
Real world efficacy of osimertinib in second line/beyond in patients with metastatic EGFR+ non-small cell lung cancer and role of paired tumour-plasma T790M testing at tyrosine kinase inhibitor resistance
Highlight box
Key findings
• This study reiterates the benefit of osimertinib post resistance to 1G/2G TKI in EGFR-mutant NSCLC in second/subsequent line; and demonstrates that tissue T790M mutation status may be more predictive of response to osimertinib than plasma T790M testing.
What is known and what is new?
• Osimertinib has demonstrated efficacy in metastatic EGFR T790M-positive NSCLC after progression on prior TKI based on the AURA3 trial. This study presents real-world data on efficacy of osimertinib in/beyond second line setting and examined outcomes of plasma and tumour T790M-positive patients treated with osimertinib.
What is the implication, and what should change now?
• Findings of this study suggest potential T790M heterogeneity at time of EGFR TKI resistance and that paired tumor-plasma T790M testing may better inform treatment response to osimertinib. Tissue T790M testing should be considered whenever feasible.
Introduction
Epidermal growth factor receptor (EGFR) mutations are the most common actionable driver mutations in non-small cell lung cancer (NSCLC), of which exon 19 deletions and exon 21 point mutations (L858R) are predominant (1,2). Current therapeutic strategies include the sequential approach starting with first-generation (1G) tyrosine kinase inhibitors (TKIs) (gefitinib, erlotinib), second-generation (2G) (afatinib, dacomitinib), followed by sequential osimertinib if found to be T790M+ (3), or upfront third-generation (3G) (osimertinib) (4,5). The EGFR T790M mutation is the primary mechanism of resistance in patients with progression on 1G/2G TKIs, for which 3G TKIs are typically efficacious (6,7).
The FLAURA study showed significantly longer median progression-free survival (mPFS) (18.9 vs. 10.3 months, HR 0.46, P<0.001) and overall survival (OS) (38.6 vs. 31.8 months, HR 0.80, P=0.046) with first-line osimertinib compared with 1G TKIs (4,5). Subgroup analysis suggests inferior outcomes with Asian ethnicity and L858R. On the other hand, in the AURA3 trial when patients received osimertinib after acquiring T790M mutation to first line EGFR TKI, the median OS (mOS) was 26.8 [95% confidence interval (CI): 23.5–31.5] vs. 22.5 (95% CI: 20.2–28.8) months with platinum-based chemotherapy (3). Several real-world studies on sequential use of 1G/2G TKIs followed by osimertinib (upon T790M acquired resistance) have also reported good clinical efficacy and survival outcomes with this approach with OS 36–61.3 months from start of first-line EGFR TKI (8-13). Nonetheless, the optimal TKI sequence is still not known, and while osimertinib may increasingly be a preferred first-line option, a concern is the lack of standard targeted therapy after progression on osimertinib.
Additionally, detection of T790M mutation from cell-free DNA (cfDNA) or circulating tumour DNA using non-invasive liquid biopsy techniques has increasingly been incorporated into routine clinical practice at the point of resistance on 1G/2G TKI. Plasma cfDNA genotyping using the Cobas EGFR mutation test v2—a semi-quantitative real-time polymerase chain reaction (PCR) test, was the first liquid biopsy to be approved as a companion diagnostic test to identify T790M mutation. Several other mutational analysis platforms including amplification refractory mutation system (ARMS), digital PCR, as well as next-generation sequencing (NGS) techniques have also been utilised for this purpose. However, few of the previous real-world studies on sequential TKI treatment had focused on differential outcomes between plasma vs. tumour T790M+ patients treated with osimertinib.
Data on efficacy of osimertinib beyond second line and outcomes between plasma vs. tumour T790M+ patients is limited, and sequential use of osimertinib after 1G/2G TKIs remains relevant. Here, we describe the real-world outcomes of use of osimertinib in second and subsequent line setting in our patients with advanced EGFR+ NSCLC after resistance to prior front-line EGFR TKI, treated at a tertiary cancer center in Asia. We present the following article in accordance with the STROBE reporting checklist (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-661/rc).
Methods
We identified 202 patients with metastatic EGFR+ NSCLC treated with osimertinib in second or subsequent line after progression on prior EGFR-TKI from July 2015 to January 2019 at the National Cancer Centre Singapore. Osimertinib was first made available to patients as part of the AZD9291 Early Access Program (EAP) in 2015. Of these, data from 192 evaluable patients were analyzed (Figure 1). All patients were started on osimertinib 80 mg once daily with dose reductions as per physician’s discretion for tolerability. Patients underwent regular radiological assessments with computed tomography (CT) scans and were analysed for response rate (RR) and PFS as per investigator-assessed Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria.
