
Lung function impairment in lung cancer screening: discordance between risk and screening outcomes when looking through a PRISm
Introduction
Retrospective (post-hoc) analyses of lung cancer screening studies enable researchers the unique opportunity of identifying sub-cohorts of patients who are at greatest risk of lung cancer. In these real-world studies (1-3), the lung cancers are diagnosed prospectively and the subjects are broadly representative of those currently participating in screening. Such an approach allows the screening community to better understand, assess and refine the factors underlying risk of lung cancer (4). There is growing interest in targeting those at greatest risk, with the primary aim of improving screening efficiency (4). It has been suggested that this risk-based targeted screening, using more than age and smoking criteria, should maximise the number of lung cancer deaths averted per person screened (5). However, such an approach assumes a linear relationship between the risk and the benefits of screening (4). We ask “Is it safe to assume that enriching screening to those at greatest risk will necessarily optimise the benefits of screening at the individual level?”.
The German Lung Cancer Screening Intervention Study (LUSI)
Kaaks and colleagues stratified 2,029 subjects randomised to the computed tomography (CT) screening arm of their study (6), according to baseline pre-bronchodilator spirometry. They defined 3 mutually exclusive groups of screening participants, eligible according to age (50–69 years old) and smoking history (>15 pack years and quit <10 years), into those with airflow limitation (AFL) [chronic obstructive pulmonary disease (COPD), forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) <70%, n=369], preserved ratio impaired spirometry (PRISm; FEV1/FVC ≥70 and FEV1% predicted <80%, n=311) or normal spirometry (n=1,307). The prevalence for the normal, COPD and PRISm groups was 65.7%, 18.6%, and 15.7% respectively, although only 40% of those with impaired lung function (COPD or PRISm) had been diagnosed with airways disease. They found those with impaired lung function in the PRISm group had an elevated risk of lung cancer, independent of smoking, and more aggressive lung cancers (also observed in those with COPD). The latter was suggested by a lower prevalence of adenocarcinomas and higher prevalence of squamous cell cancers. Importantly they also showed that relative to those with normal spirometry, those with COPD and PRISm had less early-stage lung cancers, more cardiovascular comorbidity, shorter life expectancy and greater mortality from both lung cancer and non-lung cancer causes. However, the study was somewhat underpowered for these secondary endpoints. Critically, Kaaks and colleagues were unable to determine whether the observations they report in those with PRISm might affect lung cancer mortality reduction according to screening arm, as spirometry data was not collected for those in their usual care control arm.
The National Lung Screening Trial (NLST)
We have reported a similar post-hoc analysis in a subgroup of the NLST, where over 18,000 subjects in the American College of Radiology Imaging Network (ACRIN) arm underwent pre-bronchodilator spirometry (7). In this analysis, we showed that those with PRISm (also termed GOLD U) had an elevated risk of lung cancer and that this risk roughly equated to those with mild COPD (GOLD 1, FEV1% predicted ≥80%). In this study we also showed that the increased risk of lung cancer associated with a reduced FEV1% was observed at <80% predicted (Fig. 2 in ref. 7). In this analysis we confirm the findings of Burrows and colleagues (8), showing %predicted FEV1 ≤60% confers a greater risk of lung cancer than age and pack years. In conclusion, both the LUSI and NLST post-hoc analyses support previous studies confirming that an increased impairment of lung function (reduced %predicted FEV1 <80%) is associated in a linear inverse relationship with the risk of lung cancer (9-11). It is here that our conclusions in regards the relevance of impaired lung function on outcomes from screening, markedly diverge from those who believe targeting those at greatest risk will necessarily and consistently achieve the best outcomes for participants. We believe that the increased risk of lung cancer, conferred by co-existing impairment in lung function, does not automatically lead to greater benefits from screening (12-14).
