Targeting eukaryotic protein translation in mesothelioma
Introduction
Malignant mesothelioma arises from mesothelial cells in the pleura and is not curable with current therapies. The current standard of care for unresectable mesothelioma is combination chemotherapy of cisplatin and pemetrexed resulting in a median time to progression of 7 months and overall survival of 12 months. Overall response rates to this first line systemic therapy is only 41% in Phase 3 trials (1). As is true of most malignancies, activated cap-mediated translation of polyribosomes is a hallmark of mesothelioma. Dysregulated cap-dependent protein translation is implicated in tumorigenesis in multiple cancers (2,3). The rate-limiting step in the initiation of mRNA translation is the binding of eukaryotic initiation factor 4E (eIF4E) to the 5' cap structure (m7GpppN) of mRNA. Overexpression of eIF4E is observed in many solid tumors. Under normal cellular conditions, eIF4E activity is negatively regulated by 4E-BP proteins. 4E-BP1 blocks the interaction between eIF4E and the scaffolding protein eIF4G, inhibiting the formation of the active eIF4F complex and suppressing cap-mediated translation. 4E-BP1 undergoes hierarchical phosphorylation during mitogenic stimulation, which decreases its affinity for eIF4E and results in the activation of translation (3-5). In cancer, elevated levels of active eIF4F increase the translation of “weak” mRNAs with long structured 5'-untranslated regions, which typically encode anti-apoptotic proteins and growth regulatory factors contributing to tumorigenesis (6,7). Elevated levels of eIF4E enhance translation of many malignancy-related proteins, such as vascular endothelial growth factor (VEGF), c-myc, and osteopontin (2,3,7). Activation of cap-mediated translation in general results in translation of what appears to be a limited yet vital cohort of proteins associated with maintenance of the malignant phenotype (8,9). Previous studies have shown the 5' cap-mediated translation of proteins is up-regulated in many or most cancers, including mesothelioma, and that downregulation of the eIF4F complex activity in mesothelioma is associated with loss of the malignant phenotype and increased sensitivity to cytotoxic therapies (2,10).
Mesothelioma, as most solid tumors, is associated with activation of transmembrane tyrosine kinase receptors such as EGFR and IGFR. Members of the IGF axis are particularly over-expressed and active in mesothelioma cells, and circulating levels of IGF are elevated in the blood of patients with mesothelioma (11,12). Ligand mediated activation of the IGFR axis leads directly to mTOR mediated 5' cap-mediated translation via phosphorylation of eIF4E and the resultant enhanced binding to the transcript cap. Consequently, inhibition of eIF4E following transgene expression of a dominant negative form of the endogenous 4E-BP1 binding protein blunts and eliminates the stimulatory effects of IGF on mesothelioma cell growth. As such, down-regulation of cap-mediated translation directly leads to the loss in vivo of the malignant phenotype of mesothelioma xenografts (10).
This article will review the state of the art in understanding enhanced cap-mediated protein translation in mesothelioma and the potential for its targeting as a therapeutic approach. Emphasis will be given to the role cap-mediated translation may play in altering the apoptotic potential of mesothelioma cells as well as direct inhibition of growth and stimulation. Finally, observations on the potential role protein translation may play in impacting ongoing clinical studies utilizing oncolytic viral therapy for mesothelioma will be discussed.
5' cap-mediated translation
Eukaryotic protein translation proceeds from one of two independent mechanisms. The default mechanism proceeds from the 5' end of the transcript and is mediated by the eIF4 complex (Figure 1). The eIF4 complex is composed of several molecular entities including eIF4E, eIF4A, the family of 4E-BP proteins, and eIF4G (3). The resulting lariat form is thought to be the functional unit for protein translation. The rate limiting entity in this complex is the eIF4E protein which binds directly to the 5' cap structure (m7GpppN) of the target mRNA. The eIF4G protein appears to function as a docking protein stabilizing the complex, while the eIF4A protein assists in unwinding the transcript aiding in its translation. The 4E-BP proteins may function to displace the integral interaction between eIF4E and eIF4G and thus inhibit translation activation. Activation of the complex following binding the eIF4G docking protein leads to recruitment of other integral entities, including the 40S ribosomal subunit, mTOR, and eIF4A helicase protein. This latter protein is integral in positioning and unwinding secondary structure of the nascent polyribosome prior to active translation (13).
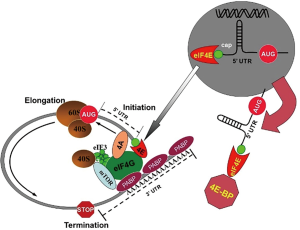
The alternative mechanism of protein translation in eukaryotes is IRES (Independent Ribosomal Entry Site). First described as a primary method for viral induction of protein synthesis after infection of eukaryotic cells, IRES sites have been described in the 5'-untranslated (5'-UTR) of limited numbers of eukaryotic transcripts, and may be accessed in direct proportion to the availability of eIF4E (14). Viral subversion of protein translation includes degradation and cleavage of vital components of eIF4 complex. However, in eukaryotic translation, the default mechanism widely accepted to involve the cap translational complex.
