Advances in radiotherapy techniques and delivery for non-small cell lung cancer: benefits of intensity-modulated radiation therapy, proton therapy, and stereotactic body radiation therapy
Introduction
The 21st century has seen several paradigm shifts in the treatment of non-small cell lung cancer (NSCLC) in early-stage inoperable disease, definitive locally advanced disease, and the postoperative setting. Patients are increasingly being treated with curative intent rather than palliation. Survival has improved in advanced stages with more aggressive approaches involving combinations of chemotherapy and thoracic radiotherapy (RT) (1). Several chemotherapy agents developed during the 1990s demonstrated enhanced activity. In addition, the birth of immunotherapy and targeted therapy has revolutionized the treatment of advanced lung cancer. Improved survival rates for inoperable patients with stage III NSCLC have been realized by using “conventional” radiation techniques (2-5) involving the standard dose of 60 Gy delivered over 6 weeks. This dose of radiation was found to be most efficacious in dose-escalation trials in the 1970s and did not change significantly for 20 years. Initial radiation therapy approaches utilized 2-dimensional (2D) imaging for the design of treatment fields. The inherent problems in visualization of tumor and nodal disease on a 2D radiograph necessitated larger radiation fields to cover uncertainty and minimize marginal failures. The tradeoff with these larger fields was an increase in toxicity, which limited use of higher radiation doses.
Local tumor control remained suboptimal in patients treated with conventional RT (even with the addition of chemotherapy) which resulted in renewed interest in strategies to improve local treatment (5). A key driver in the improvement of local control has been the significant evolution in radiation techniques in the last three decades, allowing delivery of more effective radiation doses while limiting doses to normal tissues. With the advent of image-guided radiation therapy (IGRT), techniques have moved from 2D approaches to 3-dimensional conformal RT (3DCRT). The next generation of 3DCRT, intensity-modulated RT (IMRT) and volumetric-modulated arc therapy (VMAT), have enabled even more conformal radiation delivery. Evaluation of altered dose fractionation with these technology advances led to the development of stereotactic body RT (SBRT) for early-stage lung cancer. The recent advent of pencil-beam scanning (PBS) proton therapy has opened new avenues for improving conformity and the therapeutic ratio. SBRT and PBS techniques have placed significant emphasis on motion management, which continues to be among the biggest technical challenges in the use of advanced radiation modalities in lung cancer. Novel monitoring and mitigation strategies have provided the possibility of reducing morbidity/mortality using the standard dose, as well as the possibility of safely escalating the dose to improve oncologic outcomes.
This article reviews the technical advances in RT, their clinical impact, and the associated possibilities for future research in NSCLC. We will focus on progression from 3DCRT to IMRT/VMAT in definitive management of advanced disease, the utility of SBRT in early-stage inoperable disease, and the advent of proton therapy and its role in early- and late-stage disease.
Technical comparison: 3DCRT and IMRT
3DCRT and challenges
Conventional RT for lung cancer, developed in the 1970s before adoption of computed tomography (CT) for treatment planning, was supplanted by 3DCRT, which uses 3D patient-specific geometry in treatment planning. Despite this progression from conventional RT, limited beam arrangements and uniform dose in each beam in 3DCRT can lead to high doses to organs at risk (OARs) (i.e., normal lungs, heart, spinal cord, and esophagus) because of the simple and relatively large fields (6,7). Several pioneers of the early 3DCRT era published predictors of complications (8-14). Graham et al. from Washington University in 1999 demonstrated a correlation between the volume of lung receiving 20 Gy and rates of pneumonitis that remains in use today (8). This analysis demonstrated an 8% rate of grade 3 pneumonitis in patients whose lung volume receiving greater than 20 Gy (V20) was between 22–31%, as compared to 23% for patients whose V20 was >40%. Furthermore, no patients with V20 <32% had grade 5 toxicity. Wang et al. performed a retrospective investigation in 223 NSCLC patients treated with concurrent chemotherapy and 3DCRT and found the incidence of grade 3 or higher pneumonitis for patients with V20 >28% was 37% compared to 4% in patients with V20 ≤28% (14). This high risk of complications translated to poor outcomes from increased morbidity and mortality in patients whose disease was controlled. A significant risk of local failures, suggesting a possible utility to dose escalation, was also noted; however, the already high rates of toxicity meant that newer techniques would be required that could change the therapeutic ratio. For these reasons, considerable interest focused on developing and applying treatment planning and delivery techniques that could improve dose conformality (e.g., IMRT).
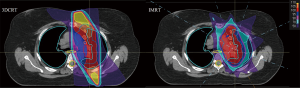
IMRT is an increasingly common method of lung cancer treatment for both early-stage and locally advanced NSCLC. IMRT treatment plans use advanced technology to modify the intensity of each photon beam via dose-rate alterations and field modulation with multileaf collimators (MLCs). The two main types of IMRT delivery are static and dynamic (or VMAT). Although VMAT has treatment time advantages over static IMRT delivery, no evidence indicates definitive superiority of one technique over the other (15-18). Regardless of IMRT technique, treatment plans are usually inversely optimized by a treatment planning system and generate conformal dose distributions with sharper dose falloff around treatment structures, thereby theoretically reducing collateral dose to normal tissue and resulting morbidity associated with radiation dose to OARs (Figure 1: esophagus and spinal cord) (13,19-21).
To test the hypothesis of reduced OAR dose with IMRT, several studies have compared the dosimetry of 3DCRT and IMRT in treating NSCLC (22-25). Grills et al. showed that IMRT can reduce the lung V20 by 15% and esophagus V50 by 40% in node-positive patients (23). Christian et al. evaluated five IMRT plans using three, five, seven, and nine equally spaced coplanar beams and one plan with non-coplanar beams and compared them to six-field, inversely planned, 3DCRT plans for 10 patients (26). Their results demonstrated that the ratio of the percentage of the planning target volume (PTV) covered by the 90% isodose line to the percentage of lung volume receiving 20 Gy (PTV 90/V20) was significantly better in all IMRT plans, except those with three fields, when compared with equivalent 3DCRT plans. Regarding the benefit of an increase in the number of beams in IMRT plans for NSCLC, they showed an increase in PTV90/V20 ratio with the increase in the number of equally spaced coplanar beams. They found that nine beams provided the optimal solution in six of the 10 cases; however, they cautioned that increasing setup times, as well as the risk of increased systematic and random errors, may mitigate the marginal increase in benefit (26). More importantly, they also noted that IMRT plans with <5 beams conferred no notable benefit “compared with beam-angle optimized 3DCRT plans” (26).
Numerous techniques have been developed recently that can leverage the advantages of IMRT with the dynamic motion of MLCs and simultaneous motion of the X-ray source. Intensity-modulated arc therapy (IMAT) is an alternative to tomotherapy proposed by Yu that delivers the radiation dose through single or multiple arcs along with MLC-based modulation to conform the beam to the target and to block critical structures (27). VMAT, as developed by Otto, is a single-arc form of IMAT that also uses a variable dose rate to modulate radiation dose delivery (28).
Motion management and mitigation
These advanced techniques, including VMAT and IMRT, allow delivery of more complex plans while simultaneously decreasing treatment times. In the treatment of NSCLC, however, they heighten concerns about the effects of motion interplay on IMRT delivery. Unlike 3DCRT plans that encompass the entire target through each beam, IMRT plans may block certain regions of the target from certain beams or arc angles (29). These concerns have led to the development of a variety of motion management and mitigation techniques. Breath-hold and abdominal compression are two common methods to reduce tumor motion and, thereby, the average dose to normal lung tissue (30,31). Other management strategies include acquiring 4-dimensional CT (4DCT) to identify tumor motion during breathing cycles and to allow a better estimation of dose delivery to tumors and normal structures (32-37). All of these methods have shown significantly reduced lung V20 (31,38). Finally, significant research exists on beam gating and tumor tracking (39-42). However, inherent irregularities in patient breathing patterns and the intrinsic delay in dose delivery (i.e., MLC and gantry motion) can lead to increased treatment times and necessitate development of class solutions that are predictive in nature (43-45). Techniques to minimize breathing irregularity including biofeedback and active breathing control may offer additional benefits in improving inter- and intra-fraction reproducibility but additional work to improve reproducibility is needed (46-48). Ongoing research may elucidate techniques that can effectively reduce normal tissue dose without compromising treatment efficiency.
