Clinical outcomes of CyberKnife stereotactic radiosurgery for elderly patients with presumed primary stage I lung cancer
Introduction
The solitary pulmonary nodule (SPN) is defined as a radiographic opacity up to 30 millimeters in diameter with at least two-thirds of its margins surrounded by lung parenchyma (1). SPNs are being increasingly detected in recent years due to the more widespread use of imaging and screening chest computed tomography (CT) scans. Surgical resection is primary treatment for a pathological diagnosis of early stage non-small cell lung cancer (NSCLC) in a medically fit patient who can withstand the stress of surgery. Stereotactic body radiation therapy (SBRT), also called stereotactic ablative radiotherapy (SABR), has had excellent success in the treatment of stage I NSCLC in medically inoperable patients, and it has been reported to have comparable local control (LC) to surgery with minimal morbidity. Multiple studies have documented that SBRT achieves a very high LC and can improve survival in medically inoperable patients with early lung cancer (2,3) who are often frail and have competing risk factors for death.
For operable patients, the equipoise to justify randomization to SBRT compared to surgery in clinical trial is clearly more difficult, and thus several studies have been terminated due to lack of enrollment (4). Recently, a pooled analysis of two randomized trials (STARS and ROSEL) was performed to assess SBRT versus surgery for operable stage I NSCLC. Notably, they found that SBRT was associated with a higher 3-year overall survival (OS) than surgery (95% vs. 79%, P=0.037) (5). This suggests that in certain patients, SBRT may achieve outcomes comparable to surgery.
A large number of lung nodules are detected due to the widespread use of chest CT scans. However, without a tissue biopsy, radiographic features alone cannot confirm the absolute presence of a malignancy. Not all lesions are amenable to endobronchial biopsy, and image-guided biopsy can fail to diagnose smaller lung lesions (≤20 mm). Given the poor functional status, comorbidities, and concerns about toxicity, including pneumothorax, infection, and bleeding many patients, especially elderly patients, refuse or do not undergo a biopsy due to concern of toxicities. When patients refuse biopsy or surgical resection, an alternative of active surveillance may be suggested. However, for NSCLC, even at early stage, the lack of treatment is often fatal (6). A recent meta-analysis assessing seven cohort studies (4,418 patients) and 15 randomized controlled trials (1,031 patients) evaluated mortality without treatment in NSCLC patients. The pooled mean survival for patients without anticancer treatment was 7.15 months (5,6). Even for T1 early stage NSCLC, the median survival among a cohort of 1,432 patients who did not undergo surgical resection or treatment with chemotherapy or radiation was only 13 months (7). Thus, definitive treatment is usually recommended, rather than surveillance. In addition, definitive treatment generally should be performed without delay because waiting times >4 weeks can cause tumor growth (8) and new nodal and distant metastases even for early-stage NSCLC (9). Several groups have reported their findings on SBRT in patients with SPNs clinically diagnosed as lung cancer who lack tissue confirmation (10-12). The 3-year LC values range from 80% to 94%, which are comparable to outcomes of SBRT for pathological diagnosed early NSCLC patients (11,12).
In this study, we performed a retrospective analysis of elderly patients with clinically diagnosed primary stage I lung cancer lacking tissue diagnosis who were treated with SBRT at our institution, and we assessed LC (in-field), survival, and toxicity.
Methods
Patients
We performed a retrospective analysis of patients with presumed primary stage I lung cancer patients underwent SBRT (CyberKnife®, Accuray, Sunnyvale, CA, USA) at our institution from March 2009 to March 2016. Prior to treatment, all patients underwent comprehensive staging, including head magnetic resonance imaging and 18fluorine-fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT), tumor markers, routine blood tests, and blood chemistry panels. The inclusion criteria were presumed primary stage I lung cancer without tissue confirmation; age ≥75 years; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0, 1, or 2. Patients who had a history of cancer or were diagnosed pathologically or suspected as having small cell lung cancer (SCLC) due to the elevated of neuron specific enolase, were excluded from this study. The patients’ conditions were comprehensively assessed by radiologists and oncologists. The study was approved by the Institutional Ethical committee Written informed consent was obtained from the patients.