We included patients who consented under the Lung Cancer Consortium Singapore (LCCS) database and clinical data including baseline characteristics, primary EGFR mutation, T790M mutation status upon progression, presence of baseline brain metastases (BM), first-line EGFR-TKI used, systemic treatment including chemotherapy use prior to osimertinib, as well as survival status, were captured. Electronic records of these patients were retrospectively reviewed and anonymized for analyses and reporting. Patients with incomplete data or lost to follow-up were excluded. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent to participate was obtained from each of the patients under our LCCS data-base. This study was approved by Centralized Institutional Review Board (CIRB), Singapore (No. CIRB 2007/444/B). Patient data were de-identified and anonymized before analysis.
Reflex testing for primary EGFR mutations at the point of diagnosis of non-squamous NSCLC was performed by direct Sanger sequencing or Roche COBAS EGFR mutation test—a real-time allele-specific PCR test, while T790M testing on histology specimens was performed mainly by the COBAS EGFR mutation test. For plasma specimens, tests on cfDNA for T790M mutation were performed by the plasma EGFR COBAS mutation test.
Statistical analysis
Baseline patient demographics, cancer type, primary EGFR mutations, T790M mutation status, type of T790M testing, presence of BM and TKI are summarized using descriptive statistics; categorical data were described using frequency and percentages while continuous data were described using median with interquartile range and range. We evaluated the overall response rates (ORR) for osimertinib as well as progression-free survival (PFS) and overall survival (OS) in this patient cohort. PFS was calculated from the start date of osimertinib to the date of documented progression of disease. OS was calculated from start date of osimertinib to date of demise and surviving patients were censored at date of last follow-up. The Logrank test was used to compare the survival between groups of patients. Univariable and multivariable analysis (MVA) were performed using the Cox proportional hazard regression model, proportional hazard assumption was assessed using the Schoenfeld residuals test.
Results
Baseline characteristics
In this cohort of 193 patients, the median age at diagnosis was 63 years (interquartile range, 55–70 years); 59.6% of patients were females, with a predominance of never-smokers (79.8%). Up to 79.3% of patients were of Eastern Cooperative Oncology Group (ECOG) performance status 0–1 at start of osimertinib use.
One hundred (51.8%) patients received osimertinib in the second line, whereas the remaining patients received it in third or subsequent lines (up to 9 prior lines of treatment). Median number of lines was 2 (range, 2–10). Prior line TKI therapy was gefitinib or erlotinib (1G-TKI) in 144 (74.6%) patients, afatinib in 47 (24.4%) and EGF816 as part of a phase I/II trial (14) in 2 (1%) patients. BM was present in 55/193 (28.5%) patients at baseline (at time of starting osimertinib), and 37 (67.3%) of them had received whole brain radiotherapy (WBRT). In the group of patients who received afatinib, there was higher proportion of BM (46.8% vs. 22.9%) compared to the 1G-TKI group. Baseline patient data is presented in Table 1.
Table 1
| Characteristics | Frequency (N=193) |
|---|---|
| Age at diagnosis, years | |
| Mean (SD) | 62 (10.4) |
| Median [interquartile range] | 63 [55–70] |
| Range | 25 to 85 |
| Gender, n (%) | |
| Female | 115 (59.6) |
| Male | 78 (40.4) |
| Ethnicity, n (%) | |
| Chinese | 166 (86.0) |
| Malay | 8 (4.1) |
| Indian | 4 (2.1) |
| Others | 15 (7.8) |
| ECOG, n (%) | |
| 0–1 | 153 (79.3) |
| 2–4 | 40 (20.7) |
| Smoking history, n (%) | |
| Non-smoker | 154 (79.8) |
| Former/current | 37 (19.2) |
| Unknown | 2 (1.0) |
| Primary EGFR mutation, n (%) | |
| Exon 19 mutation | 117 (60.6) |
| Exon 21 mutation | 62 (32.1) |
| Others | 12 (6.2) |
| Unknown | 2 (1.0) |
| T790M mutant, n (%) | |
| No | 20 (10.4) |
| Yes | 151 (78.2) |
| Unknown/NA | 22 (11.4) |
| Line of treatment of osimertinib | |
| Median | 2 |
| Range | 2 to 10 |
| Presence of baseline brain metastasis, n (%) | |
| Yes | 55 (28.5) |
| No | 138 (71.5) |
| Preceding TKI use, n (%) | |
| Gefitinib/erlotinib | 144 (74.6) |
| Afatinib | 47 (24.4) |
| Others | 2 (1.0) |
| Chemotherapy use prior to osimertinib, n (%) | |
| No | 111 (57.5) |
| Yes | 79 (40.9) |
| NA | 3 (1.6) |
TKI, tyrosine kinase inhibitor; EGFR+, epidermal growth factor receptor mutation positive; NSCLC, non-small cell lung cancer; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group; NA, not applicable.