Impact of heterogeneity correction
The increasing complexity of treatment plans results in increasing dependency on accurate dose modeling. One significant advance on this front has been determining the impact of heterogeneous tissue density on dose delivery. Differences in dose calculations with and without heterogeneity corrections for IMRT and SBRT treatments in NSCLC patients have been investigated in several studies that have uniformly demonstrated the necessity for advanced algorithms with heterogeneity corrections to achieve accurate dose calculations (49-54). Vanderstraeten et al. compared full Monte Carlo calculations with two different convolution/superposition algorithms and one pencil-beam algorithm for 10 lung cancer patients receiving IMRT (55). They found a better agreement between convolution/superposition and Monte Carlo methods for dose calculation within the target structures. They concluded that none of the dose calculation algorithms could provide results within 5% of the Monte Carlo calculations, and therefore it is imperative to be aware of the impact of the dose calculation algorithm on plan evaluation. Davidson et al. determined the accuracy of heterogeneity on dose calculations from two IMRT treatment planning systems against thermoluminescent detectors and radiochromic film measurements positioned in a lung phantom (56). They found that the collapsed cone convolution/superposition dose calculation algorithm provided clinically acceptable results within ±5% of the measurements. They also demonstrated that the pencil-beam algorithm as tested may overestimate the dose to the target. Although Monte Carlo simulations continue to serve as the gold standard for dose calculations, heterogeneity corrections have dramatically improved the accuracy of more efficient but less precise algorithms needed to successfully implement inverse planning IMRT.
Clinical evidence: 3DCRT and IMRT
Although the initial rationales for IMRT and VMAT were largely their dosimetric advantages, numerous retrospective studies have attempted to isolate the clinical benefits of IMRT over conventional external-beam radiation. Some early reports on the benefits of intensity modulation in lung cancer treatment came from the MD Anderson Cancer Center (MDACC) (13,21). Yom et al. reviewed rates of toxicity, particularly radiation pneumonitis, in 68 patients with advanced NSCLC treated with concurrent chemotherapy and IMRT from 2002 to 2005 (21). They found that patients treated with IMRT had dramatic and statistically significant decreases in the rate of grade 3 radiation pneumonitis at 1 year compared to 3DCRT patients (8% and 32%, respectively). Liao et al. then evaluated an expanded cohort of 496 patients treated between 1999 and 2006, with 318 receiving 3DCRT and 91 receiving 4DCT/IMRT. Their report demonstrated a statistically significant improvement in overall survival (OS), with a hazard ratio of 0.64 (95% CI, 0.41–0.98) when treated with IMRT (13).
The advent of 3DCRT and consequent improvements in toxicity profiles also initiated a series of phase I and II dose-escalation trials that occurred in parallel with development of IMRT. Several authors showed that with the same dose constraints, up to 35% greater RT doses could be given to the target with IMRT than 3DCRT, with the aim of improving local control (23,25,57). Armed with this favorable dosimetric data on toxicity and significant improvements already demonstrated with 3DCRT, several institutions initiated dose-escalation trials in the 1990s and 2000s.
The Radiation Therapy Oncology Group (RTOG) conducted a phase I/II study of dose escalation without concurrent chemotherapy (58) in 177 patients treated using 3DCRT to doses ranging from 70.9 to 90.3 Gy. The results demonstrated that it was safe to escalate radiation dose to 83.8 Gy with a lung V20 of <25% and to 77.4 Gy if the planned lung V20 was between 25% and 36%. Equally important, 90.3 Gy, the highest dose tested, was determined to be too toxic on the basis of two grade 5 toxicities in that population. The safety of dose escalation in the absence of concurrent chemotherapy was also verified by University of Michigan researchers, who escalated the radiation dose from 63 to 103 Gy in 2.1-Gy fractions (59). Most patients (81%) did not receive neoadjuvant chemotherapy. The authors demonstrated improved local control and OS with higher doses of radiation when patients were divided into three treatment groups (63–69, 74–84, and 92–103 Gy).
The cited studies demonstrated the potential safety and efficacy of dose escalation in lung cancer without concurrent chemotherapy; in the same period, emerging data also indicated a benefit for concurrent chemotherapy. Dose escalation in the setting of concurrent chemotherapy was believed by some to be challenging because of increased risks of cardiopulmonary and esophageal toxicity and the possibility that synergistic effects on tumors could be overshadowed by increased rates of adverse effects. Three phase I/II trials with concurrent chemotherapy were undertaken by RTOG, the University of North Carolina (UNC), and the North Central Cancer Treatment Group (NCCTG) (60-62). RTOG 0117 was designed as a combined phase I/II trial and enrolled 8 patients in cohort 1 of the phase I portion of the trial (60). These patients were treated to a dose of 75.25 Gy in 35 fractions. Two major pulmonary toxicities (grade 3 and grade 5) occurred, leading to a reduction in dose to 74 Gy in 37 fractions. An additional 9 patients were enrolled in the phase I cohort 2, with only one experiencing dose-limiting toxicity (grade 3 esophagitis). The phase II component enrolled a total of 55 patients in the 74-Gy arm, of whom 53 were evaluable (60). This portion of the study showed a median OS of 21.6 months with a more acceptable 10% ≥ grade 3 lung toxicity. Similarly, the NCCTG designed a phase I trial to escalate the RT dose from 70 to 78 Gy and found unacceptably high toxicities (50%; 2 of 4 patients) at a dose of 78 Gy (62). Like the RTOG, they concluded that 74 Gy was a safe and tolerable dose. The UNC phase I trial also demonstrated that 74 Gy was a safe dose with concurrent chemotherapy (61).
On the basis of these trials, RTOG launched a phase III trial (RTOG 0617) to determine the benefit of dose-escalated RT utilizing the 74-Gy dose and assessing the benefit of cetuximab (63). The trial was stopped prematurely when results crossed the prespecified boundary for futility. Median OS was 28.7 months in the standard dose (60 Gy) arm and 20.3 months in the dose-escalation arm. Toxicity, particularly severe esophagitis, was more prevalent in the 74 Gy arm (21%) than the 60 Gy arm (7%). Pulmonary toxicity did not differ statistically but marginally favored the standard-dose arm. Despite early termination, this study raised critical questions about dose and toxicity. Of particular importance were questions on rates of completion of prescribed chemoradiation and concerns about volume of disease, adequacy of margins, and heart dose, with associated cardiac morbidity. A recent secondary analysis of this trial further demonstrated that IMRT was associated with lower cardiac doses and pulmonary toxicities (64). The cardiac dose, particularly V40, was further linked with OS on adjusted analysis (64). Notably, this was despite larger PTV, higher PTV/volume of lung ratio, and more stage IIIB disease in patients receiving IMRT (64). Lastly, there was no difference in OS between IMRT and 3DCRT.
Additional secondary analysis on differences in quality of life (QOL) in the standard- and high-dose arms revealed a correlation between baseline QOL and outcomes (65). The authors also demonstrated that, despite the absence of dramatic differences in physician-graded toxicity profiles, patient-reported QOL was meaningfully and statistically significantly lower in the high-dose arm at 3 months. Participants in the RTOG 0617 trial were stratified by receipt of IMRT or 3DCRT. When the QOL of these two groups was compared using the Functional Assessment of Cancer Therapy-Lung Cancer Subscale, fewer patients in the IMRT arm experienced a decline (21% and 46%, respectively; P=0.003). Overall IMRT utilization was similar in the 60- and 74-Gy arms (44.1% and 46.0%, respectively). The difference in QOL, however, occurred despite certain imbalances favoring the 3DCRT group over the IMRT group such as lower PTV volumes [409 and 509 cc, respectively (P<0.001)] and fewer stage IIIB patients [31% and 43%, respectively (P=0.04)]. Finally, the lower proportion of decline in QOL was persistent for patients receiving IMRT at 12 months, and treatment modality (IMRT or 3DCRT) remained significant in multivariate logistic regression models.
