
Three non-small cell lung cancer patients who developed pulmonary thromboses during osimertinib treatment and could safely resume concomitant anticoagulation treatment: a report of three cases
Introduction
In a randomized phase III trial (the FLAURA trial), compared to first-generation epidermal growth factor receptor (EGFR)-tyrosine-kinase inhibitors (TKIs), osimertinib, a third-generation EGFR-TKI, demonstrated significant prolongation of progression-free survival (PFS) and overall survival (OS) (1). Therefore, it has become the standard treatment for EGFR mutation-positive non-small cell lung cancer (NSCLC).
Venous thromboembolism (VTE), comprising deep vein thrombosis and pulmonary embolism (PE), is an important complication associated with cancer and a leading cause of death in cancer patients. Being a cancer patient is a risk factor for VTE, with a frequency of VTE four to seven times higher than that of non-cancer patients (2). Chemotherapy is known to be a risk factor for VTE (3), and certain drugs such as bevacizumab are also risk factors (4).
A previous study identified first-generation EGFR-TKIs as risk factors for thrombosis (5). EGFR-TKIs are known to trigger platelet activation. This platelet activation may promote thrombus formation via platelet adhesion, aggregation, and release reaction (6). On the other hand, whether osimertinib is a risk factor for VTE remains unclear. Moreover, limited evidence addresses treatment strategies after VTE during osimertinib onset. In this case series, we report on three patients who developed VTE during osimertinib treatment and were able to safely continue using concomitant anticoagulation therapy. We present the following three cases in accordance with the CARE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-419/rc).
Case presentation
Case 1
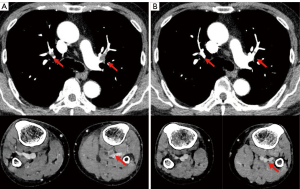
A 71-year-old man with a 35 pack-year smoking history was diagnosed in September 2018 with stage IVA lung adenocarcinoma (LUAD) (cT1cN3M1b) harboring an EGFR L858R mutation. Osimertinib (80 mg/day) was initiated in November 2018. Two months later, computed tomography (CT) revealed a good partial response in the primary tumor. Subsequently, the dose was reduced to 40 mg/day due to adverse events, such as skin rash and pruritus. In April 2021, the patient developed bilaterally asymmetrical leg edema and grade 3 thrombocytopenia. The D-dimer level was high, 17.6 µg/mL, and a subsequent contrast CT revealed thromboses in the pulmonary arteries and the vein of the lower leg (Figure 1A). The patient was immediately administered anticoagulation using apixaban, and the osimertinib treatment was discontinued. Two weeks later, contrast CT revealed a decrease in the thromboses (Figure 1B), and the osimertinib treatment was restarted, while the apixaban treatment continued. The PFS after the initiation of osimertinib treatment was 40.7 months and that following the restarting of osimertinib was 11.4 months. The patient is currently undergoing osimertinib treatment without VTE recurrence.
Case 2
A 66-year-old man, non-smoker, was diagnosed in October 2017 with stage IVB LUAD (cT4N0M1c) harboring an exon 19 deletion. After palliative radiation therapy for the spinal and brain metastases, gefitinib 250 mg/day was initiated in January 2018. After 15 months, the treatment was terminated due to bilateral pulmonary metastases. An EGFR T790M mutation was detected using liquid biopsy, and gefitinib was switched to osimertinib 80 mg/day in May 2019, achieving a partial response. Nine months after the initiation of osimertinib, the patient was incidentally diagnosed with PE on a routine CT scan (Figure 2A). The patient was administered anticoagulation with edoxaban while receiving osimertinib. After six weeks, a contrast CT revealed that the thrombus had disappeared (Figure 2B). Osimertinib and edoxaban treatment was continued without PE recurrence. Eight months after the PE onset, worsening pleural effusion and dissemination were observed, and osimertinib was discontinued.

Case 3
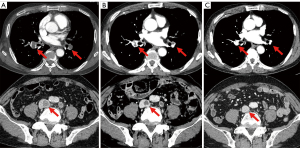
A 74-year-old man with a smoking history of 20 pack-years was diagnosed in April 2015 with stage IB LUAD (cT2aN0M0) harboring an EGFR L858R mutation. A right upper lobectomy was performed in July 2015; however the cancer recurred in May 2019, with pleural dissemination and mediastinal lymph node metastasis. Osimertinib (80 mg/day) was initiated in June 2019. Six weeks later, a CT revealed a partial response. Subsequently, the dose was reduced to 40 mg/day at the patient’s request, due to fatigue. Eight months after the initiation of the osimertinib treatment, the patient was admitted to the emergency room with edema and pain in the left lower leg. The percutaneous oxygen saturation was 88% (room air), which decreased further with exertion. The patient required 1 L/min of oxygen therapy via a nasal cannula. The circulation dynamics were stable. A contrast CT scan revealed thromboses in both pulmonary arteries, the common iliac vein, and the persistent left inferior vena cava. The patient was diagnosed with VTE and was administered anticoagulation with apixaban while receiving osimertinib (Figure 3A). Seven days after admission, a contrast CT revealed a decrease in the thromboses (Figure 3B), and oxygen administration was terminated when the patient recovered from respiratory failure. Osimertinib and apixaban treatment was then continued. Five months after the VTE onset, a contrast CT revealed that the thromboses had disappeared (Figure 3C). Seven months after the onset of VTE, there was an enlargement of the mediastinal lymph node metastases. After radiotherapy for the mediastinal lymph node metastases, osimertinib was continued, without apixaban. Fourteen months after the onset of VTE, the mediastinal lymph node metastases worsened again, and osimertinib was discontinued. No VTE recurrence was observed with the osimertinib treatment, even without the use of apixaban.