EGFR mutation status
Of the 193 patients, majority harbored exon 19 (60.6%, n=117) and exon 21 (32.1%, n=62) mutations, whereas patients with exon 18 mutations (G719A, p.E709_T710>D) or dual co-existing EGFR mutations accounted for most of the remaining patients. Of note, 6 (3.1%) patients had de novo T790M mutation detected upon diagnosis which co-occurred with another sensitizing EGFR mutation. In the group of patients who received upfront afatinib, there was a higher proportion of double (compound) EGFR mutations (6.4% vs. 2.8%) compared to 1G-TKI group.
In this cohort, 171 (88.6%) patients underwent testing for T790M mutation of which the mutation was detected in 151 (78.2%) of all patients; 95 (49.2%) patients had T790M detected based on re-biopsy (comprising both histology as well as cytology specimens) and 63 (32.6%) from plasma EGFR testing. T790M status was negative in 20 (10.4%) and not tested in 22 (11.4%) patients who received osimertinib.
Overall efficacy of osimertinib
After a median follow up of 36.6 (95% CI: 31.8–41.6) months, mPFS was 10.3 (95% CI: 8.64–11.50) months on osimertinib (Figure 2) and mOS was 20.0 (95% CI: 15.61–23.13) months for the whole cohort (Figure 3).
Osimertinib was used in second line in 52% of patients, achieving an mPFS of 10.0 (95% CI: 6.34–12.29) months and OS of 20.5 (95% CI: 15.18–23.89) months. In the remaining 48% patients who received osimertinib in the third/subsequent line, outcomes were similar with mPFS of 10.3 (95% CI: 8.05–14.03) months and mOS of 18.7 (95% CI: 14.13–23.75) months. Interestingly there was also no significant difference in RR to osimertinib at 43% in both groups.
Physician-assessed ORR to osimertinib was 43% (95% CI: 35.9–50.3%): 48.3% in T790M+ vs. 20% in T790M-negative patients (Table 2). In patients who received osimertinib, 72.5% achieved disease control [best response being partial response (PR) or stable disease (SD)] (Table 3). Duration of response achieved was 11.1 (95% CI: 8.28–13.67) months in T790M+ patients.
Table 2
| Variables | EGFR T790M positive | EGFR T790M negative | HR (95% CI) | P value |
|---|---|---|---|---|
| ORR | 48.3% | 20% | ||
| Median PFS | 11.2 months | 3.1 months | 0.52 (0.32, 0.85) | 0.01 |
| Median OS | 22.6 months | 7.9 months | 0.43 (0.26, 0.72) | 0.001 |
EGFR, epidermal growth factor receptor; HR, hazards ratio; CI, confidence interval; ORR, overall response rate; PFS, progression-free survival; OS, overall survival.
Table 3
| Best overall response | Frequency |
|---|---|
| PR, n (%) | 83 (43.0) |
| SD, n (%) | 57 (29.5) |
| PD, n (%) | 40 (20.7) |
| Not evaluable/not applicable, n (%) | 13 (6.7) |
| Best overall response rate (95% CI) | 43% (35.9–50.3%) |
| Disease control rate (95% CI) | 72.5% (65.7–78.7%) |
PR, partial response; SD, stable disease; PD, progressive disease; CI, confidence interval.
By MVA, ECOG ≥2 (adjusted HR 2.53, P<0.001) upon starting osimertinib was associated with a shorter OS of 6.5 months compared to 23.1 months in patients who were ECOG 0–1, whereas presence of T790M+ portends a longer OS (adjusted HR 0.50, P=0.008) (Table 4). The presence of T790M mutation was significantly associated with longer PFS compared to T790M-negative patients (adjusted HR 0.57, P=0.027). Conversely ECOG ≥2 (adjusted HR 2.10, P<0.001) and first-line afatinib use (adjusted HR 1.58, P=0.009) compared to 1G-TKI use, were significantly associated with shorter PFS by MVA (Table 5).
Table 4
| Variables | Adjusted HR (95% CI) | P value |
|---|---|---|
| EGFR T790M negative | 1 | |
| EGFR T790M positive | 0.50 (0.30, 0.83) | 0.008 |
| ECOG 0–1 | 1 | |
| ECOG 2–4 | 2.53 (1.71, 3.75) | <0.001 |
HR, hazards ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group.