Future directions
Strong emphasis has been placed on determining the cause of decreased survival in the high-dose arm of the RTOG 0617 trial. This is likely to drive further work in not only identifying dosimetric parameters but also innovations in reliably characterizing and quantifying cardiopulmonary toxicity from RT. One increasingly used method is cardiac magnetic resonance imaging, which offers the possibility of evaluating characteristics such as late fibrosis and tissue perfusion. These metrics may help increase sensitivity for detection of radiation-associated cardiac complications beyond frank ischemic changes. Motion management and mitigation will also play a significant role in decreasing dose to surrounding uninvolved lung, and predictive strategies will be integral to minimizing target volumes. Functional lung imaging may help leverage the inherent heterogeneity of lung function to minimize consequences from normal tissue irradiation. Parallel research into development of radiation toxicity mitigators is underway and may further improve the therapeutic ratio and potentially allow re-evaluation of dose escalation in the future. The next generation of treatment planning is already being investigated and could help further reduce intermediate dose regions despite the potential downside of a larger low-dose bath. One such modality, 4π, involves the use of a highly non-coplanar planning system that utilizes the entire 4π solid angle space in an attempt to improve high-dose conformality at the expense of increased treatment time (66). Much work remains to be done, but dosimetric studies are increasingly highlighting the advantages of 4π treatment planning techniques (67).
SBRT
Introduction
SBRT or stereotactic ablative RT (SABR) is a technique for delivering a high biologically effective dose (BED; usually BED >100 Gy10 in contrast to a BED of 72 Gy10 with 60-Gy conventional fractionation) to well-localized early-stage NSCLC lesions. SBRT has developed into an excellent option for patients with early-stage NSCLC, especially in cases deemed medically or surgically inoperable. Stereotactic treatment offers the advantage of higher doses per fraction, decreased overall treatment time, and steep dose gradients. However, uncertainty remains over SBRT’s superiority to other modes of treatment, such as surgical resection, which remains the standard of care for stage I disease.
SBRT technique
SBRT can be delivered using a 3DCRT, IMRT, or VMAT approach. Typically for the 3DCRT technique, 8–15 static beams are used to generate conformal dose distributions and steep dose gradients. Six megavolt energies are desired over higher energies because of the sharper penumbra resulting from less lateral electron transport (i.e., secondary electrons are lower energy and travel shorter distances). The individual beams are non-opposed, separated by 20º–30º, and can be coplanar or non-coplanar. Non-coplanar beams have the advantage of increasing the conformality of the high-dose region but should be used with caution because of inherent shortcomings, including difficulties with portal imaging and associated increase in setup uncertainty, potential for collision and inadequate gantry clearance, possibly longer beam paths, and theoretically longer treatment times.
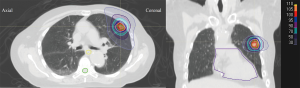
Beam weighting is adjusted to achieve optimal coverage while minimizing dose to critical structures. The prescription point is also an important consideration for SBRT. In the United States, dose is usually prescribed to between 60% and 90% isodose lines, although the initial Japanese (JCOG 0403) study prescribed to the isocenter (68). Additionally, coverage of the PTV is usually set so that 95% of the PTV is covered by the prescription dose and 99% of the PTV receives at least 90% of the dose. The hot spot is ideally placed in the gross tumor volume (GTV) or should fall within the PTV. To generate rapid falloff, the 50% isodose line can be analyzed to make it conformal around the lesion with few spikes (Figure 2). In order to generate the steep gradient, there is very little margin around the target to account for penumbra. Alternative techniques involve prescribing to lower isodose lines, which can also improve dose falloff (69). The dose prescription for SBRT fractionation varies depending on whether the tumor is peripherally located in the chest (25–34 Gy × 1 fraction, 18 Gy × 3 fractions, 12–12.5 Gy × 4 fractions, or 10–12 Gy × 5 fractions) or centrally located (10–12 Gy × 5 fractions). Because of the high doses involved, tumor size is typically limited to <5 cm in diameter, which prevents overinclusion of treated healthy tissue (70-72).
IMRT/VMAT-based SBRT techniques may have advantages compared with 3DCRT in paraspinal patients in whom motion is limited and where dose constraints to esophagus or spinal cord cannot be achieved. IMRT has the advantage of better coverage of irregular-shaped targets. This approach has also been utilized for peripheral tumors to help reduce dose to the ribs/chest wall. VMAT has the advantage of delivering a beam of radiation over a 358º arc with simultaneous movement of the MLC with varying gantry speed and dose rate. This leads to a reduction in treatment time with increased OAR sparing. And while analyses comparing the impact of technique on normal lung dosimetry are limited, initial results are mixed and warrant careful consideration of the low-dose bath (17,73). Using IMRT/VMAT requires attention to positional misses and uncertainty in dose delivery because of the interplay between MLC movement and respiratory tumor motion, as well as dosimetric inaccuracy resulting from tissue heterogeneity and small field sizes. Furthermore, compared with conventionally fractionated therapy, fewer fractions limit the degree of dose-averaging for SBRT regimens. IMRT and VMAT also require significantly more technical resources for planning, quality assurance, and delivery of treatment (74). Lastly, clinical data comparing techniques are limited, and while retrospective data suggest adequate rates of tumor control and toxicity (75), careful consideration of motion mitigation and caution when using modulated beams in tumors with significant (>1 cm) motion are recommended.
The challenges of SBRT treatment planning (i.e., geometric miss, dose heterogeneity, and normal lung dose) are accentuated by the high dose per fraction and low number of fractions. Reliable geometry is of paramount importance in safe and accurate delivery of SBRT, and the regular use of Winston-Lutz tests to check the isocentricity of delivery (<1 mm) and online image guidance to accurately verify tumor and OAR location before and potentially during treatment play significant roles in the reliability of the system (70,76). SBRT dose calculations must also be very precise and, therefore, should include a heterogeneity correction, because lung density can vary up to 0.1× or 0.1 times that of surrounding tissue. This leads to an increased range of photons and secondary electrons that can blur beam edges. Tissue heterogeneity correction depends on beam energy, field size, path length, and lung density and can be calculated accurately using Monte Carlo and superposition/convolution algorithms. The dose calculation grid is frequently set to ≤2 mm for acceptable accuracy (within 1%) (77).
Clinical evidence for SBRT
A number of retrospective reports suggest that conventional RT for early-stage NSCLC results in poor rates of local control and OS. For example, retrospective data from Duke University looked at 156 patients with stage I medically inoperable NSCLC who received a median dose of 64 Gy (range, 50–80 Gy) in 1.2-Gy twice-daily or 3-Gy daily fractions (78). At these doses, deaths were attributed to a high rate of local failure (42%), and the researchers observed that patients with improved local control, which correlated to radiation dose received, also had improved 5-year cause-specific survival (CSS) rates. Population data further validated this; a Surveillance, Epidemiology, and End Results study looked at 4,357 patients with stage I and II NSCLC who did not undergo surgical resection but were treated with conventional RT (79). The researchers concluded that radiation offers a 5–7-month survival benefit but no cure; patients who did and did not receive RT had similar outcomes (5-year OS, 15%). Various literature reviews report 5-year OS to be 30–40% in early-stage NSCLC treated with conventional RT, with doses ≥65 Gy necessary for long-term control (80-82). These data compared adversely with historical surgical series (83-85). Moreover, despite higher doses of conventional radiation, local failure rates remained high (30–70%) (80) and clinicians started exploring the practicality of radiosurgery in treatment of early-stage NSCLC. The use of SBRT for lung cancer was first published in 1991 from clinical work started in Sweden for 42 tumors in 31 cancer patients (86). Various sites, including lung, were treated using a stereotactic body frame for fixation, and prescribed doses, ranging from 7.7 to 30 Gy/fraction (mean, 14.2 Gy), were given for 1–4 fractions. This early work demonstrated an excellent local control rate (80%) and, more important, revealed minimal complications, suggesting the safety of such an approach. During this time, early studies were also underway in Japan, and the combination of Swedish and Japanese experience spearheaded exploration of SBRT for early-stage NSCLC (87,88).
