised, international, multicentre phase 2 trial. Lancet Oncol 2015;16:1651-8. [Crossref] [PubMed]
- Cho BCJ, Donahoe L, Bradbury PA, et al. Surgery for malignant pleural mesothelioma after radiotherapy (SMART): final results from a single-centre, phase 2 trial. Lancet Oncol 2021;22:190-7. [Crossref] [PubMed]
- Ambrogi MC, Bertoglio P, Aprile V, et al. Diaphragm and lung-preserving surgery with hyperthermic chemotherapy for malignant pleural mesothelioma: A 10-year experience. J Thorac Cardiovasc Surg 2018;155:1857-1866.e2. [Crossref] [PubMed]
- Klotz LV, Lindner M, Eichhorn ME, et al. Pleurectomy/decortication and hyperthermic intrathoracic chemoperfusion using cisplatin and doxorubicin for malignant pleural mesothelioma. J Thorac Dis 2019;11:1963-72. [Crossref] [PubMed]
- Lapidot M, Gill RR, Mazzola E, et al. Pleurectomy Decortication in the Treatment of Malignant Pleural Mesothelioma: Encouraging Results and Novel Prognostic Implications Based on Experience in 355 Consecutive Patients. Ann Surg 2022;275:1212-20. [Crossref] [PubMed]
- Gill RR, Yeap BY, Bueno R, et al. Quantitative Clinical Staging for Patients With Malignant Pleural Mesothelioma. J Natl Cancer Inst 2018;110:258-64. [Crossref] [PubMed]
- Raskin J, Surmont V, Cornelissen R, et al. A randomized phase II study of pleurectomy/decortication preceded or followed by (neo-)adjuvant chemotherapy in patients with early stage malignant pleural mesothelioma (EORTC 1205). Transl Lung Cancer Res 2018;7:593-8. [Crossref] [PubMed]
- Verma V, Ahern CA, Berlind CG, et al. Treatment of malignant pleural mesothelioma with chemotherapy preceding versus after surgical resection. J Thorac Cardiovasc Surg 2019;157:758-766.e1. [Crossref] [PubMed]
- Voigt SL, Raman V, Jawitz OK, et al. The Role of Neoadjuvant Chemotherapy in Patients With Resectable Malignant Pleural Mesothelioma-An Institutional and National Analysis. J Natl Cancer Inst 2020;112:1118-27. [Crossref] [PubMed]
- Magouliotis DE, Tasiopoulou VS, Athanassiadi K. Updated meta-analysis of survival after extrapleural pneumonectomy versus pleurectomy/decortication in mesothelioma. Gen Thorac Cardiovasc Surg 2019;67:312-20. [Crossref] [PubMed]
- Lee DS, Carollo A, Alpert N, et al. VATS Pleurectomy Decortication Is a Reasonable Alternative for Higher Risk Patients in the Management of Malignant Pleural Mesothelioma: An Analysis of Short-Term Outcomes. Cancers (Basel) 2021;13:1068. [Crossref] [PubMed]
- Lim E, Darlison L, Edwards J, et al. Mesothelioma and Radical Surgery 2 (MARS 2): protocol for a multicentre randomised trial comparing (extended) pleurectomy decortication versus no (extended) pleurectomy decortication for patients with malignant pleural mesothelioma. BMJ Open 2020;10:e038892. [Crossref] [PubMed]
- Bovolato P, Casadio C, Billè A, et al. Does surgery improve survival of patients with malignant pleural mesothelioma?: a multicenter retrospective analysis of 1365 consecutive patients. J Thorac Oncol 2014;9:390-6. [Crossref] [PubMed]
- Kuribayashi K, Doi H, Kijima T. Types of surgery post-neoadjuvant chemotherapy for pleural mesothelioma. Expert Rev Respir Med 2019;13:1189-94. [Crossref] [PubMed]
- Schwartz RM, Watson A, Wolf A, et al. The impact of surgical approach on quality of life for pleural malignant mesothelioma. Ann Transl Med 2017;5:230. [Crossref] [PubMed]
- Batirel HF, Metintas M, Caglar HB, et al. Adoption of pleurectomy and decortication for malignant mesothelioma leads to similar survival as extrapleural pneumonectomy. J Thorac Cardiovasc Surg 2016;151:478-84. [Crossref] [PubMed]
- Shah R, Klotz LV, Chung I, et al. A Phase II Trial of Nivolumab With Chemotherapy Followed by Maintenance Nivolumab in Patients With Pleural Mesothelioma After Surgery: The NICITA Study Protocol. Clin Lung Cancer 2021;22:142-6. [Crossref] [PubMed]