Table 5
| Variables | Adjusted HR (95% CI) | P value |
|---|---|---|
| EGFR T790M negative | 1 | |
| EGFR T790M positive | 0.57 (0.34, 0.94) | 0.027 |
| ECOG 0–1 | 1 | |
| ECOG 2–4 | 2.10 (1.45, 3.04) | <0.001 |
HR, hazards ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; ECOG, Eastern Cooperative Oncology Group.
Efficacy of osimertinib in T790M+ 2nd/3rd line osimertinib by subgroups
Common sensitizing EGFR mutations in exon 19 and exon 21 have similar PFS of 10.3 months (HR 0.97, P=0.9), whereas other mutations are associated with a shorter PFS of 1.7 months (HR 1.39, P=0.3) though numbers were small (n=12). There was no significant difference between OS achieved in patients with exon 19 mutations vs. exon 21 mutations vs. others at 19.7 vs. 20.5 vs. 15.2 months, respectively (P=0.8).
Prior chemotherapy was also not associated with any statistically different ORR, PFS or OS in patients who received osimertinib. Patients with baseline BM had a shorter OS of 16.3 months compared to 22.4 months in patients without baseline BM (HR 1.58, P=0.01). An ECOG status ≥2 upon diagnosis was associated with a significantly shorter PFS of 3.0 months (HR 2.34, P<0.001) and OS of 6.5 months (HR 2.79, P<0.001) with osimertinib treatment compared to PFS of 11.4 months and OS of 23.1 months in patients with ECOG 0–1. Smoking history did not significantly affect PFS or OS on osimertinib.
Plasma vs. tumour T790M+ subgroups
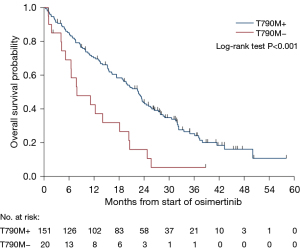
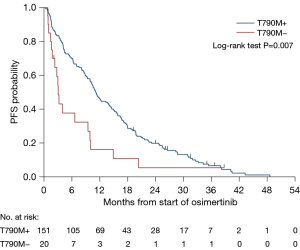
In T790M+ patients, treatment with osimertinib resulted in a statistically significantly improved mOS of 22.6 months vs. only 7.9 months in T790M-negative patients (HR 0.43, P<0.001) (Figure 4), and mPFS was 11.2 months compared to 3.1 months (HR 0.52, P=0.007) (Figure 5). This trend was shown in T790M+ proven on tumour samples, however, plasma T790M+ was not associated with a significant difference in either PFS (HR 0.67, P=0.2) or OS (HR 0.73, P=0.3). There were 21 patients with unknown T790M mutation status, OS was 14.1 months (HR 0.70, P=0.3) and PFS was 8.0 months (HR 0.83, P=0.6).
ORR in patients who were tumour T790M+ was 52% vs. 44% in those plasma T790M+. mPFS and mOS in tumour T790M+ patients were 14.5 and 23.5 months, respectively; and in plasma T790M+ patients were 8.0 and 18.7 months, respectively, though unable to prove statistical significance as the 2 groups overlap. Twenty-two patients underwent both plasma and tissue testing for T790M mutation, however concordance rate of T790M testing by the 2 methods was only 40.9%. Of the 10 patients who were T790M+ on plasma testing but had discordant results on tissue testing, ORR to osimertinib was only 30% compared to 52% ORR in patients who were T790M+ on tissue testing (Table 6).
Table 6
| Plasma | |||
|---|---|---|---|
| EGFR T790M negative | EGFR T790M positive | ||
| Tumour | EGFR T790M negative | 1/1 (100%) | 3/10 (30%) |
| EGFR T790M positive | 2/3 (67%) | 5/8 (63%) | |
EGFR, epidermal growth factor receptor.
Prior EGFR TKI therapy
Seventy-five percent of patients received prior 1G EGFR-TKI gefitinib or erlotinib, 24% patients received prior 2G EGFR-TKI afatinib and 1% received other EGFR-TKIs (EGF816). Patients received prior line TKI for a median duration of 13.1 months (interquartile range, 7.7–18.6 months), with no significant difference between duration of treatment with prior gefitinib/erlotinib (12.6 months) vs. afatinib (13.6 months).