A phase I dose escalation study from the University of Indiana was conducted on operable but medically ineligible stage IA and IB patients (tumor size <7 cm) (89). For T1 tumors, the maximum tolerated dose (MTD) was not reached (maximum dose =60 Gy), but for tumors >5 cm, the MTD was 72 Gy in 3 fractions. At the time of publication, only one local failure occurred in doses ≥16 Gy. This work led to RTOG 0236, a phase II trial recruiting resectable but medically inoperable patients whose primary tumor was <5 cm in size and ≥2 cm from the bronchial tree (because of high rates of grade 5 toxicities seen with centrally located tumors) (69). The radiation dose was 60 Gy in 20-Gy fractions without heterogeneity corrections (18 Gy ×3 with corrections), and 3-year local control was 90.6%, with survival at 55.8% (90).
In this setting of numerous trials with no clear consensus on optimal dosing, retrospective data published from Japan looked at 245 stage I patients treated with 18–75 Gy targeted at the isocenter, given in 1–22 fractions (87). The group observed a local failure rate of only 8.1% for a BED10 ≥100 Gy vs. 26.4% when the BED10 was <100 Gy. This trend was also seen in survival outcomes, where 3-year OS was 88.4% vs. 69.4% with BEDs10 ≥ or <100 Gy, respectively.
In this setting of dose escalation, numerous subsequent retrospective analyses began to demonstrate improving local control rates and survival. Grills et al. reviewed 124 early-stage NSCLC patients who were not eligible for a lobectomy and underwent either SBRT (n=55) or a wedge resection (n=69) (91). The authors noted better local control with SBRT (recurrence of 4% vs. 20%) and similar CSS in both cohorts (93% vs. 94%) (91). This equivalence was further supported by data from Onishi et al., who retrospectively evaluated operable stage IA and IB patients treated with a mean BED10 of 116 Gy (range, 100–141 Gy) and reported excellent 5-year local control rates of 92%, with OS ranging from 62% to 72%, similar to surgical outcomes (92). A separate group also performed a propensity-matched analysis that compared 64 SABR patients with 64 patients who underwent a video-assisted thoracoscopic surgery lobectomy and determined that post-SABR locoregional control rates were superior at 1 and 3 years (96.8% and 93.3% vs. 86.9% and 82.6%, respectively) with similar OS (93).
Because of this equivalence in survival and improved local control compared with historical surgical data, the STARS trial out of MDACC and the ROSEL trial from The Netherlands attempted to compare SBRT to surgical lobectomy in a randomized trial. Additionally an American College of Surgeons Oncology Group and RTOG combined trial (ACOSOG Z4099/RTOG1021) was also initiated to compare clinical results of SBRT to sublobar resection. Despite early termination, an exploratory QOL analysis of the 22 enrolled patients on the ROSEL trial suggested a possible advantage to SBRT, particularly in health-related QOL (94). All analyses of these trials, however, are extremely limited due to being underpowered, as all the trials closed early due to poor accrual.
To mitigate this statistical limitation, a pooled analysis of the STARS and ROSEL trials was performed with a combined total of 58 T1–T2 (<4 cm) operable patients (95). Patients were randomized in a 1:1 fashion to surgery or SBRT (54 Gy/3 fractions for peripheral lesions given over 5–8 days vs. 50 Gy/4 fractions or 60 Gy/5 fractions for central lesions). Surprisingly, OS for the SBRT cohort was superior to the surgical cohort, with 3-year OS of 95% in the SBRT cohort vs. 79% with surgery (P<0.05). Toxicity rates were also lower in the SBRT arm than the surgery arm (10% and 44%, respectively, grade 3 or greater toxicities). The surgical arm also had one grade 5 toxicity. These randomized trials, although underpowered, suggest that SBRT may be better tolerated than surgery, with the possibility of improved survival.
Future work
Technological improvements will continue to drive significant innovation in the field of SBRT. To address the above technical challenges in 3DCRT/IMRT/VMAT planning and delivery for SBRT, such as reliable setup, motion management, and accurate target and normal structure delineation, new tools will be needed to improve the therapeutic ratio. Reliable and consistent patient immobilization systems, tumor motion management strategies (such as abdominal compression, breath-hold, respiratory gating, coaching with audiovisual feedback, and intra-fraction tumor-tracking real-time imaging techniques with dynamic beam and/or couch compensation), and improved imaging modalities (such as 18F-FDG PET for better identification of GTV) all appear to be potential strategies to improve outcomes and decrease toxicity from SBRT. Further clinical research is needed to directly answer the question about equivalence with surgical management, and, to that end, multiple randomized trials, including STABLE-MATES, SABRTooth, VALOR (Veterans Affairs Lung Cancer Surgery Or Stereotactic Radiotherapy), and POSTILV (A Randomized Phase II Trial In Patients With Operable Stage I Non-Small Cell Lung Cancer: Radical Resection Versus Ablative Stereotactic Radiotherapy-RTOG3502), are planned or underway (96). Additionally, emerging data looking at expanding the cohort of patients eligible for SBRT [e.g., patients with central tumors (97) or tumors >5 cm (98)] are promising; and further clinical data are imminent.
Proton therapy
Introduction
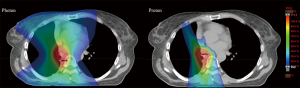
Proton therapy offers a unique pattern of energy deposition, with the majority of dose delivered at the end of range, with virtually no exit dose. This property makes the modality particularly attractive for clinical use in the thorax, where numerous radiosensitive critical structures reside in close proximity to the target (i.e., uninvolved lung, heart, esophagus, spinal cord, major vessels, and chest wall) (Figure 3). Dose distributions associated with proton therapy allow the possibility of dose escalation while maintaining current levels of normal tissue exposure. As noted, recent clinical trials suggest that RT can achieve local disease control rates similar to surgical approaches with potentially less toxicity in early-stage NSCLC (90,95). Such results have correlated with significant dose escalation, in the range of BED >100 Gy10, over doses traditionally achieved with non-stereotactic techniques or in locally advanced disease (88). Results from RTOG 0617, however, have given clinicians pause in attempting to achieve higher doses with traditional 3DCRT or IMRT, considering the worsened outcomes with 74 vs. 60 Gy (63). These outcomes were attributed to, and correlated on multivariate analysis, with increased exposure of normal tissues, such as the heart and esophagus, to significant doses of radiation. These may be areas where, in well-selected patients, proton therapy could offer substantial dosimetric advantages.
Proton therapy technique
Modalities
Proton therapy can now be delivered through several methodologies. The most widely used, passive scattering (PS-PT), employs a single beam that is spread out in the depth dimension by a range-modulator wheel (spread-out Bragg peak) prior to widening in the other dimensions by a scatterer. The lateral edge of the beam is then shaped by an aperture and the distal edge by a compensator. Of note, it is not possible to conform the proximal edge of a PS-PT beam.
On the other hand, the rapidly expanding technique of PBS proton therapy, also known as “spot scanning”, employs scanning magnets to deliver discrete spots of proton beams across a 2D rectilinear grid in the vertical and lateral directions. The range is set for each layer by the energy selection system. This approach allows for both improved proximal dose conformality and intensity-modulated proton therapy (IMPT) within the target. By utilizing multifield optimization and a few (usually 2–4) highly heterogeneous fields that sum to the desired improved dose distribution, IMPT has shown dosimetric improvements over IMRT and PS-PT in multiple in silico studies (99,100). However, these advantages do not come without some increase in uncertainties and diminution in robustness of plan delivery. These uncertainties are highlighted in lung cancers (101-104). In particular, several studies have demonstrated the heightened sensitivity of IMPT to changes in heterogeneity and motion interplay effects as compared with PS-PT (105-109).
Several methods are available to mitigate these uncertainties: robust beam angle selection utilizing water-equivalent thickness optimization, 4DCT-based robust optimization, layer or volumetric “repainting” delivery, spot-sequence delivery optimization, increased fractionation, spot-size modulation, mini-ridge filter utilization, and respiratory gating or breath-hold-based treatment, to name a few (105,110-114). Unfortunately, most of these methods require additional treatment planning software and devices or increase time and logistical burden on planning, quality assurance, and treatment delivery.