Treatment
SBRT was performed (CyberKnife®, Accuray, Sunnyvale, CA, USA) using technology which was previously described by our group (13). A total of nine patients who were ineligible for the “X sight lung” option were thus implanted with one to three gold fiducials inside or near the tumor to define the tumor position and to use for tumor tracking during SBRT. Approximately 1 week after fiducial placement, CT simulation was performed for treatment planning (BrillianceTM Big Bore, Philips, Netherlands). Gross tumor volume (GTV) was defined as the tumor volume delineated on lung windows settings. The planning target volume (PTV) was obtained by expanding the GTV by 3 mm uniformly in all directions. The dose was prescribed based on the isodose line and covered the PTV. SBRT was delivered to a total dose of 40 to 60 Gy over 2 to 5 days. The dose equivalence was used as a linear quadratic model and considered by assuming α/β=10 Gy for the tumor. The biological effective dose (BED) ranged from 83–150 Gy, and the median BED was 132 Gy. Dose and fractionation schedules were developed based on the patient’s performance status, tumor size, and location.
Follow-up (FU) and statistics
The endpoints of this study were LC, cause-specific survival, OS and treatment toxicity. All patients underwent clinical examination and CT scan for evaluation of treatment results 4–6 weeks after SBRT, then every 3 months for the first 2 years, and then the every 6–8 months until death.
Acute and late toxicity was assessed according to the RTOG and RTOG/EORTC toxicity scales. Responses were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) (14). Local failure was defined as growth following initial tumor shrinkage or progression on two consecutive scans, with the date of local failure backdated to the earliest scan showing progression. Regional recurrences were defined as hilar, mediastinal, or supraclavicular nodal enlargement on CT. Distant failures were defined as any failure outside of the thorax, as well as malignant pleural or pericardial effusions and disease in different lobes. The OS was assessed from the start of SBRT until death, censoring the last FU date. The cancer-specific survival (CSS) was assessed from the start of SBRT until cancer progression death, censoring the last FU date. The progression-free survival (PFS) was calculated from that same time until disease progression. The OS and CSS curves were estimated by Kaplan-Meier analysis and were compared using the log-rank test and the Cox model. The influence of variables on survival was investigated using univariate analysis (Cox model). Statistical analysis was performed with commercial software, (SPSS® version 21.0, SPSS Inc., Chicago IL, USA), and P<0.05 was considered statistically significant for all analyses.
Results
Patient and tumor characteristics
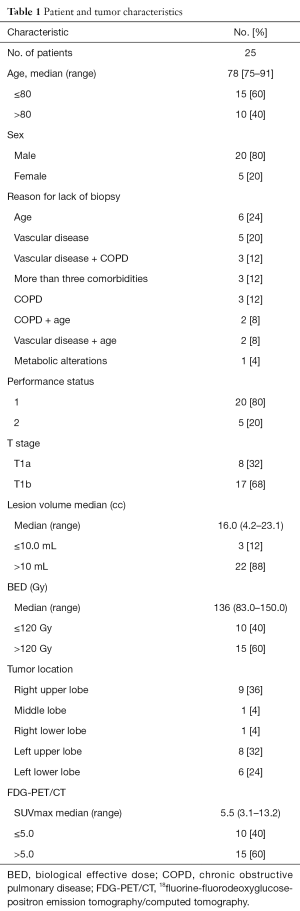
From March 2009 to March 2016, 25 patients with a median age of 78 years from our CyberKnife center were enrolled in the study. The patient characteristics are detailed in Table 1. The median FU was 36.0 months (range, 4 to 84 months). The most common tumor localization was the upper lobe [17 of 25 patients (68%)]. The main cause of inoperability and lack of tissue confirmation of lung cancer was the presence of comorbidity [19 of 25 patients (76%)]. Six patients (10.5%) refused biopsy due to concerns of toxicity. Twenty-three patients (92%) were ineligible for surgery on account of their advanced age and/or comorbidities. Two patients (8%) refused primary surgery.
Full table
LC
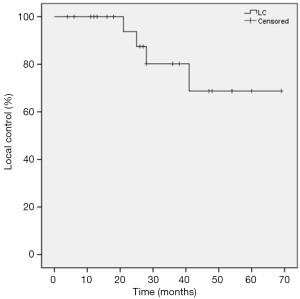
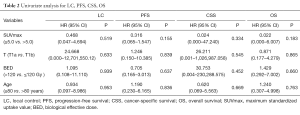
Local progression occurred in four patients (16%), regional recurrence in two patients (8%) and distant metastasis in six patients (24%). Among patients (n=8) with tumor sizes ≤20 mm, no local progression occurred. Overall, the 1-year actuarial LC rate was 100%, 3-year actuarial LC rate was 78.8%, and 5-year actuarial LC rate was 65.7%. Actuarial LC of the SPNs is shown in Figure 1. In univariate analysis, pre-treatment aximum standardized uptake value (SUVmax) (<5 vs. ≥5), age (< 80 vs. ≥80), BED (120 vs. ≤120 Gy), and stage (T1a vs. T1b) were not significantly related to LC (Table 2).