Patients who received prior afatinib showed a trend towards a lower ORR of 32% with osimertinib use vs. 46.5% in patients who received prior 1G EGFR TKI (P=0.08) despite a similar rate of T790M positivity (79.9% with 1G-TKI and 72.3% with afatinib use). Patients receiving prior EGF816 achieved an ORR of 50%. PFS achieved on osimertinib was significantly longer at 10.5 months in patients who received prior 1G EGFR-TKI compared to 6.3 months in patients with prior 2G EGFR-TKI, with a hazard ratio of 1.57 (P=0.009), however type of first-line TKI (whether 1G or 2G TKI) did not have a significant influence on OS—20 months with 1G TKI and 19 months with afatinib (P=0.2).
Discussion
The data demonstrates that osimertinib is effective in patients with EGFR+ metastatic NSCLC (mNSCLC) who develop T790M mutation upon progression on prior 1G/2G TKI in second line and later setting. Despite nearly half of patients receiving osimertinib in third line or beyond, the mPFS of 10 months achieved was comparable to that reported in the AURA3 trial where osimertinib was given predominantly (95% of patients) in the second line setting (3). mOS with osimertinib was 20 months—lower compared to that (26.8 months) in AURA3 (15), likely contributed by a significant proportion of our patients (20%) with poor performance status. Known to be a negative prognostic factor in NSCLC (16,17), poor ECOG of ≥2 was also significantly associated with shorter OS in our patients by MVA (HR 2.53, P<0.001).
The development of T790M mutation is both a robust prognostic and predictive biomarker for efficacy of osimertinib, its presence resulting in significantly higher RR, longer PFS and OS in the patients. ORR achieved was 48% in patients with T790M+ mutation and 24% in the cohort of 44 patients with negative or unknown T790M status. In patients without T790M mutation in our study, osimertinib resulted in RR of 20%—consistent with results from the phase I AURA trial (18), poor OS of 14.1 months and PFS of 3.1 months only. Detecting the presence of acquired T790M mutation is hence critical to identify patients most likely to benefit from second-line osimertinib, in line with current recommendations (19,20).
In this cohort, only 49% of patients diagnosed with T790M+ had undergone tissue biopsy at TKI resistance. Although tissue biopsy remains the current standard for molecular analysis, obtaining tumour tissue biopsy can present challenges due to inaccessibility of tumour, risk of complications from the invasive procedure, or inadequacy of tissue for molecular analysis. The use of liquid biopsies for genotyping is an appealing alternative and increasingly utilized in clinical practice. Circulating free tumor-derived DNA (ctDNA) found in blood plasma has been approved by regulatory agencies for T790M detection to identify patients for osimertinib after progression on first-line 1G/2G EGFR TKI (19,20).
In our study, the Cobas EGFR mutation test was used for T790M mutation testing in both tumour tissue as well as plasma cfDNA. It has reported sensitivity of 70–80% for genotyping and concordance ranging 51–86% between tissue and plasma (21-25). The concordance of tumour and plasma testing for T790M mutation in our cohort appeared to be suboptimal at only 40.9%, which could be a function of tumour burden and intratumoral heterogeneity for T790M-mediated resistance (26), with the caveat of test sensitivity and the small number of patients with paired tumour-plasma T790M testing. A retrospective analysis from patients in AURA3 (25) had also demonstrated that EGFR Cobas plasma test was less sensitive and had lower concordance of 51% with Cobas tissue T790M, compared to plasma droplet digital PCR and plasma NGS.
We found that T790M positivity on tumour was more predictive for treatment response compared to plasma T790M positivity, consistent with results from earlier studies (27,28). In the subgroup of patients with both tumour and plasma testing results, 8 patients who had concordant findings of T790M+ in both plasma and tumour achieved an ORR of 63% (5 of 8 patients) compared to only 30% (3 of 10 patients) who were plasma T790M+ but had negative tumour T790M testing. The converse was true—in the 3 patients who were tumour T790M+ but plasma T790M−, the ORR was still 67%. Plasma T790M+ patients on osimertinib had numerically shorter PFS/OS compared to tissue T790M+ patients in our study however we were unable to verify the statistical significance due to overlap between these 2 subgroups and a significant proportion of patients not having known plasma T790M mutation status. This may be attributed to higher level of ctDNA shed by the tumor in plasma T790M+ patients and may reflect a higher tumour burden as suggested by an association of plasma T790M positivity with higher number of metastatic sites (29). Thress et al. also reported lower clinical ORR of 38% in patients with plasma T790M+ but tumour T790M−, as well as lower rate of plasma T790M mutation detection in patients with disease limited to the thorax vs. extrathoracic metastatic disease (24), suggesting potential tumour heterogeneity, presence of other resistance mechanisms and that plasma ctDNA is better able to reflect total tumour burden compared to tissue biopsy. Despite that, currently there remains limited data on outcomes of patients with plasma T790M+/tissue T790M− status treated with osimertinib, which requires further validation in a larger prospective study. Furthermore, there is also the likelihood of false negative rates of approximately 30% with cfDNA-based liquid biopsies compared with traditional tissue biopsies (30,31), consistent with the ORR shown in the tumour T790M+/plasma T790M− group. This underscores the value of paired tumour and plasma testing for T790M status to guide T790M-directed therapy in the TKI resistance setting. Aside from T790M mutation, tissue testing may also offer additional insights into resistance mechanisms including information about the transcriptomic subtype, tumour microenvironment, as well as histologic transformation (32).