Dosimetric studies
Multiple dosimetric planning efforts have revealed the theoretical benefits of proton therapy and especially PBS-PT over 3DCRT and IMRT techniques. For example, planning comparisons in patients with stage I disease demonstrated reductions in mean dose to ipsilateral lung, total lung, heart, esophagus, and spinal cord for proton therapy over 3DCRT (115). Important dosimetric surrogates for pulmonary complications (V5 and V20) were also substantially reduced. Additional work from MDACC and the University of Florida has exhibited the potential for PT to reduce dose to other structures of concern, such as the chest wall in SBRT approaches (116,117).
Similar results were demonstrated in the locally advanced setting. In fact, proton therapy has shown the potential for targeting more comprehensive volumes, including prophylactic treatment of at-risk nodal volumes, with persistently reduced dosimetric markers for complication when compared with photon approaches (117,118). Another approach being evaluated is photon-SBRT with proton mediastinal nodal irradiation (119). These data also encouraged investigators to compare dose-escalated proton planning with 3DCRT and IMRT in both early-and late-stage disease (120,121). In stage I tumors, dose was escalated from 66 Gy to 87.5 CGE without increases in lung V5, V10, or V20 (121). Similarly, in stage III tumors, 74 CGE was achieved versus 63 Gy with 3DCRT, again without worsening of lung dosimetric constraints (121). Spinal cord, heart, esophageal, and integral doses were also all improved with proton therapy. IMPT has shown a particular ability to reduce projected complication rates in early-stage, late-stage, and postoperative patients with lung cancer. As a result, dosimetric studies for dose escalation with IMPT have shown great promise over comparative plans with 3DCRT, IMRT, and PS-PT (99,100,118,122).
When compared with photon techniques, proton therapy in general substantially reduces moderate-to-low-dose exposure of normal lung and nearby critical tissues when targeting lung cancers. Conformality of high-dose regions, however, is compromised due to the increased uncertainty that results from a combination of highly heterogeneous beam paths in the thorax and the finite range of the proton beams. Mitigation of these uncertainties necessitates motion-robust planning approaches that inherently degrade the high-dose conformality. However, the resulting improvements in dosimetric surrogates for complication from photon experiences would seem to allow for further target dose escalation without increasing toxicity.
Clinical outcomes
Numerous clinical trials have been initiated to investigate proton therapy in lung cancer patients; however, initial results have been mixed. A phase I trial performed at MDACC demonstrated in 25 patients the potential for a moderately hypofractionated course (15 fractions of 3–4 Gy/fraction) of proton therapy without concurrent chemotherapy (biologic agents allowed) (123). Two high-grade toxicities occurred, including a tracheoesophageal fistula in a patient who also received bevacizumab, as well as a case of radiation pneumonitis. A Japanese study escalated dose in stage IA and IB patients to 70–94 CGE (3.5–4.9 CGE/fraction) in 37 patients (124). The authors demonstrated excellent local control and low rates of toxicity. Specifically, they achieved 80% local control and 84% survival at 2 years, with only 6 patients experiencing grade 2 and 3 (3 patients each) pulmonary toxicities. Of these 6, 5 patients had stage IB disease, highlighting the significance of the dose-volume effect. Work at Loma Linda University utilizing PS-PT has shown the efficacy and safety of dose escalation from 50 to 70 CGE in 10 fractions in early-stage lung cancers (125,126). They demonstrated a 4-year OS rate of 51%, and none of the 111 patients required steroid treatment for pneumonitis (125). Further evidence, primarily out of Japan, has strengthened the case for comparable efficacy of proton therapy and photon SBRT, although some high-grade toxicities have been encountered at relatively acceptable rates (125,127,128).
In locally advanced disease, a recent National Cancer Database analysis suggested a possible improvement in OS for stage II and III patients receiving proton therapy compared with photon therapies, however this difference was not significant on propensity-matched analysis (129). Early clinical trials have also been relatively positive. Following the previously cited study at MDACC, Chang et al. published the results of a phase II effort investigating concurrent chemoradiotherapy utilizing proton therapy in unresectable stage III NSCLC (130). This study employed a total dose of 74 CGE, similar to the high-dose arm in RTOG 0617 that demonstrated increased complications and worsened survival with photons. In contrast, Chang et al. encountered no grade 4 or 5 toxicities. Grade 3 toxicities were limited to 5 patients with dermatitis, 5 with esophagitis, and 1 with pneumonitis out of total 44 patients enrolled. OS and progression-free survival were 86% and 63%, respectively, at 1 year, with only 4 (9.1%) local-only recurrences. Median survival was 29.4 months. A similar study from the University of Florida closed early after enrolling 14 patients but employed 74 to 80 CGE in conventional fractionation in patients with stage III disease (131). Median OS and progression-free survival were 33 and 14 months, respectively, with no acute grade 3 toxicities and only two patients experiencing late grade 3 toxicities (one gastrointestinal, one pulmonary). Another 15-patient effort from the University of Tsukuba demonstrated similar results at the 74-CGE mark (132).
Multiple phase II and III clinical trials have been initiated to compare proton results to those with photons or to further test dose escalation, especially in the setting of concurrent chemotherapy. Recently, Chang et al. nicely summarized trials underway or recently completed (133). The only randomized data to date were presented at the 2016 meeting of the American Society for Clinical Oncology. Disappointingly, this MDACC/Massachusetts General Hospital trial failed to demonstrate a reduction in toxicity with PS-PT versus IMRT, despite relatively similar outcomes (134). It is notable that target volumes were larger in the proton therapy group (P=0.071) and that higher doses were generally prescribed in the proton cohort with higher resultant lung volumes receiving 30 and 80 Gy.
Future directions
Proton therapy, mainly through PS-PT experiences, has demonstrated largely acceptable toxicity rates with similar-to-improved outcomes in several small institutional trials. With rapidly expanding availability, the shift toward PBS-PT techniques, improvements in gating/breath-hold approaches, and the potential for daily volumetric image guidance, great promise remains for the application of proton therapy in early, locally advanced, and recurrent lung cancers. Further evidence and clinical investigation are anticipated.
Conclusions
Technological advances in RT, starting with volumetric imaging, have revolutionized the paradigm for lung cancer treatment. These improvements have allowed development of a variety of techniques that can enhance the therapeutic ratio. Application of these techniques has allowed physicians to reduce toxicity by sparing normal tissue in certain cases and to dose escalate the BED to improve tumor control in others. Clinical validation of these advantages has been demonstrated in the form of IMRT and SBRT, respectively. Emerging technologies, such as highly non-coplanar planning (4π) and PBS, continue to push the boundaries of the therapeutic ratio. And although they have raised new challenges regarding precision of delivery, dosimetric comparisons have been promising and clinical data are eagerly awaited. Finally, these technological advances in radiation therapy are paving the way to safely and effectively expand our multimodality treatment arsenal to integrate burgeoning systemic therapies, including immunotherapy.