Full table
Survival
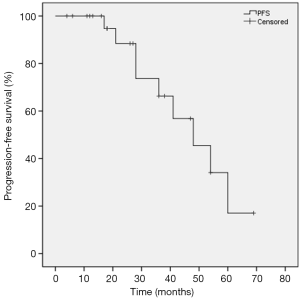
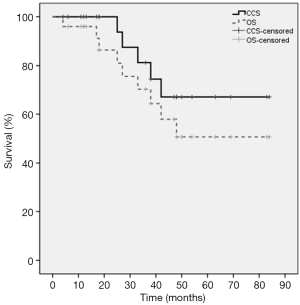
Median FU for all patients was 36.0 months. At the time of analysis, 5 of the 25 patients (20%) died of disease progression and 4 patients died of comorbidities. The 3-year PFS was 66.3% and the 5-year PFS was 17.1%. The median PFS time was 48.0 months (95% CI: 31.2–64.8). The 1-, 3-, and 5-year OS rates were 96%, 70.2%, and 50.7%, respectively. The 1-, 3-, and 5-year CSS rates were 100%, 81.3%, and 67.0%, respectively. The Kaplan-Meier PFS and the CSS and OS curves are shown in Figures 2,3, respectively. In univariate analysis, SUVmax (<5 vs. ≥5), age (<80 vs. ≥80), BED (>120 vs. ≤120 Gy) and stage (T1a vs. T1b) were not significantly related to PFS, CSS or OS (Table 2).
Toxicity
There have been no cases of acute or late grade 4 toxicity or possible treatment-related death. The most common acute toxicity was grades 1–2 fatigue (5/25,20%). Acute grades 1–2 radiation pneumonitis occurred in two patients (8%), and acute grade 3 radiation pneumonitis was observed in two patients (8%), who needed to be treated with steroid inhalers and oral steroids for a short duration of time. Late grade 3 radiation pneumonitis was observed in one patient (4%) at 6 months after SBRT.
Discussion
SBRT is an accepted standard therapy for stage I NSCLC in patients deemed medically unfit for or refusing surgery. Multiple studies have confirmed that SBRT is safe and effective for a clinically diagnosed primary stage I lung cancer (10-12). In this study, we addressed the question of whether SBRT may achieve good LC, survival, and toxicity profile in an even more frail elderly patient population who refused or cannot undergo biopsy of their presumed early stage NSCLC. We demonstrate that SBRT for elderly patients with presumed primary stage I lung cancer who lack tissue confirmation achieved good LC and CSS with minimal toxicity.
Pathologic diagnosis is the most accurate diagnosis for lung tumors. However, there is an inherent false-negative rates for biopsy, in addition to unique risks and potential morbidities associated with both CT-guided and EBUS-directed lung biopsies (15). Therefore, it is necessary to improve the sensitivity and specificity of the procedures and to increase the rate of accurate diagnosis, as well as to minimize procedure-associated morbidities such as pneumothorax, bleeding, and infection. Chest CT is one of the most reliable modalities for identifying pulmonary malignancies, and given advances in the improved resolution of CT scans with thin slice thickness, high resolution, and contrast enhancement, serial images showing growth of a lung nodule in this patient population may supplant a tissue diagnosis in certain cases. High resolution CT can evaluate the detailed characteristics of lung nodules, such as their size, morphology, and type of opacity. FDG-PET/CT scanning is also increasingly used to differentiate pulmonary malignancies from benign nodules by means of having higher glucose metabolism. American College of Chest Physicians (ACCP) review calculated the sensitivity and specificity of FDG-PET/CT scanning to be 94.2% and 83.3%, respectively, for the identification of malignant pulmonary nodules (16). Several quantitative prediction models using clinical and radiological criteria have been developed to assist clinicians in discriminating malignant from benign nodules (17-20). Three models incorporate clinical and CT nodule characteristics, such as age, smoking, history of cancer, nodular diameter, location and morphology (17), and a fourth model (Herder et al.) added FDG-PET/CT to the Mayo Clinic model. Recently, a study to compare the performance of these models in a population of patients recruited from a UK teaching hospital showed that the highest accuracy was seen for the model described by Al-Ameri et al. incorporating FDG avidity (21) into the model to predict, based on imaging parameters, who had NSCLC. ACCP also recommended that those with a risk greater than 60% of having a pulmonary malignancy should receive further treatment (16).