Interestingly, our study showed that patients who received prior first line afatinib had inferior PFS (but not OS) compared to those who received 1G TKI. This observation may be confounded by presence of higher proportion of patients with compound EGFR mutations who received afatinib which is associated with poor clinical outcomes in lung adenocarcinoma (33,34). Furthermore, there was a higher prevalence of CNS metastases in the group who received afatinib compared to 1G TKI (46.8% vs. 22.9%). Osimertinib has shown superior intracranial activity and brain penetrance (3,35), but prior to the advent of osimertinib, afatinib was preferred over 1G TKI for EGFR+ mNSCLC patients with de novo BM, based on clinical observations from trials showing intracranial efficacy with afatinib in patients with BM (36-39). This selection bias likely resulted in more patients with BM being treated with afatinib, conceivably resulting in poorer PFS (6.3 vs. 10.5 months, HR 1.58, P=0.009) with first-line afatinib compared to 1G TKI.
Several real-world studies have shown good outcomes with a sequential TKI approach which potentially helps to prolong time to chemotherapy in patients with acquired T790M mutation on EGFR TKI, particularly with sequential afatinib and osimertinib (11,12,40,41). The RESET (40) and UpSwing (42) observational studies demonstrated favourable OS of more than 35 months (from time of start of afatinib) in T790M-positive patients on osimertinib after afatinib failure. Our findings too, support the role for sequential TKI strategy especially in countries where there are resource constraints.
Limitations of our study include its retrospective nature which could have resulted in inadvertent selection bias. Definitive conclusions regarding the subgroups of interest cannot be drawn due to the small numbers particularly for the patients with paired tumor and plasma T790M testing, and we acknowledge that our findings for this subgroup remain hypothesis-generating. Data regarding toxicity and patient-reported outcomes were also not included. There was also limited data regarding mechanisms of post-osimertinib resistance due to limited number of biopsies performed after osimertinib failure.
Conclusions
The findings of our study are particularly relevant to countries where sequential 3G TKI strategy is being practised in the setting of acquired T790M resistance to 1G/2G TKI. Our results further emphasize the complementary role of plasma cfDNA to tissue T790M testing, and the potential for additional insight from paired plasma-tumour biopsies with regards to genomic heterogeneity and acquired T790M-mediated resistance.
Acknowledgments
This data was presented as a poster at ISALC 2020 World Conference on Lung Cancer.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-661/rc
Data Sharing Statement: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-661/dss
Peer Review File: Available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-661/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-22-661/coif). JM reports travel support for meetings from AstraZeneca. GGYL reports honoraria from Amgen and consulting/advisory role for Merck, AstraZeneca, Pfizer, Bristol Myers Squibb and Roche. MKA reports support for meetings from AstraZeneca, Boehringer Ingelheim, Ipsen; honoraria from Boston Scientific and consulting/advisory role for Merck. RK reports research funding support from Sanofi (Inst), Janssen Pharmaceuticals (Inst); honoraria from Astellas Pharma, Novartis, Janssen Pharmaceuticals, MSD Oncology, Bristol-Myers Squibb, consulting/advisory role for Pfizer, Astellas Pharma, Novartis, Mundipharma. TR reports honoraria and Speakers’ Bureau from Novartis, consulting/advisory role for Ipsen and Eisai. TKHL reports honoraria for AstraZeneca and consulting/advisory role for MSD. DSWT reports research funding from Novartis (Inst), GlaxoSmithKline (Inst), AstraZeneca (Inst); honoraria from Bristol-Myers Squibb, Takeda Pharmaceuticals, Novartis, Roche and Pfizer; consulting/advisory role for Novartis, Merck, Loxo, AstraZeneca, Roche, Pfizer. DWTL reports research funding from Bristol-Myers Squibb (Inst), honoraria from Boehringer Ingelheim, consulting/advisory role for Roche, AstraZeneca, MSD Oncology, Novartis and Boehringer Ingelheim and stock ownership from Clearbridge Biomedics. QSN reports research funding from Novartis, MSD Oncology and Bayer; honoraria from MSD Oncology, AstraZeneca and Pierre Fabre and consulting/advisory role for Boehringer Ingelheim. WLT reports educational grant support from AstraZeneca; honoraria from Novartis, Merck, and Amgen; support for meetings from AstraZeneca, Ipsen, Boehringer Ingelheim, Bristol-Myers Squibb (Inst), and DKSH. