CK-coated magnetic-based beads as a tool to isolate circulating tumor cells (CTCs) in human tumors
Introduction
Circulating tumor cells (CTCs) were first described in 1869 by Thomas Ashworth, an Australian physician. When observing under the microscope the blood of a man with metastatic cancer, he discovered “cells identical with those of the cancer itself” and postulated that they “may tend to throw some light upon the mode of origin of multiple tumors existing in the same person” (1). In the following 150 years, researchers have demonstrated the presence of CTCs in the blood of most cancer patients and play a key role in the metastatic dissemination of carcinomas. In addition, after the development of technologies with the necessary sensitivity and reproducibility, the diagnostic potential of these cells is beginning to be exploited (2).
Two main issues complicate successful development of new therapies or therapeutic combinations in many types of tumors. One is the lack of surrogate, pharmacodynamic markers that could provide an early indication of response to treatment and help guide clinical decision making (3). The second issue is the difficulty of obtaining serial biopsies, which are essential to explore predictive biomarkers, to understand acquired resistance mechanisms and to select appropriate therapies upon progression (4,5). CTC enumeration and characterization have potential to address these problematic issues. Monitoring the number of CTCs of patients in treatment strongly suggest that, in several tumor types, CTC detection and enumeration has prognostic value and may serve as an surrogate response biomarker. In addition, research in the field of CTC characterization could reveal novel drug targets and inform on the mechanisms of metastasis. CTCs may be considered as a sort of “liquid biopsy” that can be analyzed to provide real-time information on patient’s disease status (6). As CTCs are involved in the establishment of metastasis, such analyses could be more relevant for certain purposes than those performed on tumor samples at presentation. For instance, CTCs could be especially useful to identify emergent treatment resistance (7-9).
A variety of techniques to detect CTCs in peripheral blood have been available for a number of years, based on immunomagnetic bead separation, filtration based size separation, antigens cell sorting using flow cytometry and density gradient centrifugation (10). However, these methods have been hard to reproduce across laboratories, making data comparison difficult. Recently, the U.S. Food and Drug Administration has approved the CellSearch® System (Veridex LLC, Raritan, NJ), to predict progression-free survival (PFS) and overall survival (OS) in patients with metastatic breast (11), colorectal (12), gastric (13), prostate (14), small cell lung (SCLC) (15,16) and non-small cell lung cancer (NSCLC) (17). This has facilitated a more reliable and reproducible CTC counting across multiple sites and the CellSearch® System is currently considered the standard method for detecting CTCs in the clinical setting. In this platform, magnetic beads coated with epithelial cell-adhesion molecule (EpCAM) antibody are used to isolate CTCs from the background of billions of peripheral white blood cells. With this system it is possible to reproducibly find a single circulating tumor cell in a 7.5 mL blood sample. In most studies, values of 2 to 5 CTCs per 7.5 mL blood have been reported to be the optimal cutoff value discriminating poor prognosis.
Despite its usefulness, the CellSearch® System has also some drawbacks. Epithelial, EpCAM positive cells can also be found in healthy donors (18), EpCAM-based assays are not able to detect normal-like tumor cells (19), a precise cytological analysis of isolated cells is not possible due to technological limitations and some data suggest that epithelial antigens are lost in some CTCs due to the epithelial-mesenchymal transition (EMT), a crucial event in metastases (19-21). Finally, in the case of advanced NSCLC, CTCs can be demonstrated in only around 30% of patients. In an effort to overcome these drawbacks, new methods of isolation by size have been developed and are commercially available, based on filtration (22). They are low-cost technologies that do not require large or expensive apparatus. Blood flow passes through a micro porous membrane filter allowing size-selective isolation of rare tumor cells under standardized conditions. The device is completely accessible and it has been reported that cells isolated onto the filter, that are well preserved morphologically, can be analyzed using a variety techniques such as IHQ, FISH or molecular biology techniques (22). Finally, the system allows isolation of live cells that can be grown in culture. Another alternative to CellSearch® is the use of magnetic beads coated with a mixture of cytokeratines (CKs) instead of EpCAM, that can lead a more specific isolation of CTCs and allows for cytological analysis of the resulting cells (23,24).