In a subset of the NLST cohort, when comparing outcomes according to screening arm, it has been reported that the relative reduction in lung cancer deaths from CT-based screening [relative to those randomised to the chest X-ray (CXR) arm] was reduced by about one half for those with AFL (COPD) (15) and that this is primarily due to a markedly reduced benefit in those with severe or very severe COPD (GOLD 3–4) (14). We have previously reported that with increasing risk of lung cancer, according to the PLCOM2012 model, there is increasing prevalence of COPD (52% in PLCOM2012 quintile 5 in the NLST) (14). This is relevant because as you enrich for COPD, the surgical rate for lung cancer declines, the histology of the lung cancers is more likely to be aggressive [more squamous cell and more non-small cell lung cancer-not otherwise specified (NSCLC-NOS) with less adenocarcinomas, i.e., “histology shift”] and there is a greater death rate from non-lung cancer causes (16). Others have reported a higher complication rate in this group (17). In a further post-hoc analysis of this NLST subgroup, the great majority (>80%) of the lung cancer deaths averted in the CT arm (relative to the CXR arm) was found in those with normal lung function and undiagnosed COPD (18). Several investigators have found the majority of lung cancer screening participants with AFL have never been previously diagnosed with COPD (18-20). We conclude from these analyses that targeting those at greatest risk for lung cancer, using traditional risk factors based on clinical parameters (e.g., PLCOM2012) but excluding spirometry, enriches the screening population with people who have a high prevalence of largely unrecognised COPD. This is problematic as many in this group will have worse outcomes from screening (less reduction in lung cancer deaths and more complications) due to the many factors outlined above. This raises the question “How do those with PRISm do in regards to screening outcomes according to the NLST data?”.
Screening outcomes for those in the NLST—looking through a PRISm
When the ACRIN sub-cohort of the NLST (n=18,463) was subdivided into the same three mutually exclusive groups described by Kaaks and colleagues (6), the prevalence for the normal, AFL (COPD) and PRISm groups was 49.4%, 33.4% and 17.2% respectively. The higher rates of COPD and PRISm in the NLST compared to LUSI are expected given the older age and pack year eligibility requirement of the NLST (Table 1). In agreement with the results from LUSI, we found there was a linear increase in lung cancer prevalence (Figure 1) as the mean %predicted FEV1 reduced across the 3 groups. Those in the PRISm group had more aggressive (or advanced) lung cancer as suggested by lower rates of stage 1–2 disease at diagnosis, a higher prevalence of squamous cell and NSCLC-NOS and less adenocarcinoma (“Histology shift”). These findings also accord with those reported in the LUSI study (6). In addition, those with PRISm had lower rates of surgery for their lung cancers which may relate to the greater cardiovascular comorbid disease and more aggressive/advanced lung cancers (Table 1). Comparable to the findings reported in the LUSI study, we found that the characteristics of lung cancer in those with PRISm (stage, histology and surgery), were comparable to those with COPD and statistically different to those with normal spirometry. However, when we analysed the outcomes after stratification by screening arm, those with normal spirometry did better with CT compared to CXR screening (45% relative reduction in lung cancer mortality, P=0.0082). The relative reduction in lung cancer mortality for those with impaired lung function (PRISm and COPD) was less than half (14% and 18% respectively) and too underpowered to be significant. More importantly our findings “when looking through a PRISm”, strongly challenge the assumption that increased risk leads to greater benefits from screening.