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent to participate was obtained from each of the patients under our Lung Cancer Consortium Singapore (LCCS) data-base. This study was approved by Centralized Institutional Review Board (CIRB), Singapore (No. CIRB 2007/444/B). Patient data were de-identified and anonymized before analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [Crossref] [PubMed]
- Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154-62. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum–Pemetrexed in EGFR T790M–Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med 2020;382:41-50. [Crossref] [PubMed]
- Skoulidis F, Papadimitrakopoulou V. Targeting the Gatekeeper: Osimertinib in EGFR T790M Mutation–Positive Non–Small Cell Lung Cancer. Clin Cancer Res 2017;23:618-22. [Crossref] [PubMed]
- Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014;4:1046-61. [Crossref] [PubMed]
- Tamiya M, Tamiya A, Suzuki H, et al. Which Is Better EGFR-TKI Followed by Osimertinib: Afatinib or Gefitinib/Erlotinib? Anticancer Res 2019;39:3923-9. [Crossref] [PubMed]
- Marinis F, Wu YL, de Castro G, et al. ASTRIS: a global real-world study of osimertinib in >3000 patients with EGFR T790M positive non-small-cell lung cancer . Future Oncol 2019;15: [Crossref] [PubMed]
- Huang YH, Tseng JS, Hsu KH, et al. The impact of different first-line EGFR-TKIs on the clinical outcome of sequential osimertinib treatment in advanced NSCLC with secondary T790M. Sci Rep 2021;11:12084. [Crossref] [PubMed]
- Hochmair MJ, Morabito A, Hao D, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: final analysis of the GioTag study. Future Oncol 2020;16:2799-808. [Crossref] [PubMed]
- Popat S, Jung HA, Lee SY, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG). Lung Cancer 2021;162:9-15. [Crossref] [PubMed]
- Magios N, Bozorgmehr F, Volckmar AL, et al. Real-world implementation of sequential targeted therapies for EGFR-mutated lung cancer. Ther Adv Med Oncol 2021;13:1758835921996509. [Crossref] [PubMed]
- Tan DS, Leighl NB, Riely GJ, et al. Safety and efficacy of nazartinib (EGF816) in adults with EGFR-mutant non-small-cell lung carcinoma: a multicentre, open-label, phase 1 study. Lancet Respir Med 2020;8:561-72. [Crossref] [PubMed]
- Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum-pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR-tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol 2020;31:1536-44. [Crossref] [PubMed]
- Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol 2010;5:620-30. [Crossref] [PubMed]
- Simmons CP, Koinis F, Fallon MT, et al. Prognosis in advanced lung cancer – A prospective study examining key clinicopathological factors. Lung Cancer 2015;88:304-9. [Crossref] [PubMed]
- Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR Inhibitor–Resistant Non–Small-Cell Lung Cancer. N Engl J Med 2015;372:1689-99. [Crossref] [PubMed]
- National Comprehensive Cancer Network. National Comprehensive Cancer Network Clinical Practice Guidelines NSCLC . National Comprehensive Cancer Network Clinical Practice Guidelines NSCLC. [Online] 16 March, 2022. [Cited: 23 March, 2022]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- European Society for Medical Oncology. ESMO Clinical Practice Guidelines Metastatic NSCLC. ESMO Clinical Practice Guidelines Metastatic NSCLC. [Online] 15 September, 2020. [Cited: 23 March, 2022]. Available online: https://www.esmo.org/content/download/347819/6934778/1/ESMO-CPG-mNSCLC-15SEPT2020.pdf
- Szpechcinski A, Bryl M, Wojcik P, et al. Detection of EGFR mutations in liquid biopsy samples using allele-specific quantitative PCR: A comparative real-world evaluation of two popular diagnostic systems. Adv Med Sci 2021;66:336-42. [Crossref] [PubMed]