In this study, we evaluated the feasibility of using CK-coated magnetic-based beads (MACS technology) to isolate CTCs in the clinical setting, as well as its usefulness as a source of genetic material for testing mutations in relevant genes such as EGFR or K-ras. MACS technology are 50-nm super paramagnetic particles conjugated to specific antibodies against a particular antigen on the cell surface. Due to the small size, they do not activate cells and they will not saturate cell surface epitopes. Columns contain a matrix composed of ferromagnetic spheres covered with a cell-friendly coating. When placed on a magnetic separator, the spheres amplify the magnetic field by 10,000-fold, thus inducing a high gradient within the column. This is crucial for isolation of cells which are only minimally labeled, leaving enough epitopes free for concurrent antibody staining.
Methods
Study design
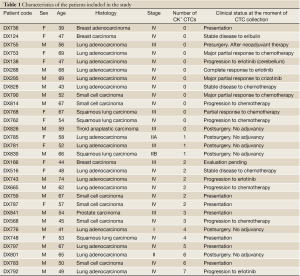
This was a study conducted with patients at the Dexeus University Hospital (Barcelona, Spain). Patients with a confirmed, histologically proven lung cancer were eligible; some patients with other malignancies were also enrolled (Table 1). All patients gave written, informed consent. Enrichment, detection and microdissection of all samples was performed in GENYO (Granada, Spain).
Enrichment and detection of CTCs
A total of 10 mL of heparinized peripheral blood was collected from each patient. Samples were processed within 24 hours with double density gradient (Histopaque 1077 over Histopaque 1119). For CTCs enrichment, we used the Carcinoma Cell Enrichment and Detection kit, MACS technology (Miltenyi Biotec) (23,24). Enrichment of cells of epithelial origin after ficoll gradient was performed by positive immunomagnetic cell separation, utilizing magnetic beads labeled with a multi-cytokeratin-specific antibody (CK3-11D5) which recognizes cytokeratin 7, 8, 18 and 19. Cytokeratin positive cells were further identified by immunocytochemical methods on Pen-membrane slides, and contaminated WBC excluded by negative selection for CD45. Additionally, cytokeratin-expressing cells were revealed by incubation with freshly prepared Fast Red TR/Naphthol AS-MX substrate solution for 15 minutes in a humidity chamber at room temperature. Pen-membrane slides were washed once with PBS and stained with Mayer’s haematoxylin solution (Sigma) for 30 seconds at room temperature. The epithelial tumor cells could quickly and easily be identified and enumerated based on their strong coloration.
Microdissection and genetic analyses of CTCs
CTCs were captured from the pen-membranes by laser microdissection into 10 µL of PCR buffer (Ecogen, Barcelona, Spain) plus proteinase K and incubated 4 hours to overnight at 60 °C. Proteinase was inactivated at 95 °C for 10 minutes, and the cell extract submitted to PCR. EGFR mutations were determined as described (25). Basically, a first round of PCR amplification was performed for EGFR exons 19 and 21. EGFR exon 19 status was determined by a second round of PCR amplification with use of a carboxyfluorescein (FAM)-labelled primer, followed by length analysis in an ABI prism 3130 DNA Analyzer (Applied Biosystems, Foster City, CA, USA). Exon 21 point mutations were detected with a 5'-nuclease PCR assay (TaqMan assay; Applied Biosystems) using FAM and VIC minor groove binder (MGB)-labelled probes for the wild-type and mutant sequence, respectively. Mutations in the K-ras gene were determined by standard methods: PCR followed by agarose gel electrophoresis and sequencing.
Results
Isolation of CTCs
The CTCs isolation criteria were intact cells positive both for DAPI staining and CKs but negative for CD45 by immunofluorescence (IF). An example of a positive cell is shown in Figure 1. The expression of vimentin, an epithelial-to-mesenchymal marker, was also determined. In two patients, circulating cells positive for vimentin were discovered.