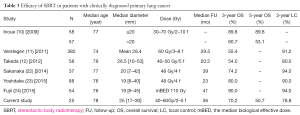
A number of investigators worldwide have described outcomes after SBRT in patients without a pathological diagnosis (Table 3), (10-12,22-24). In those studies, SBRT was reportedly well tolerated, with 3-LC rates between 80% and 94%. The 3-year OS rates were in the range of 54% to 90%. The survival results of the current study are comparable to those of published series despite the generally more advanced age in the current study population. Over the last decade, the use of lung SBRT without biopsy has increased (25). Inoue et al. (10) analyzed the outcomes of 115 stage I clinically diagnosed lung cancer patients treated with SBRT. The 3-year and 5-year OS rates for patients with a tumor size ≤20 mm in diameter (n=58) were both 89.8%, and those with tumors >20 mm (n=57) were 60.7% and 53.1% (P<0.0005), respectively. Sakanaka et al. reported the results of 37 patients clinically diagnosed with primary stage I lung cancer empirically treated with SBRT. After a median FU of 36 months, the 3-year OS was 89.9% in patients with T1a tumors versus 51.7% in patients with T1b/T2a tumors (22). The researchers suggested that tumor size was a prognostic factor for OS in SBRT for clinically diagnosed primary lung cancer. Of note this is compatible with a previous report of SBRT for pathologically diagnosed NSCLC (26). Verstegen et al. reported a comparison between 209 clinically diagnosed patients and 382 pathologically confirmed NSCLC patients who underwent SBRT, and concluded that OS and LC were similar in large groups of patients with or without pathological diagnosis (11), suggesting that risk of overtreatment of truly benign nodules is low if strict radiological and patient characteristics are used to guide treatment decisions for nodules lacking tissue confirmation. Other studies also reported that there was no difference in OS between confirmed NSCLC patients and clinically diagnosed patients (12,27). A recent meta-analysis confirmed an association of high pre-RT SUVmax of primary tumor with poor OS and LC in NSCLC patients receiving RT. Such an association seems to be particularly strong for patients with stage I NSCLC receiving SBRT (28). In the present study, four patients had local recurrences, with the time to recurrence from treatment of 21, 25, 28, and 41 months. All patients with local recurrences had T1b tumors that were among the largest tumor sizes in the present cohort. Despite having a numerically notable effect on outcomes, likely due to the small patient sample size and inadequate power, tumor size (T1a vs. T1b) and SUVmax (<5 vs. ≥5) were not significantly related to survival and LC.
Full table
Elderly patients and those with poor pulmonary function or multiple comorbidities often are not candidates for biopsy. Therefore, elderly patients with clinically diagnosed lung cancer are now offered SBRT, a minimally invasive definitive therapy for early stage NSCLC. A National Cancer Data Base analysis showed that a significant improvement in survival was noted for elderly patients who receive SBRT relative to observation alone. SBRT should be considered as part of a patient’s treatment options for early stage NSCLC, and providers should be aware of this minimally invasive treatment option for elderly patients with early stage NSCLC (29). Mancini et al. demonstrated that elderly patients (≥75 years) treated with SBRT for early-stage NSCLC appear to have equivalent OS, LC and toxicity rates as compared to younger patients. For elderly patients, the rate of grade ≥3 pneumonitis was 8.7% (30). In our study, two of the 25 patients (8%) similarly developed grade 3 radiation pneumonitis, and the toxicities seen in the current study are comparable to those of previous trials.
Conclusions
The results of the present study support the efficacy and safety of SBRT in elderly patients with clinically diagnosed primary stage I lung cancer. In cases where tissue diagnosis is possible, histological confirmation of malignancies should be the gold-standard for work-up for a patient with suspected early stage NSCLC. However, patients should be counseled about the pros and cons of empiric SBRT without tissue confirmation in situations where a tissue diagnosis is not technically feasible or biopsy could be associated with an unacceptable risk profile. This research has several limitations, including a limited sample size, and further studies of empiric SBRT are needed to be conducted to appropriate dose levels for elderly patients with a poor performance status lacking tissue confirmation of malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
Ethical Statement:The study was approved by the Institutional Ethical Committee (No. 2016NZGKJ-016) and written informed consent was obtained from all patients.