Acknowledgements
We would like to thank Nancy Knight, PhD, for editorial review of the manuscript and Sarah Snider, JD, for assistance with preparation of the figures.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 4.2016. J Natl Compr Canc Netw 2016;14:255-64. [Crossref] [PubMed]
- Perez CA, Pajak TF, Rubin P, et al. Long-term observations of the patterns of failure in patients with unresectable non-oat cell carcinoma of the lung treated with definitive radiotherapy. Report by the Radiation Therapy Oncology Group. Cancer 1987;59:1874-81. [Crossref] [PubMed]
- Perez CA, Stanley K, Rubin P, et al. A prospective randomized study of various irradiation doses and fractionation schedules in the treatment of inoperable non-oat-cell carcinoma of the lung. Preliminary report by the Radiation Therapy Oncology Group. Cancer 1980;45:2744-53. [Crossref] [PubMed]
- Schaake-Koning C, van den Bogaert W, Dalesio O, et al. Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 1992;326:524-30. [Crossref] [PubMed]
- Dillman RO, Seagren SL, Propert KJ, et al. A randomized trial of induction chemotherapy plus high-dose radiation versus radiation alone in stage III non-small-cell lung cancer. N Engl J Med 1990;323:940-5. [Crossref] [PubMed]
- Rengan R, Rosenzweig KE, Venkatraman E, et al. Improved local control with higher doses of radiation in large-volume stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;60:741-7. [Crossref] [PubMed]
- Wang L, Correa CR, Zhao L, et al. The effect of radiation dose and chemotherapy on overall survival in 237 patients with Stage III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;73:1383-90. [Crossref] [PubMed]
- Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 1999;45:323-9. [Crossref] [PubMed]
- Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys 2001;51:650-9. [Crossref] [PubMed]
- Jin H, Tucker SL, Liu HH, et al. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol 2009;91:427-32. [Crossref] [PubMed]
- Kim TH, Cho KH, Pyo HR, et al. Dose-volumetric parameters for predicting severe radiation pneumonitis after three-dimensional conformal radiation therapy for lung cancer. Radiology 2005;235:208-15. [Crossref] [PubMed]
- Kong FM, Hayman JA, Griffith KA, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys 2006;65:1075-86. [Crossref] [PubMed]
- Liao ZX, Komaki RR, Thames HD Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys 2010;76:775-81. [Crossref] [PubMed]
- Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT). Int J Radiat Oncol Biol Phys 2006;66:1399-407. [Crossref] [PubMed]
- Bertelsen A, Hansen O, Brink C. Does VMAT for treatment of NSCLC patients increase the risk of pneumonitis compared to IMRT? - a planning study. Acta Oncol 2012;51:752-8. [Crossref] [PubMed]
- Chan OS, Lee MC, Hung AW, et al. The superiority of hybrid-volumetric arc therapy (VMAT) technique over double arcs VMAT and 3D-conformal technique in the treatment of locally advanced non-small cell lung cancer--a planning study. Radiother Oncol 2011;101:298-302. [Crossref] [PubMed]
- Holt A, van Vliet-Vroegindeweij C, Mans A, et al. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys 2011;81:1560-7. [Crossref] [PubMed]
- Jiang SB, Pope C, Al Jarrah KM, et al. An experimental investigation on intra-fractional organ motion effects in lung IMRT treatments. Phys Med Biol 2003;48:1773-84. [Crossref] [PubMed]
- Galvin JM, Ezzell G, Eisbrauch A, et al. Implementing IMRT in clinical practice: a joint document of the American Society for Therapeutic Radiology and Oncology and the American Association of Physicists in Medicine. Int J Radiat Oncol Biol Phys 2004;58:1616-34. [Crossref] [PubMed]
- Veldeman L, Madani I, Hulstaert F, et al. Evidence behind use of intensity-modulated radiotherapy: a systematic review of comparative clinical studies. Lancet Oncol 2008;9:367-75. [Crossref] [PubMed]
- Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2007;68:94-102. [Crossref] [PubMed]
- Chapet O, Khodri M, Jalade P, et al. Potential benefits of using non coplanar field and intensity modulated radiation therapy to preserve the heart in irradiation of lung tumors in the middle and lower lobes. Radiother Oncol 2006;80:333-40. [Crossref] [PubMed]
- Grills IS, Yan D, Martinez AA, et al. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys 2003;57:875-90. [Crossref] [PubMed]
- Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2004;58:1258-67. [Crossref] [PubMed]
- Schwarz M, Alber M, Lebesque JV, et al. Dose heterogeneity in the target volume and intensity-modulated radiotherapy to escalate the dose in the treatment of non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2005;62:561-70. [Crossref] [PubMed]
- Christian JA, Bedford JL, Webb S, et al. Comparison of inverse-planned three-dimensional conformal radiotherapy and intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2007;67:735-41. [Crossref] [PubMed]
- Yu CX. Intensity-modulated arc therapy with dynamic multileaf collimation: an alternative to tomotherapy. Phys Med Biol 1995;40:1435-49. [Crossref] [PubMed]
- Otto K. Volumetric modulated arc therapy: IMRT in a single gantry arc. Med Phys 2008;35:310-7. [Crossref] [PubMed]
- Palta JR, Deye JA, Ibbott GS, et al. Credentialing of institutions for IMRT in clinical trials. Int J Radiat Oncol Biol Phys 2004;59:1257,9; author reply 1259-61.
- Mah D, Hanley J, Rosenzweig KE, et al. Technical aspects of the deep inspiration breath-hold technique in the treatment of thoracic cancer. Int J Radiat Oncol Biol Phys 2000;48:1175-85. [Crossref] [PubMed]
- Rosenzweig KE, Hanley J, Mah D, et al. The deep inspiration breath-hold technique in the treatment of inoperable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2000;48:81-7. [Crossref] [PubMed]
- Chavaudra J, Bridier A. Definition of volumes in external radiotherapy: ICRU reports 50 and 62. Cancer Radiother 2001;5:472-8. [Crossref] [PubMed]
- Keall PJ, Mageras GS, Balter JM, et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76. Med Phys 2006;33:3874-900. [Crossref] [PubMed]
- Liu HH, Balter P, Tutt T, et al. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for radiotherapy of lung cancer. Int J Radiat Oncol Biol Phys 2007;68:531-40. [Crossref] [PubMed]
- Rietzel E, Liu AK, Chen GT, et al. Maximum-intensity volumes for fast contouring of lung tumors including respiratory motion in 4DCT planning. Int J Radiat Oncol Biol Phys 2008;71:1245-52. [Crossref] [PubMed]
- Underberg RW, Lagerwaard FJ, Slotman BJ, et al. Use of maximum intensity projections (MIP) for target volume generation in 4DCT scans for lung cancer. Int J Radiat Oncol Biol Phys 2005;63:253-60. [Crossref] [PubMed]
- Webb S. Motion effects in (intensity modulated) radiation therapy: a review. Phys Med Biol 2006;51:R403-25. [Crossref] [PubMed]
- Barnes EA, Murray BR, Robinson DM, et al. Dosimetric evaluation of lung tumor immobilization using breath hold at deep inspiration. Int J Radiat Oncol Biol Phys 2001;50:1091-8. [Crossref] [PubMed]
- Ohara K, Okumura T, Akisada M, et al. Irradiation synchronized with respiration gate. Int J Radiat Oncol Biol Phys 1989;17:853-7. [Crossref] [PubMed]
- Kubo HD, Hill BC. Respiration gated radiotherapy treatment: a technical study. Phys Med Biol 1996;41:83-91. [Crossref] [PubMed]
- Keall PJ, Kini VR, Vedam SS, et al. Motion adaptive x-ray therapy: a feasibility study. Phys Med Biol 2001;46:1-10. [Crossref] [PubMed]
- Neicu T, Shirato H, Seppenwoolde Y, et al. Synchronized moving aperture radiation therapy (SMART): average tumour trajectory for lung patients. Phys Med Biol 2003;48:587-98. [Crossref] [PubMed]