Table 1
| Variable | Normal referent [n=9,128 (49.4%)] | PRISm [n=3,175 (17.2%)] | AFL [n=6,160 (33.4%)] | P value (normal vs. PRISm) | P value (normal vs. AFL) | P value (PRISm vs. AFL) |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Mean age, years (SD) | 60.8 (4.8) | 61.6 (4.9) | 62.6 (5.2) | <0.0001 | <0.0001 | <0.0001 |
| Male gender, n (%) | 4,917 (53.9) | 1,620 (51.0) | 3,670 (59.6) | 0.0057 | <0.0001 | <0.0001 |
| Current smoker, n (%) | 4,123 (45.2) | 1,680 (52.9) | 3,470 (56.3) | <0.0001 | <0.0001 | 0.0007 |
| Mean pack years, years (SD) | 52.2 (21.1) | 58.3 (25.0) | 59.9 (25.2) | <0.0001 | <0.0001 | 0.0025 |
| Mean cigarettes/day (SD) | 27.4 (10.6) | 28.9 (12.0) | 28.5 (11.1) | <0.0001 | <0.0001 | 0.13 |
| Mean years quit (SD) | 4.1 (5.3) | 3.2 (4.9) | 2.8 (4.6) | <0.0001 | <0.0001 | 0.0002 |
| Mean Smoking duration, years (SD) | 38.7 (7.2) | 40.9 (7.1) | 42.4 (7.2) | <0.0001 | <0.0001 | <0.0001 |
| Family history of lung cancer, n (%) | 2,140 (23.4) | 726 (22.9) | 1,454 (23.6) | 0.51 | 0.82 | 0.42 |
| Personal history of COPD#, n (%) | 1,049 (11.5) | 680 (21.4) | 1,977 (32.1) | <0.0001 | <0.0001 | <0.0001 |
| Mean BMI, kg/m2 (SD) | 28.1 (4.9) | 29.5 (5.8) | 26.7 (4.8) | <0.0001 | <0.0001 | <0.0001 |
| Education level, n (%) | ||||||
| High school or less | 2,386 (26.1) | 1,050 (33.1) | 2,040 (33.1) | <0.0001 | <0.0001 | 0.10 |
| Post high school/some college | 3,173 (34.8) | 1,121 (35.3) | 2,042 (33.1) | |||
| College/postgrad/professional | 3,326 (36.4) | 917 (28.9) | 1,913 (31.1) | |||
| Other/unknown | 243 (2.7) | 87 (2.7) | 165 (2.7) | |||
| Lung function/AFL | ||||||
| Mean FEV1% predicted (SD) | 95.6 (12.4) | 69.9 (8.4) | 66.1 (19.7) | <0.0001 | <0.0001 | <0.0001 |
| Mean FEV1/FVC (SD) | 78.23 (4.96) | 76.23 (4.98) | 59.38 (9.26) | <0.0001 | <0.0001 | <0.0001 |
| Mean FVC% predicted (SD) | 93.6 (12.5) | 70.2 (9.3) | 84.1 (21.3) | <0.0001 | <0.0001 | <0.0001 |
| Pre morbid disease (self-reported), n (%) | ||||||
| COPD | 204 (2.2) | 202 (6.4) | 838 (13.6) | <0.0001 | <0.0001 | <0.0001 |
| Chronic bronchitis | 681 (7.5) | 436 (13.7) | 954 (15.5) | 0.0034 | <0.0001 | 0.024 |
| Emphysema | 320 (3.5) | 215 (6.8) | 1,072 (17.4) | <0.0001 | <0.0001 | <0.0001 |
| Adult asthma | 366 (4.0) | 265 (8.3) | 616 (10.0) | <0.0001 | <0.0001 | 0.0096 |
| Heart disease | 1,021 (11.2) | 568 (17.9) | 847 (13.8) | <0.0001 | <0.0001 | <0.0001 |
| Hypertension | 3,126 (34.2) | 1,385 (43.6) | 2,237 (36.3) | <0.0001 | <0.0001 | <0.0001 |
| Stroke | 209 (2.3) | 150 (4.7) | 191 (3.1) | <0.0001 | 0.0021 | <0.0001 |
| Diabetes | 777 (8.5) | 492 (15.5) | 470 (7.6) | <0.0001 | 0.051 | <0.0001 |
| Lung cancer characteristics | ||||||
| Lung cancer cases, n (%) | 223 (2.4) | 141 (4.4) | 393 (6.4) | <0.0001 | <0.0001 | 0.0001 |
| Lung cancer prevalence per 1,000‡ (95% CI) | 44 (39, 49) | 60 (50, 69) | 75 (68, 83) | <0.0001 | <0.0001 | <0.0001 |
| Lung cancer deaths, n (%) | 82 (0.9) | 75 (2.4) | 186 (3.0) | <0.0001 | <0.0001 | 0.0001 |
| Lung cancer lethality [95% CI] (%) | 37 [31, 43] | 53 [45, 61] | 47 [42, 52] | 0.002 | 0.011 | 0.23 |
| Stage I–II, n [%] | 129 [55] | 53 [36] | 183 [46] | 0.0004 | 0.035 | 0.035 |
| Adenocarcinoma/BAC, n [%] | 121 [52] | 53 [36] | 141 [36] | 0.0034 | <0.0001 | 0.91 |
| Squamous, n [%] | 36 [15] | 36 [25] | 93 [24] | 0.025 | 0.014 | 0.80 |
| Non-small cell lung cancer-NOS, n [%] | 28 [12] | 24 [16] | 79 [20] | 0.22 | 0.0092 | 0.34 |
| Surgery for lung cancer, n [%] | 149 [67] | 62 [44] | 191 [49] | <0.0001 | <0.0001 | 0.35 |
| Mortality#, n (%) | ||||||
| Total mortality | 433 (4.7) | 273 (8.6) | 666 (10.8) | <0.0001 | <0.0001 | 0.0008 |
| Lung cancer deaths | 93 (1.0) | 80 (2.5) | 198 (3.2) | <0.0001 | <0.0001 | 0.061 |
| Cardiovascular deaths | 101 (1.1) | 69 (2.2) | 154 (2.5) | <0.0001 | <0.0001 | 0.33 |