References
- Patel VK, Naik SK, Naidich DP, et al. A practical algorithmic approach to the diagnosis and management of solitary pulmonary nodules: part 1: radiologic characteristics and imaging modalities. Chest 2013;143:825-39. [Crossref] [PubMed]
- Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol 2009;27:3290-6. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Simone CB 2nd, Dorsey JF. Additional data in the debate on stage I non-small cell lung cancer: surgery versus stereotactic ablative radiotherapy. Ann Transl Med 2015;3:172. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Wao H, Mhaskar R, Kumar A, et al. Survival of patients with non-small cell lung cancer without treatment: a systematic review and meta-analysis. Syst Rev 2013;2:10. [Crossref] [PubMed]
- Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non-small cell lung cancer: implications for early detection. Chest 2007;132:193-9. [Crossref] [PubMed]
- Geiger GA, Kim MB, Xanthopoulos EP, et al. Stage migration in planning PET/CT scans in patients due to receive radiotherapy for non-small-cell lung cancer. Clin Lung Cancer 2014;15:79-85. [Crossref] [PubMed]
- Murai T, Shibamoto Y, Baba F, et al. Progression of non-small-cell lung cancer during the interval before stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;82:463-7. [Crossref] [PubMed]
- Inoue T, Shimizu S, Onimaru R, et al. Clinical outcomes of stereotactic body radiotherapy for small lung lesions clinically diagnosed as primary lung cancer on radiologic examination. Int J Radiat Oncol Biol Phys 2009;75:683-7. [Crossref] [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Radiother Oncol 2011;101:250-4. [Crossref] [PubMed]
- Takeda A, Kunieda E, Sanuki N, et al. Stereotactic body radiotherapy (SBRT) for solitary pulmonary nodules clinically diagnosed as lung cancer with no pathological confirmation: comparison with non-small-cell lung cancer. Lung Cancer 2012;77:77-82. [Crossref] [PubMed]
- Shen ZT, Wu XH, Li B, et al. Clinical outcomes of CyberKnife stereotactic body radiotherapy for peripheral stage I non-small cell lung cancer. Med Oncol 2015;32:55. [Crossref] [PubMed]
- Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205-16. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-148S.
- Gould MK, Donington J, Lynch WR, et al. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e93S-120S.
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref] [PubMed]
- Gould MK, Ananth L, Barnett PG, et al. A clinical model to estimate the pretest probability of lung cancer in patients with solitary pulmonary nodules. Chest 2007;131:383-8. [Crossref] [PubMed]
- McWilliams A, Tammemagi MC, Mayo JR, et al. Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 2013;369:910-9. [Crossref] [PubMed]
- Herder GJ, van Tinteren H, Golding RP, et al. Clinical prediction model to characterize pulmonary nodules: validation and added value of 18F-fluorodeoxyglucose positron emission tomography. Chest 2005;128:2490-6. [Crossref] [PubMed]
- Al-Ameri A, Malhotra P, Thygesen H, et al. Risk of malignancy in pulmonary nodules: A validation study of four prediction models. Lung Cancer 2015;89:27-30. [Crossref] [PubMed]
- Sakanaka K, Matsuo Y, Nagata Y, et al. Safety and effectiveness of stereotactic body radiotherapy for a clinically diagnosed primary stage I lung cancer without pathological confirmation. Int J Clin Oncol 2014;19:814-21. [Crossref] [PubMed]
- Yoshitake T, Nakamura K, Shioyama Y, et al. Stereotactic body radiation therapy for primary lung cancers clinically diagnosed without pathological confirmation: a single-institution experience. Int J Clin Oncol 2015;20:53-8. [Crossref] [PubMed]
- Fujii O, Demizu Y, Hashimoto N, et al. Particle therapy for clinically diagnosed stage I lung cancer: comparison with pathologically proven non-small cell lung cancer. Acta Oncol 2015;54:315-21. [Crossref] [PubMed]
- Rutter CE, Corso CD, Park HS, et al. Increase in the use of lung stereotactic body radiotherapy without a preceding biopsy in the United States. Lung Cancer 2014;85:390-4. [Crossref] [PubMed]
- Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;79:1104-11. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol 2009;4:976-82. [Crossref] [PubMed]
- Na F, Wang J, Li C, et al. Primary tumor standardized uptake value measured on F18-Fluorodeoxyglucose positron emission tomography is of prediction value for survival and local control in non-small-cell lung cancer receiving radiotherapy: meta-analysis. J Thorac Oncol 2014;9:834-42. [Crossref] [PubMed]
- Nanda RH, Liu Y, Gillespie TW, et al. Stereotactic body radiation therapy versus no treatment for early stage non-small cell lung cancer in medically inoperable elderly patients: A National Cancer Data Base analysis. Cancer 2015;121:4222-30. [Crossref] [PubMed]
- Mancini BR, Park HS, Harder EM, et al. Elderly patients undergoing SBRT for inoperable early-stage NSCLC achieve similar outcomes to younger patients. Lung Cancer 2016;97:22-7. [Crossref] [PubMed]