- Sawant A, Venkat R, Srivastava V, et al. Management of three-dimensional intrafraction motion through real-time DMLC tracking. Med Phys 2008;35:2050-61. [Crossref] [PubMed]
- Dietrich L, Tücking T, Nill S, et al. Compensation for respiratory motion by gated radiotherapy: an experimental study. Phys Med Biol 2005;50:2405. [Crossref] [PubMed]
- Keall P, Vedam S, George R, et al. The clinical implementation of respiratory-gated intensity-modulated radiotherapy. Med Dosim 2006;31:152-62. [Crossref] [PubMed]
- Cheung PC, Sixel KE, Tirona R, et al. Reproducibility of lung tumor position and reduction of lung mass within the planning target volume using active breathing control (ABC). Int J Radiat Oncol Biol Phys 2003;57:1437-42. [Crossref] [PubMed]
- Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys 1999;44:911-9. [Crossref] [PubMed]
- Kashani R, Balter JM, Hayman JA, et al. Short-term and long-term reproducibility of lung tumor position using active breathing control (ABC). Int J Radiat Oncol Biol Phys 2006;65:1553-9. [Crossref] [PubMed]
- Keall PJ, Webb S. Optimum parameters in a model for tumour control probability, including interpatient heterogeneity: evaluation of the log-normal distribution. Phys Med Biol 2007;52:291-302. [Crossref] [PubMed]
- Lax I, Panettieri V, Wennberg B, et al. Dose distributions in SBRT of lung tumors: Comparison between two different treatment planning algorithms and Monte-Carlo simulation including breathing motions. Acta Oncol 2006;45:978-88. [Crossref] [PubMed]
- Li J, Galvin J, Harrison A, et al. Dosimetric verification using monte carlo calculations for tissue heterogeneity-corrected conformal treatment plans following RTOG 0813 dosimetric criteria for lung cancer stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys 2012;84:508-13. [Crossref] [PubMed]
- Matsuo Y, Takayama K, Nagata Y, et al. Interinstitutional variations in planning for stereotactic body radiation therapy for lung cancer. Int J Radiat Oncol Biol Phys 2007;68:416-25. [Crossref] [PubMed]
- Panettieri V, Wennberg B, Gagliardi G, et al. SBRT of lung tumours: Monte Carlo simulation with PENELOPE of dose distributions including respiratory motion and comparison with different treatment planning systems. Phys Med Biol 2007;52:4265-81. [Crossref] [PubMed]
- Xiao Y, Papiez L, Paulus R, et al. Dosimetric evaluation of heterogeneity corrections for RTOG 0236: stereotactic body radiotherapy of inoperable stage I-II non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2009;73:1235-42. [Crossref] [PubMed]
- Vanderstraeten B, Reynaert N, Paelinck L, et al. Accuracy of patient dose calculation for lung IMRT: A comparison of Monte Carlo, convolution/superposition, and pencil beam computations. Med Phys 2006;33:3149-58. [Crossref] [PubMed]
- Davidson SE, Ibbott GS, Prado KL, et al. Accuracy of two heterogeneity dose calculation algorithms for IMRT in treatment plans designed using an anthropomorphic thorax phantom. Med Phys 2007;34:1850-7. [Crossref] [PubMed]
- Marnitz S, Stuschke M, Bohsung J, et al. Intraindividual comparison of conventional three-dimensional radiotherapy and intensity modulated radiotherapy in the therapy of locally advanced non-small cell lung cancer a planning study. Strahlenther Onkol 2002;178:651-8. [Crossref] [PubMed]
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys 2005;61:318-28. [Crossref] [PubMed]
- Kong FM, Ten Haken RK, Schipper MJ, et al. High-dose radiation improved local tumor control and overall survival in patients with inoperable/unresectable non-small-cell lung cancer: long-term results of a radiation dose escalation study. Int J Radiat Oncol Biol Phys 2005;63:324-33. [Crossref] [PubMed]
- Bradley JD, Bae K, Graham MV, et al. Primary analysis of the phase II component of a phase I/II dose intensification study using three-dimensional conformal radiation therapy and concurrent chemotherapy for patients with inoperable non-small-cell lung cancer: RTOG 0117. J Clin Oncol 2010;28:2475-80. [Crossref] [PubMed]
- Rosenman JG, Halle JS, Socinski MA, et al. High-dose conformal radiotherapy for treatment of stage IIIA/IIIB non-small-cell lung cancer: technical issues and results of a phase I/II trial. Int J Radiat Oncol Biol Phys 2002;54:348-56. [Crossref] [PubMed]
- Schild SE, McGinnis WL, Graham D, et al. Results of a Phase I trial of concurrent chemotherapy and escalating doses of radiation for unresectable non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1106-11. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non–small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol 2017;35:56-62. [Crossref] [PubMed]
- Movsas B, Hu C, Sloan J, et al. Quality of Life Analysis of a Radiation Dose-Escalation Study of Patients With Non-Small-Cell Lung Cancer: A Secondary Analysis of the Radiation Therapy Oncology Group 0617 Randomized Clinical Trial. JAMA Oncol 2016;2:359-67. [Crossref] [PubMed]
- Dong P, Lee P, Ruan D, et al. 4π non-coplanar liver SBRT: a novel delivery technique. Int J Radiat Oncol Biol Phys 2013;85:1360-6. [Crossref] [PubMed]
- Dong P, Lee P, Ruan D, et al. 4π noncoplanar stereotactic body radiation therapy for centrally located or larger lung tumors. Int J Radiat Oncol Biol Phys 2013;86:407-13. [Crossref] [PubMed]
- Hiraoka M, Ishikura S. A Japan clinical oncology group trial for stereotactic body radiation therapy of non-small cell lung cancer. J Thorac Oncol 2007;2:S115-7. [Crossref] [PubMed]
- Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol 2006;24:4833-9. [Crossref] [PubMed]
- Sethi RA, Barani IJ, Larson DA, et al. Handbook of Evidence-Based Stereotactic Radiosurgery and Stereotactic Body Radiotherapy. Springer, 2016:245.
- Benedict SH, Yenice KM, Followill D, et al. Stereotactic body radiation therapy: the report of AAPM Task Group 101. Med Phys 2010;37:4078-101. [Crossref] [PubMed]
- Merrow CE, Wang IZ, Podgorsak MB. A dosimetric evaluation of VMAT for the treatment of non-small cell lung cancer. J Appl Clin Med Phys 2012;14:4110. [PubMed]
- Ong CL, Verbakel WF, Cuijpers JP, et al. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol 2010;97:437-42. [Crossref] [PubMed]
- Bortfeld T. Image Guided IMRT: concepts and clinical applications. New York, NY: Springer, 2005.
- Yamashita H, Haga A, Takahashi W, et al. Volumetric modulated arc therapy for lung stereotactic radiation therapy can achieve high local control rates. Radiat Oncol 2014;9:243. [Crossref] [PubMed]
- Dahele M, Pearson S, Purdie T, et al. Practical considerations arising from the implementation of lung stereotactic body radiation therapy (SBRT) at a comprehensive cancer center. J Thorac Oncol 2008;3:1332-41. [Crossref] [PubMed]
- Pass HI, Carbone DP, Johnson DH, et al. Principles and practice of lung cancer: the official reference text of the IASLC. 4th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2010.
- Sibley GS, Jamieson TA, Marks LB, et al. Radiotherapy alone for medically inoperable stage I non-small-cell lung cancer: the Duke experience. Int J Radiat Oncol Biol Phys 1998;40:149-54. [Crossref] [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [Crossref] [PubMed]
- Qiao X, Tullgren O, Lax I, et al. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer 2003;41:1-11. [Crossref] [PubMed]
- Zimmermann FB, Bamberg M, Molls M, et al. Radiation therapy alone in early stage non-small cell lung cancer. Semin Surg Oncol 2003;21:91-7. [Crossref] [PubMed]
- Jeremic B, Classen J, Bamberg M. Radiotherapy alone in technically operable, medically inoperable, early-stage (I/II) non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2002;54:119-30. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615,22; discussion 622-3.