| Respiratory deaths | 17 (0.2) | 18 (0.6) | 86 (1.4) | 0.0005 | <0.0001 | 0.0003 |
| Non-lung cancer deaths | 110 (1.2) | 47 (1.5) | 118 (1.9) | 0.23 | 0.0004 | 0.13 |
| Outcomes favouring CT | ||||||
| Reduction in lung cancer deaths | 45%ɸ | 14% | 18% | – | – | – |
| Relative risk (95% CI) | 0.55 (0.35, 0.86) | 0.86 (0.55, 1.35) | 0.82 (0.61, 1.08) | – | – | – |
| Lung cancer deaths averted per 100 screened (95% CI) | 53 (33, 78) | 37 (17, 80) | 67 (40, 98) | – | – | – |
†, usable spirometry—98% of total cohort; #, includes deaths from lung cancer identified at post-mortem (n=28); ‡, adjusted for smoking and age; ɸ, P=0.0082. PRISm, preserved ratio impaired spirometry; AFL, airflow limitation; SD, standard deviation; COPD, chronic obstructive pulmonary disease; BMI, body mass index; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; CI, confidence interval; BAC, bronchioloalveolar carcinoma; NOS, not otherwise specified; CT, computed tomography.
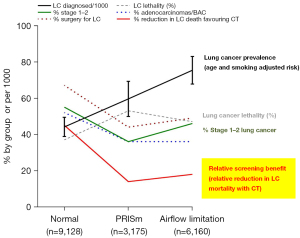
The disparate outcomes are best demonstrated in Figure 1 which shows that while there is a linear relationship showing increasing lung cancer risk (lung cancer adjusted prevalence, black line) across the 3 groups, those with PRISm have the lowest rate of stage 1–2 disease at diagnosis, the lowest prevalence of adenocarcinoma and lowest surgical rate for their lung cancer (Table 1). Moreover, while the relative reduction in lung cancer mortality favouring the CT arm is reduced in those with PRISm and COPD (14% and 18% respectively), compared to those with normal spirometry (45%), the absolute benefits reveal an entirely unexpected finding (Table 1). The number of lung cancer deaths averted in the CT arm relative to the CXR was 53/100 in those with normal spirometry and 67/100 in those with COPD, but was only 37/100 in those with PRISm. Compared to the linear relationship between risk of lung cancer across the 3 groups (Figure 1, black line), this finding suggests that both the absolute and relative benefit of screening might be lowest in those with PRISm (Table 1). This provides an example of the discordance between the risk of lung cancer and the benefit of screening. The most apparent difference between those with PRISm and those with COPD is the very low rate of stage 1–2 disease, two thirds that observed in those with normal spirometry (36% vs. 46% vs. 55% respectively, P=0.035 and P=0.0004). Interestingly, this coincided with the greatest lung cancer “lethality” (lung cancer deaths divided by total lung cancers according to each group) in those with PRISm. We suggest that lung cancer lethality may reflect some aspect of lung cancer biology (possibly aggressiveness) by partially correcting for clinical factors underlying risk. Further analyses are planned to better understand why those with PRISm did so badly relative to those with normal spirometry and AFL. In both the LUSI and NLST analyses, PRISm was associated with greater body mass index (BMI) and more cardiovascular comorbidity, in particular diabetes. Based on a multivariable logistic regression (data not shown), while both more advanced lung cancer (reduced early-stage cancer and more aggressive histology) and lower surgical rates were features of those with COPD and PRISm (Table 1), reduced stage 1–2 lung cancer was the only lung cancer characteristic to be linked to lung cancer death in all 3 groups after adjusting for other relevant variables (sex, age, smoking and screening arm). The link between diabetes and lung cancer mortality is currently poorly understood but may relate to elevated systemic inflammation which we and others have previously linked to the risk of both COPD and lung cancer (21).