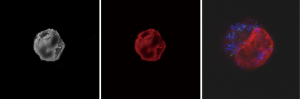
CTC positive and negative cases
The clinical characteristics of the 30 patients analyzed, together with the results obtained are summarized in Table 1. CTCs were identified 17 of the 30 patients included in the study (57%). In 11 patients the number of CTCs detected was 1 to 3, in 5 patients 4 to 6 and only in one sample more than 6 CTCs could be isolated (Figure 1). In the case of surgically resected NSCLC, the percentage of patients where CTCs were identified raised to 71% with an average of 3 CTCs per positive sample (Table 2). In contrast, it was only 50% in stage IV, unresectable NSCLC, with the same average number of CTCs per positive patient. Regarding other pathologies, CTCs were isolated in 71% of the SCLC patients analyzed, and in 40% of samples from other malignancies.
Full Table

Full Table
CTC status and outcome
The successful detection of CTCs seemed to correlate with the clinical outcome of the patient. More than 80% of patients were positive at presentation or after surgery (no adjuvance). The percentage dropped to 57% in patients progressing upon chemotherapy, and was only 13% (1 of 8) in those responding to treatment (Table 3).

Full Table
DNA amplification of CTCs
Finally, we analyzed the usefulness of the CTCs as a source of genetic material in 8 CTC-positive patients. To do so, we incubated the CTCs in PCR buffer plus proteinase K and used the cell lysate to analyze mutations in EGFR (exons 19 and 21) and K-ras (exon 2). Successful amplification was only observed in the three patients where the number of CTCs was 4 or higher, in concordance with the previously published sensitivity of our assay (25). In all cases, the mutational status of the CTCs and the primary tumor were coincident (Table 4).

Full Table
Discussion
To our knowledge, our study is the first to evaluate the feasibility of using CK-coated magnetic-based beads to isolate CTCs in lung cancer patients. Previous studies have generally employed the FDA-approved CellSearch® System (17,26,27) in which the magnetic beads are coated with epithelial cell-adhesion molecule (EpCAM) antibody. One of the drawbacks of this methodology is that it does not detect those CTCs where epithelial antigens -such as EpCAM- get lost in due to the epithelial–mesenchymal transition (EMT), a key event in the metastatic process (19-21). In two patients of our study, we found CTCs positive by immunofluorescence for vimentin and two EMT markers. Of note, one of them was EML4-ALK positive patient with a long-term response to crizotinib. In this patient, no CTCs positive for epithelial markers could be found. Our results are in line with those reported by Krebs et al. [2012] (28). In a population of 40 chemonaïve, stages IIIA-IV NSCLC patients, they detected CTCs in 32 of 40 (80%) patients using a filtration-based system, compared to 9 (23%) using CellSearch®. A subpopulation of CTCs isolated by the filtration device did not express epithelial markers. Taken together, these data suggest that the CellSearch® technology should be complemented with a mesenchymal-antigen or a filtration based system for successful and complete isolation of CTCs in all patients.
We found that the percentage of CTC positive patients correlated with the clinical history of the disease. In our study, CTCs could be detected in more than 80% of stage III-IV lung cancer patients at presentation, with a range of 1 to 6 cells. This percentage is higher than the 61% reported in a study where CTCs were isolated by the CellSearch® System in 34 advanced NSCLC patients (17), with a range of 1 to 62. CTCs were also found in 12% of healthy donors (1-2 cells). In another, more recent publication the threshold was established at ≥2 CTCs, and 45% of the 87 stage III-IV patients were considered positive at presentation. In addition, patients with ≥5 CTCs after one cycle of chemotherapy were found to have a significantly worse overall survival (26). In contrast, a single-arm phase II clinical trial of erlotinib and pertuzumab after relapse to chemotherapy, 78% of patients had CTCs and higher baseline counts were associated with response to treatment by Response Evaluation Criteria in Solid Tumors (RECIST) (27).
In our hands, the number of CTC-positive patients remained high (again, more than 80%) when blood samples were taken immediately after surgery, but it dramatically dropped to 13% in patients responding to chemotherapy or TKIs. In patients relapsing, it raised again to 57%. A decrease in the number of CTCs in serial blood samples have been described in advanced NSCLC after partial or complete response to therapies, and it was associated with a with positron emission tomographic (FDG-PET) and RECIST responses, and longer progression-free survival (26,27).