- Li X, Zhou C. Comparison of cross-platform technologies for EGFR T790M testing in patients with non-small cell lung cancer. Oncotarget 2017;8:100801-18. [Crossref] [PubMed]
- Karlovich C, Goldman JW, Sun JM, et al. Assessment of EGFR Mutation Status in Matched Plasma and Tumor Tissue of NSCLC Patients from a Phase I Study of Rociletinib (CO-1686). Clin Cancer Res 2016;22:2386-95. [Crossref] [PubMed]
- Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: A cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer 2015;90:509-15. [Crossref] [PubMed]
- Papadimitrakopoulou VA, Han JY, Ahn MJ, et al. Epidermal growth factor receptor mutation analysis in tissue and plasma from the AURA3 trial: Osimertinib versus platinum-pemetrexed for T790M mutation-positive advanced non-small cell lung cancer. Cancer 2020;126:373-80. [Crossref] [PubMed]
- Diaz LA Jr, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 2014;32:579-86. [Crossref] [PubMed]
- Liu Y, Sun L, Xiong ZC, et al. Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving EGFR-TKIs. Onco Targets Ther 2017;10:2267-79. [Crossref] [PubMed]
- Kim H, Jung HA, Lee SH, et al. Comprehensive evaluation of the clinical utility of plasma EGFR test in non-small cell lung cancer patients with acquired resistance to first-line EGFR inhibitors. Transl Lung Cancer Res 2021;10:878-88. [Crossref] [PubMed]
- Minari R, Mazzashchi G, Bordi P, et al. Detection of EGFR-Activating and T790M Mutations Using Liquid Biopsy in Patients With EGFR-Mutated Non-Small-Cell Lung Cancer Whose Disease Has Progressed During Treatment With First- and Second-Generation Tyrosine Kinase Inhibitors: A Multicenter Real-L. Clinical Lung Cancer 2020;21:e464-e473. [Crossref] [PubMed]
- Oxnard GR, Thress KS, Alden RS, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2016;34:3375-82. [Crossref] [PubMed]
- Sacher AG, Paweletz C, Dahlberg SE, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol 2016;2:1014-22. [Crossref] [PubMed]
- Chua KP, Teng YHF, Tan AC, et al. Integrative Profiling of T790M-Negative EGFR-Mutated NSCLC Reveals Pervasive Lineage Transition and Therapeutic Opportunities. Clin Cancer Res 2021;27:5939-50. [Crossref] [PubMed]
- Kim EY, Cho EN, Park HS, et al. Compound EGFR mutation is frequently detected with co-mutations of actionable genes and associated with poor clinical outcome in lung adenocarcinoma. Cancer Biol Ther 2016;17:237-45. [Crossref] [PubMed]
- Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:45-51. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018; Epub ahead of print. [Crossref] [PubMed]
- Schuler M, Wu YL, Hirsh V, et al. First-Line Afatinib versus Chemotherapy in Patients with Non-Small Cell Lung Cancer and Common Epidermal Growth Factor Receptor Gene Mutations and Brain Metastases. J Thorac Oncol 2016;11:380-90. [Crossref] [PubMed]
- Hoffknecht P, Tufman A, Wehler T, et al. Efficacy of the irreversible ErbB family blocker afatinib in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)-pretreated non-small-cell lung cancer patients with brain metastases or leptomeningeal disease. J Thorac Oncol 2015;10:156-63. [Crossref] [PubMed]
- Hochmair M, Holzer S, Burghuber OC. Complete remissions in afatinib-treated non-small-cell lung cancer patients with symptomatic brain metastases. Anticancer Drugs 2016;27:914-5. [Crossref] [PubMed]
- Tan WL, Ng QS, Lim C, et al. Influence of afatinib dose on outcomes of advanced EGFR-mutant NSCLC patients with brain metastases. BMC Cancer 2018;18:1198. [Crossref] [PubMed]
- Kim T, Jang TW, Choi CM, et al. Sequential treatment of afatinib and osimertinib or other regimens in patients with advanced non-small-cell lung cancer harboring EGFR mutations: Results from a real-world study in South Korea. Cancer Med 2021;10:5809-22. [Crossref] [PubMed]
- Yamamoto N, Mera T, Märten A, et al. Observational Study of Sequential Afatinib and Osimertinib in EGFR Mutation-Positive NSCLC: Patients Treated with a 40-mg Starting Dose of Afatinib. Adv Ther 2020;37:759-69. [Crossref] [PubMed]
- Miura S, Märten A, Popat S. 420TiP UpSwinG: Real-world study of TKI activity in patients with EGFR mutation-positive (EGFRm+) NSCLC with uncommon mutations, and sequencing of afatinib followed by osimertinib. Ann Oncol 2020;31:S1405-S1406. [Crossref]