Ethical considerations
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
We report on three patients who developed VTE during osimertinib treatment and were able to safely continue using osimertinib while undergoing concomitant anticoagulation therapy. The clinical backgrounds of the three cases are shown in Table 1. The PFS from VTE onset in each of the three cases was 11.4+, 7.7, and 6.1 months, respectively. The OS from VTE onset was 11.4+, 26.0, and 25.9+ months, respectively. It was difficult to explain VTE as Trousseau syndrome caused by lung cancer because all patients developed VTE during disease control with osimertinib treatment. The risk of developing VTE when the disease is controlled is low (7). Additionally, the Khorana score, a risk factor for VTE, was one in all patients, indicative of a low-risk group (8). The cumulative probability of VTE after 6 months was low (3.8%) in the low-risk group. Thus, these patients were considered to have a VTE event that was attributable to osimertinib. We could not identify any predictive factors for VTE in these three cases. Moreover, the time from osimertinib initiation to VTE onset also varied from 7.7 to 29.3 months. Shiroyama et al. reported a case of VTE on the 16th day of osimertinib treatment (9). These findings indicate that VTE during osimertinib can occur even after long-term osimertinib use.
Table 1
| Characteristics | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age (years) | 71 | 66 | 74 |
| Sex | Male | Male | Male |
| Disease stage | IVA | IVB | Postoperative recurrence |
| Site of metastasis | Abdominal lymph node | Pulmonary metastasis, brain, bone | Pleural dissemination |
| Tumor histology | Adenocarcinoma | Adenocarcinoma | Adenocarcinoma |
| Smoking status (pack-years) | 35 | 0 | 20 |
| ECOG PS at the start of osimertinib | 1 | 1 | 1 |
| EGFR mutation status at the start of osimertinib | L858R in exon 21 | exon 19 deletion T790M | L858R in exon 21 |
| Lines of osimertinib treatment | 1st line | 2nd line | 1st line |
| ORR of osimertinib at the onset of VTE | PR | PR | PR |
| Dose of osimertinib at the onset of VTE (mg/body/day) | 40 | 80 | 40 |
| The time from the start of osimertinib to the onset of VTE (mo) | 29.3 | 16 | 7.7 |
| Severity of VTE | |||
| Respiratory failure | No | No | Yes |
| Circulatory failure | No | No | No |
| Treatment for VTE | Apixaban | Edoxaban | Apixaban |
| Outcomes of VTE | Recovery | Recovery | Recovery |
| Recurrence of VTE | No | No | No |
| PFS from VTE onset (mo) | 11.4+ | 7.7 | 6.1 |
| PFS from the start of osimertinib (mo) | 40.7+ | 23.7 | 13.9 |
| OS from initial treatment (mo) | 40.7+ | 42 | 33.6+ |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; mo, months; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; PS, performance status; VTE, venous thromboembolism.
The risk of VTE associated with osimertinib remains unclear. In the FLAURA study, VTE was observed in 1.4% (4/279) of patients (1). Petrelli et al. reviewed the data of 17 prospective and retrospective studies investigating osimertinib in NSCLC and reported that the incidence of VTE was 2.4% (10). When the report was limited to randomized trials, the relative risk of developing VTE with osimertinib compared to that in the control group, tended to be higher at 1.45 (95% confidence interval, 0.56–3.78; P=0.45), although the difference was not statistically significant. Conversely, a prospective observational study of 126 EGFR-mutated NSCLC patients treated with osimertinib reported a higher incidence rate of VTE (10/126, 7.9%) (11). At our hospital, three of 95 patients (3.2%) developed VTE during first- and second-line treatment with osimertinib, which was higher than that observed in the FLAURA study. Osimertinib may have a higher risk of developing VTE in the real world and this should be considered in clinical practice.
There is an ASCO Clinical Practice Guideline for VTE prophylaxis (12). The guideline does not recommend routine anticoagulation for VTE prophylaxis in all cancer patients. However, pharmacologic thromboprophylaxis may be offered in high-risk cases such as hospitalized patients who have active malignancy and acute medical illness or reduced mobility, and high-risk outpatients with cancer (Khorana score of 2 or higher). For example, apixaban therapy resulted in a significantly lower rate of VTE than did placebo among intermediate-to-high-risk ambulatory patients with cancer who were starting chemotherapy in the AVERT study (13).