Finally, we evaluated the feasibility of using the CTCs isolated by CK-coated magnetic-based beads as a source of material for genetic testing, specifically EGFR and k-ras mutation detection. At this respect, we tested 8 patients by standard techniques: PCR followed by sequencing or fragment analysis and 5’ nuclease assay. Only the three samples that had 4 or more CTCs could be successfully amplified, in line with the previously described sensitivity of our technique. This compares poorly to the results obtained with the CellSearch® System, where mutations could be detected in a significant number of CTC positive patients, probably due to a higher CTC count (27).
In summary, we have shown that CK-coated magnetic-based beads allow for isolation of CTCs of with epithelial and mesenchymal features, and CTC positivity by this methodology might correlate with the clinical history of the malignancy. However, the total number of cells isolated does not seem to be sufficient for successful genetic testing by standard techniques in a majority of patients. Therefore, more sensitive approaches such as digital PCR or NGS are needed.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ashworth TR. A case of cancer in which cells similar to those in the tumours were seen in the blood after death. Aust Med J 1869;14:146-9.
- Hiltermann TJ, van der Wekken AJ, Groen HJ. Moving forward with circulating tumor cells and lung cancer. J Thorac Dis 2012;4:440-1.
- Berghmans T, Pasleau F, Paesmans M, et al. Surrogate markers predicting overall survival for lung cancer: ELCWP recommendations. Eur Respir J 2012;39:9-28.
- Eberhard DA, Giaccone G, Johnson BE, et al. Biomarkers of response to epidermal growth factor receptor inhibitors in Non-Small-Cell Lung Cancer Working Group: standardization for use in the clinical trial setting. J Clin Oncol 2008;26:983-94.
- Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3:75ra26.
- Jiang Y, Palma JF, Agus DB, et al. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem 2010;56:1492-5.
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21.
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9.
- Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res 2006;12:4218-24.
- Riethdorf S, Pantel K. Advancing personalized cancer therapy by detection and characterization of circulating carcinoma cells. Ann N Y Acad Sci 2010;1210:66-77.
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-9.
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-21.
- Matsusaka S, Chìn K, Ogura M, et al. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci 2010;101:1067-71.
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-9.
- Hiltermann TJ, Pore MM, van den Berg A, et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann Oncol 2012;23:2937-42.
- Hou JM, Krebs MG, Lancashire L, et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J Clin Oncol 2012;30:525-32.
- Tanaka F, Yoneda K, Kondo N, et al. Circulating tumor cell as a diagnostic marker in primary lung cancer. Clin Cancer Res 2009;15:6980-6.
- Steinarsdóttir M, Jónasson JG, Vidarsson H, et al. Cytogenetic changes in nonmalignant breast tissue. Genes Chromosomes Cancer 2004;41:47-55.
- Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 2009;101:61-6.
- Mego M, Mego M, Mego M, et al. Circulating tumor cells (CTCs) and epithelial-mesenchymal transition (EMT) in breast cancer: Describing the heterogeneity of microscopic disease. Cancer Res 69 (Meeting Abstract Supplement), 3011, December 15, 2009.
- Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2002;2:442-54.
- Desitter I, Guerrouahen BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res 2011;31:427-41.
- Serrano MJ, Sánchez-Rovira P, Delgado-Rodriguez M, et al. Detection of circulating tumor cells in the context of treatment: prognostic value in breast cancer. Cancer Biol Ther 2009;8:671-5.
- Gaforio JJ, Serrano MJ, Sanchez-Rovira P, et al. Detection of breast cancer cells in the peripheral blood is positively correlated with estrogen-receptor status and predicts for poor prognosis. Int J Cancer 2003;107:984-90.
- Molina-Vila MA, Bertran-Alamillo J, Reguart N, et al. A sensitive method for detecting EGFR mutations in non-small cell lung cancer samples with few tumor cells. J Thorac Oncol 2008;3:1224-35.
- Krebs MG, Sloane R, Priest L, et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J Clin Oncol 2011;29:1556-63.
- Punnoose EA, Atwal S, Liu W, et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin Cancer Res 2012;18:2391-401.
- Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol 2012;7:306-15.