Conclusions—increasing risk does not guarantee increasing benefit
In conclusion, we suggest that assuming increasing risk of lung cancer confers a greater benefit from screening may not be justified for a significant proportion of high risk ever smokers otherwise eligible for lung cancer screening. This may be due to the deleterious effects of comorbid diseases such as COPD, and possibly diabetes, on lung cancer outcomes. This has been shown to be the case in a large real-world screening population (NLST) and confirms that impaired lung function (COPD or PRISm) may have an important effect on whether a high-risk smoker benefits from screening. We also conclude that while a reduced %predicted FEV1 is certainly linked to an increasing risk of lung cancer, those with PRISm provide an excellent example of the discordance between increasing risk and increasing benefit from screening. We hypothesise that this might be due to the deleterious effects of having both impaired lung function and diabetes. While it may be argued that targeted screening, using a risk based approach will increase the number of lung cancers identified according to the number screened (i.e., greater screening efficiency), this does not mean that a greater benefit from screening can be assumed at the individual level (12-14)—see Figure 1. When costs, life-expectancy and patient preference are considered, those of intermediate risk (deciles 3–8 or quintiles 2–4) actually do better than those in deciles 1–2 and 9–10 (22-24). Very little is spoken about the harms of screening, or potential for overtreatment, in these subgroups of otherwise eligible smokers at the greatest risk. We suggest that by comparing the outcomes of screening, subgroups of high risk smokers can be identified who will benefit most and this does not always correlate with their underlying risk for lung cancer (25). Unlike other forms of cancer screening, screening for lung cancer includes many people with largely unrecognised impairment in their lung function (e.g., COPD or PRISm) and greater comorbid disease (including diabetes) (14). These comorbidities are associated with more aggressive or advanced lung cancers and reduced life expectancy, which for many may attenuate the benefits of screening (12,22,25). Spirometry helps identify those with impaired lung function and reduced life expectancy. The decision to screen on an individual level requires careful assessment of both the risk and the evidence for clear benefit, and if the latter continues to be ignored, we are in danger of causing more harm than good for a sizable proportion of the screening community. Collectively, the results from the LUSI and NLST trials suggest that impaired lung function (reduced %predicted FEV1) is an important comorbid condition that disproportionately impacts outcomes from screening and should be considered in weighing up the risks, harms and benefits of lung cancer screening.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Translational Lung Cancer Research. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-634/coif). The authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Becker N, Motsch E, Trotter A, et al. Lung cancer mortality reduction by LDCT screening-Results from the randomized German LUSI trial. Int J Cancer 2020;146:1503-13. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Tammemägi MC. Selecting lung cancer screenees using risk prediction models-where do we go from here. Transl Lung Cancer Res 2018;7:243-53. [Crossref] [PubMed]
- Atwater T, Massion PP. Biomarkers of risk to develop lung cancer in the new screening era. Ann Transl Med 2016;4:158. [Crossref] [PubMed]
- Kaaks R, Christodoulou E, Motsch E, et al. Lung function impairment in the German Lung Cancer Screening Intervention Study (LUSI): prevalence, symptoms, and associations with lung cancer risk, tumor histology and all-cause mortality. Transl Lung Cancer Res 2022;11:1896-911. [Crossref] [PubMed]