- Wisnivesky JP, Yankelevitz D, Henschke CI. The effect of tumor size on curability of stage I non-small cell lung cancers. Chest 2004;126:761-5. [Crossref] [PubMed]
- Nesbitt JC, Putnam JB Jr, Walsh GL, et al. Survival in early-stage non-small cell lung cancer. Ann Thorac Surg 1995;60:466-72. [Crossref] [PubMed]
- Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [Crossref] [PubMed]
- Onishi H, Araki T, Shirato H, et al. Stereotactic hypofractionated high-dose irradiation for stage I nonsmall cell lung carcinoma: clinical outcomes in 245 subjects in a Japanese multiinstitutional study. Cancer 2004;101:1623-31. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [Crossref] [PubMed]
- McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non-small-cell lung carcinoma: phase I study. Int J Radiat Oncol Biol Phys 2005;63:1010-5. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol 2010;28:928-35. [Crossref] [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [Crossref] [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [Crossref] [PubMed]
- Louie AV, van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: Results from the ROSEL multicenter randomized trial. Radiother Oncol 2015;117:44-8. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Moghanaki D, Chang JY. Is surgery still the optimal treatment for stage I non-small cell lung cancer? Transl Lung Cancer Res 2016;5:183-9. [Crossref] [PubMed]
- Bezjak A, Paulus R, Gaspar L, et al. Primary study endpoint analysis for NRG Oncology/RTOG 0813 trial of stereotactic body radiation therapy (SBRT) for centrally located non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys 2016;1:5-6. [Crossref]
- Verma V, McMillan MT, Grover S, et al. Stereotactic Body Radiation Therapy and the Influence of Chemotherapy on Overall Survival for Large (≥ 5 Centimeter) Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:146-54. [Crossref] [PubMed]
- Register SP, Zhang X, Mohan R, et al. Proton stereotactic body radiation therapy for clinically challenging cases of centrally and superiorly located stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2011;80:1015-22. [Crossref] [PubMed]
- Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys 2010;77:357-66. [Crossref] [PubMed]
- Szeto YZ, Witte MG, van Kranen SR, et al. Effects of anatomical changes on pencil beam scanning proton plans in locally advanced NSCLC patients. Radiother Oncol 2016;120:286-92. [Crossref] [PubMed]
- Mori S, Dong L, Starkschall G, et al. A serial 4DCT study to quantify range variations in charged particle radiotherapy of thoracic cancers. J Radiat Res 2014;55:309-19. [Crossref] [PubMed]
- Dowdell S, Grassberger C, Sharp G, et al. Fractionated Lung IMPT Treatments: Sensitivity to Setup Uncertainties and Motion Effects Based on Single-Field Homogeneity. Technol Cancer Res Treat 2016;15:689-96. [Crossref] [PubMed]
- Santiago A, Fritz P, Muhlnickel W, et al. Changes in the radiological depth correlate with dosimetric deterioration in particle therapy for stage I NSCLC patients under high frequency jet ventilation. Acta Oncol 2015;54:1631-7. [Crossref] [PubMed]
- Dowdell S, Grassberger C, Sharp GC, et al. Interplay effects in proton scanning for lung: a 4D Monte Carlo study assessing the impact of tumor and beam delivery parameters. Phys Med Biol 2013;58:4137-56. [Crossref] [PubMed]
- Li Y, Kardar L, Li X, et al. On the interplay effects with proton scanning beams in stage III lung cancer. Med Phys 2014;41:021721. [Crossref] [PubMed]
- Hui Z, Zhang X, Starkschall G, et al. Effects of interfractional motion and anatomic changes on proton therapy dose distribution in lung cancer. Int J Radiat Oncol Biol Phys 2008;72:1385-95. [Crossref] [PubMed]
- Matney J, Park PC, Bluett J, et al. Effects of respiratory motion on passively scattered proton therapy versus intensity modulated photon therapy for stage III lung cancer: are proton plans more sensitive to breathing motion? Int J Radiat Oncol Biol Phys 2013;87:576-82. [Crossref] [PubMed]
- Furukawa T, Inaniwa T, Sato S, et al. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 2010;37:4874-9. [Crossref] [PubMed]
- Yu J, Zhang X, Liao L, et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys 2016;43:1111-8. [Crossref] [PubMed]
- Liu W, Schild SE, Chang JY, et al. Exploratory Study of 4D versus 3D Robust Optimization in Intensity Modulated Proton Therapy for Lung Cancer. Int J Radiat Oncol Biol Phys 2016;95:523-33. [Crossref] [PubMed]
- Kardar L, Li Y, Li X, et al. Evaluation and mitigation of the interplay effects of intensity modulated proton therapy for lung cancer in a clinical setting. Pract Radiat Oncol 2014;4:e259-68. [Crossref] [PubMed]
- Li H, Zhu XR, Zhang X. Reducing Dose Uncertainty for Spot-Scanning Proton Beam Therapy of Moving Tumors by Optimizing the Spot Delivery Sequence. Int J Radiat Oncol Biol Phys 2015;93:547-56. [Crossref] [PubMed]
- Grassberger C, Dowdell S, Lomax A, et al. Motion interplay as a function of patient parameters and spot size in spot scanning proton therapy for lung cancer. Int J Radiat Oncol Biol Phys 2013;86:380-6. [Crossref] [PubMed]
- Welsh J, Amini A, Ciura K, et al. Evaluating proton stereotactic body radiotherapy to reduce chest wall dose in the treatment of lung cancer. Med Dosim 2013;38:442-7. [Crossref] [PubMed]
- Hoppe BS, Huh S, Flampouri S, et al. Double-scattered proton-based stereotactic body radiotherapy for stage I lung cancer: a dosimetric comparison with photon-based stereotactic body radiotherapy. Radiother Oncol 2010;97:425-30. [Crossref] [PubMed]
- Nichols RC, Huh SH, Hoppe BS, et al. Protons safely allow coverage of high-risk nodes for patients with regionally advanced non-small-cell lung cancer. Technol Cancer Res Treat 2011;10:317-22. [PubMed]
- Kesarwala AH, Ko CJ, Ning H, et al. Intensity-modulated proton therapy for elective nodal irradiation and involved-field radiation in the definitive treatment of locally advanced non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer 2015;16:237-44. [Crossref] [PubMed]
- Caringi A, Goldsmith B, Kirk M, et al. Combined Photon SBRT and Proton Mediastinal Radiation for Stage III Lung Cancer: The Best of Both Worlds? J Thorac Oncol 2012;7:S237.
- Wu CT, Motegi A, Motegi K, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced non-small cell lung cancer. Jpn J Clin Oncol 2016. [Epub ahead of print]. [Crossref] [PubMed]
- Chang JY, Zhang X, Wang X, et al. Significant reduction of normal tissue dose by proton radiotherapy compared with three-dimensional conformal or intensity-modulated radiation therapy in Stage I or Stage III non–small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:1087-96. [Crossref] [PubMed]
- Berman AT, Teo BK, Dolney D, et al. An in-silico comparison of proton beam and IMRT for postoperative radiotherapy in completely resected stage IIIA non-small cell lung cancer. Radiat Oncol 2013;8:144,717X-8-144.
- Nihei K, Ogino T, Ishikura S, et al. High-dose proton beam therapy for Stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys 2006;65:107-11. [Crossref] [PubMed]
- Hata M, Tokuuye K, Kagei K, et al. Hypofractionated high-dose proton beam therapy for stage I non-small-cell lung cancer: preliminary results of a phase I/II clinical study. Int J Radiat Oncol Biol Phys 2007;68:786-93. [Crossref] [PubMed]
- Bush DA, Cheek G, Zaheer S, et al. High-dose hypofractionated proton beam radiation therapy is safe and effective for central and peripheral early-stage non-small cell lung cancer: results of a 12-year experience at Loma Linda University Medical Center. Int J Radiat Oncol Biol Phys 2013;86:964-8. [Crossref] [PubMed]
- Bush DA, Slater JD, Bonnet R, et al. Proton-beam radiotherapy for early-stage lung cancer. Chest 1999;116:1313-9. [Crossref] [PubMed]
- Hatayama Y, Nakamura T, Suzuki M, et al. Clinical Outcomes and Prognostic Factors of High-Dose Proton Beam Therapy for Peripheral Stage I Non-Small-Cell Lung Cancer. Clin Lung Cancer 2016;17:427-32. [Crossref] [PubMed]
- Westover KD, Seco J, Adams JA, et al. Proton SBRT for medically inoperable stage I NSCLC. J Thorac Oncol 2012;7:1021-5. [Crossref] [PubMed]
- Higgins KA, O'Connell K, Liu Y, et al. National Cancer Database Analysis of Proton Versus Photon Radiation Therapy in Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;97:128-37. [Crossref] [PubMed]
- Chang JY, Komaki R, Lu C, et al. Phase 2 study of high-dose proton therapy with concurrent chemotherapy for unresectable stage III nonsmall cell lung cancer. Cancer 2011;117:4707-13. [Crossref] [PubMed]
- Hoppe BS, Henderson R, Pham D, et al. A Phase 2 Trial of Concurrent Chemotherapy and Proton Therapy for Stage III Non-Small Cell Lung Cancer: Results and Reflections Following Early Closure of a Single-Institution Study. Int J Radiat Oncol Biol Phys 2016;95:517-22. [Crossref] [PubMed]
- Oshiro Y, Okumura T, Kurishima K, et al. High-dose concurrent chemo-proton therapy for Stage III NSCLC: preliminary results of a Phase II study. J Radiat Res 2014;55:959-65. [Crossref] [PubMed]
- Chang JY, Jabbour SK, De Ruysscher D, et al. Consensus Statement on Proton Therapy in Early-Stage and Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;95:505-16. [Crossref] [PubMed]
- Liao Z, Lee J, Komaki R, et al. Bayesian randomized trial comparing intensity modulated radiation therapy versus passively scattered proton therapy for locally advanced non-small cell lung cancer. J Clin Oncol 2016;34:abstr 8500.