Evidence regarding a treatment strategy for VTE during osimertinib is lacking. There is only one report of successful retreatment with osimertinib after the development of VTE during osimertinib (9). In addition, there are no reports of switching to other EGFR-TKIs after the onset of VTE. VTE is a serious complication that can be fatal, and we should be cautious about continuing treatment after the appearance of VTE. On the other hand, EGFR-TKI is a key drug in lung cancer treatment, and successful continuation leads to significant benefits. Understanding the risks and benefits of osimertinib continuation with concomitant anticoagulation therapy is necessary to select the best treatment option.
All patients in the current case series were treated successfully with a combination of osimertinib and direct oral anticoagulants. None of the patients experienced VTE recurrence, and all had PFS of 6 months or longer. One patient was still receiving osimertinib therapy 12 months after the onset of VTE. This report supports the hypothesis that continuation of osimertinib with concomitant anticoagulation therapy may be a treatment option for patients with VTE during osimertinib.
In this study, we have reported on three cases of VTE during osimertinib. They were successfully treated via concomitant direct oral anticoagulation and achieved prolonged PFS with osimertinib. Osimertinib may cause VTE and should be used with caution. In such cases, the patient may continue treatment using concomitant anticoagulation therapy. This finding allows the oncologist to better weigh risks and benefits in a new light in view of this observation.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-419/rc
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-419/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-419/coif). SS received honoraria for lectures from AstraZeneca, Chugai Pharma, Taiho Pharmaceutical, and the MSD, outside of the submitted work. SW received honoraria for lectures from Eli Lilly, Pfizer, Novartis Pharma, AstraZeneca, Chugai Pharma, Bristol-Myers, Boehringer Ingelheim, the MSD, Ono Pharmaceutical, Daiichi Sankyo, and Taiho Pharmaceutical, outside of the submitted work. TK received grants from Chugai Pharma, Boehringer Ingelheim, Taiho Pharmaceutical, Daiichi Sankyo, Shionogi, TEIJIN PHARMA, KYORIN Pharmaceutical, Asahi Kasei Pharma, Sanofi, MSD, Ono Pharmaceutical, and Nobelpharma; honoraria for lectures from Terumo, Eli Lilly, AstraZeneca, Daiichi Sankyo, KYORIN Pharmaceutical, Novartis, MERCK, Bristol-Myers, NIPRO, Eisai, Ono Pharmaceutical, Chugai Pharma, GlaxoSmithKline, Sumitomo Dainippon Pharma, and Kyowa Kirin; honoraria for Participation on a Data Safety Monitoring Board from Janssen Pharmaceutical; and honoraria for Receipt of drugs from Nobelpharma, outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patients for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA 2005;293:715-22. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Li LJ, Chen DF, Wu GF, et al. Incidence and risk of thromboembolism associated with bevacizumab in patients with non-small cell lung carcinoma. J Thorac Dis 2018;10:5010-22. [Crossref] [PubMed]
- Lei H, Zhang M, Wu Z, et al. Development and Validation of a Risk Prediction Model for Venous Thromboembolism in Lung Cancer Patients Using Machine Learning. Front Cardiovasc Med 2022;9:845210. [Crossref] [PubMed]
- Yang Y, Zhou Z, Niu XM, et al. Clinical analysis of postoperative venous thromboembolism risk factors in lung cancer patients. J Surg Oncol 2012;106:736-41. [Crossref] [PubMed]
- Chew HK, Wun T, Harvey D, et al. Incidence of venous thromboembolism and its effect on survival among patients with common cancers. Arch Intern Med 2006;166:458-64. [Crossref] [PubMed]
- Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood 2010;116:5377-82. [Crossref] [PubMed]
- Shiroyama T, Hayama M, Satoh S, et al. Successful retreatment with osimertinib after osimertinib-induced acute pulmonary embolism in a patient with lung adenocarcinoma: A case report. Respir Med Case Rep 2016;20:25-7. [Crossref] [PubMed]
- Petrelli F, Cabiddu M, Borgonovo K, et al. Osimertinib-related venous thromboembolism in non small lung cancer. Thromb Res 2022;210:63-6. [Crossref] [PubMed]
- Lorenzi M, Ferro A, Cecere F, et al. First-Line Osimertinib in Patients with EGFR-Mutant Advanced Non-Small Cell Lung Cancer: Outcome and Safety in the Real World: FLOWER Study. Oncologist 2021; Epub ahead of print. [Crossref] [PubMed]
- Key NS, Khorana AA, Kuderer NM, et al. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol 2020;38:496-520. [Crossref] [PubMed]
- Carrier M, Abou-Nassar K, Mallick R, et al. Apixaban to Prevent Venous Thromboembolism in Patients with Cancer. N Engl J Med 2019;380:711-9. [Crossref] [PubMed]