- Hopkins RJ, Duan F, Chiles C, et al. Reduced Expiratory Flow Rate among Heavy Smokers Increases Lung Cancer Risk. Results from the National Lung Screening Trial-American College of Radiology Imaging Network Cohort. Ann Am Thorac Soc 2017;14:392-402. [Crossref] [PubMed]
- Burrows B, Knudson RJ, Cline MG, et al. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis 1977;115:195-205. [PubMed]
- Mannino DM, Aguayo SM, Petty TL, et al. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med 2003;163:1475-80. [Crossref] [PubMed]
- de Torres JP, Bastarrika G, Wisnivesky JP, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest 2007;132:1932-8. [Crossref] [PubMed]
- Calabrò E, Randi G, La Vecchia C, et al. Lung function predicts lung cancer risk in smokers: a tool for targeting screening programmes. Eur Respir J 2010;35:146-51. [Crossref] [PubMed]
- Rivera MP, Tanner NT, Silvestri GA, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med 2018;198:e3-13. [Crossref] [PubMed]
- Silvestri GA, Young RP. Strange Bedfellows: The Interaction between COPD and Lung Cancer in the Context of Lung Cancer Screening. Ann Am Thorac Soc 2020;17:810-2. [Crossref] [PubMed]
- Young RP, Hopkins RJ. Chronic obstructive pulmonary disease (COPD) and lung cancer screening. Transl Lung Cancer Res 2018;7:347-60. [Crossref] [PubMed]
- Young RP, Duan F, Greco E, et al. Lung cancer-specific mortality reduction with CT screening: outcomes according to airflow limitation in the ACRIN-NLST study (N=18,475). Am J Respir Crit Care Med 2016;193:A6166.
- Young RP, Duan F, Chiles C, et al. Airflow Limitation and Histology Shift in the National Lung Screening Trial. The NLST-ACRIN Cohort Substudy. Am J Respir Crit Care Med 2015;192:1060-7. [Crossref] [PubMed]
- Iaccarino JM, Steiling KA, Wiener RS. Lung Cancer Screening in a Safety-Net Hospital: Implications of Screening a Real-World Population versus the National Lung Screening Trial. Ann Am Thorac Soc 2018;15:1493-5. [Crossref] [PubMed]
- Young RP, Hopkins RJ, Gamble GD, et al. Incorporating Baseline Lung Function in Lung Cancer Screening: Does a "Lung Health Check" Help Predict Outcomes? Chest 2021;159:1664-9. [Crossref] [PubMed]
- Balata H, Harvey J, Barber PV, et al. Spirometry performed as part of the Manchester community-based lung cancer screening programme detects a high prevalence of airflow obstruction in individuals without a prior diagnosis of COPD. Thorax 2020;75:655-60. [Crossref] [PubMed]
- Ruparel M, Quaife SL, Dickson JL, et al. Prevalence, Symptom Burden, and Underdiagnosis of Chronic Obstructive Pulmonary Disease in a Lung Cancer Screening Cohort. Ann Am Thorac Soc 2020;17:869-78. [Crossref] [PubMed]
- Young RP, Hopkins RJ. The Mevalonate Pathway and Innate Immune Hyper-Responsiveness in the Pathogenesis of COPD and Lung Cancer: Potential for Chemoprevention. Curr Mol Pharmacol 2017;10:46-59. [Crossref] [PubMed]
- Cheung LC, Berg CD, Castle PE, et al. Life-Gained-Based Versus Risk-Based Selection of Smokers for Lung Cancer Screening. Ann Intern Med 2019;171:623-32. [Crossref] [PubMed]
- Caverly TJ, Fagerlin A, Wiener RS, et al. Comparison of Observed Harms and Expected Mortality Benefit for Persons in the Veterans Health Affairs Lung Cancer Screening Demonstration Project. JAMA Intern Med 2018;178:426-8. [Crossref] [PubMed]
- Kumar V, Cohen JT, van Klaveren D, et al. Risk-Targeted Lung Cancer Screening: A Cost-Effectiveness Analysis. Ann Intern Med 2018;168:161-9. [Crossref] [PubMed]
- Young RP, Hopkins R. The potential impact of chronic obstructive pulmonary disease in lung cancer screening: implications for the screening clinic. Expert Rev Respir Med 2019;13:699-707. [Crossref] [PubMed]