It has been established in the last decade that most cancers possess hyper-activation of the eIF4 complex (2,3). The signaling pathways controlling activation target phosphorylation of both the eIF4E protein, resulting in enhanced binding to the 5' cap, as well as 4E-BP proteins leading to inactivation of the repressor function and release from the eIF4 complex (8,9). The resulting enhancement of cap-direct translation results in preferential expression of a limited cohort of proteins thought to be directly related to the development and maintenance of cancer. Cap-mediated translation of proteins involves recruitment of preferentially translated transcripts during the activation process. For example, proteins such as c-myc, ornithine decarboxylase, and cyclin D1 are preferentially expressed by enhanced 5' cap-mediated translation in a variety of cancers (2,3,7). Current theory indicates many transcripts necessary for “housekeeping” functions possess relatively “weak” secondary 5' prime structures (Figure 1), while the activated malignant cell will preferentially activate growth related transcripts following cellular activation (13). Modulation of this activity by either genetic or pharmacologic means will result in decreased malignant phenotype in animal models. As an example of the power of activation of cap-mediated translation in the neoplastic phenotype, transduction and enforced expression of exogenous eIF4E is transformative in 3T3 cells (15).
Similar to other cancers, mesothelioma demonstrates enhanced translation activation. Protein translation is not only central to neoplasia, but obviously a hallmark of cell growth and stimulation in all eukaryotes. Powerful growth modulators such as IGF ligand pathways are activated in mesothelioma cells and directly feed through the Akt pathway to mTOR mediated activation of translation (13). Indeed, targeting of the IGF pathway at several levels leads directly to decreased oncogenic protein translation in mesothelioma cells, as well as apoptotic cell death (15). In view of the important role translational control plays in the neoplastic process a number of investigators have developed or tested agents designed to disrupt and modulate this activity, including mesothelioma.
Antisense targeting of eIF4E
eIF4E is the limiting factor in mediating 5' cap translation. As such, it provides the most attractive direct target for anti-neoplastic therapy. Phosphorylation and activation of eIF4E by the mTOR kinase is a powerful downstream signal for cell growth mediated by activation of cell surface tyrosine kinases (8,9). Malignant transformation occurs following eIf4E over-expression in 3T3 cells mimicking the enhanced activation of eIF4E observed in many cancers (15). In addition, enforced expression of 5' cap-mediated translation in malignant cells results in expression of a select cohort of preferentially translated proteins important in the neoplastic process (2,3,7).
Direct knockdown of eIF4E using a targeted antisense strategy has been developed and tested in vitro and animal models with notable success. Generation of a third generation stabilized antisense oligonucleotides (ASO) results in a therapeutic approach that has advanced to clinical trials (16). Graff and colleagues demonstrated excellent results in silencing expression of eIF4E in a variety of cancer cells. Associated with this, in vivo activity in murine models was also excellent and associated with diminished expression of a number of oncogenic proteins. Similar results were demonstrated for NSCLC cells as well.
Mesothelioma cell have also has been proven to be sensitive to inhibition of cap-mediated translation. Targeting with an antisense molecule results in down regulation of eIFE protein levels. This correlates with inhibition of in vitro cell proliferation. As previously noted, eIF4E is the rate limiting entity in the cap mediated complex. As such, diminished levels of this protein will be associated with decreased polyribosome translation. Proteins downregulated at this level include critical signals for cell survival, apoptosis, and proliferation. Targeting of translation in mesothelioma cells lowers the apoptosis threshold and renders them more susceptible to cytotoxic agents such as pemetrexed and gemcitabine.
The utility of this approach for patient care is limited by many of the difficulties associated with antisense approaches in other disease. Antisense agents targeting BCL2, for example, have proven difficult to deliver requiring prolonged infusions with little associated clinical benefit (17). The third generation methoxy multimer antisense approach is designed to increase stability and results in a product requiring intermittent dosing more consistent with standard oncology regimens (16). In mammalian cultured cells, these ASOs specifically targeted the eIF4E mRNA for destruction, repressing expression of eIF4E-regulated proteins (e.g., VEGF, cyclin D1, survivin, c-myc, Bcl-2), inducing apoptosis, and preventing endothelial cells from forming vessel-like structures. Most importantly, intravenous ASO administration selectively and significantly reduced eIF4E expression in human tumor xenografts, significantly suppressing tumor growth. Despite reducing eIF4E levels by 80% in mouse liver, eIF4E-specific ASO administration did not affect body weight, organ weight, or liver transaminase levels, thereby providing the first in vivo evidence that cancers may be more susceptible to eIF4E inhibition than normal tissues. Using this approach, agents using third-generation ASOs with a phosphorothioate backbone and flanking bases modified with a methoxyethyl group at the 2' position of the sugar can be combined with standard cytotoxic chemotherapy thus exploiting the pro-apoptotic effects of dampening of cap-mediated translation.
Studies in mesothelioma cells have yielded similar results. Enhanced cell killing, synergy with cytotoxic agents, and downregulation of critical proteins have noted reported (18). Diminished binding of the eIF4E to the 5' cap of artificial transcripts with consequent displacement of the critical eIF4G docking protein is noted both in vitro and in vivo. As promising as this approach appears, however, there are no trials in development to further test this class of agents in human mesothelioma, largely due to the limitations in delivery of effective levels of the therapeutic agent (16). In light of these limitations, application of synthetic small molecule chemistry to design inhibitors of cap-mediated translation has attracted greater attention.
Disruptors of 5' cap complex
The critical role of eIF4E is mediated through its direct binding to the 5' cap structure (m7GpppN) of mRNA transcripts. Following this binding, recruitment of other molecules that are part of the EIF4 complex occurs, including the necessary attachment of the eIFG docking protein. Several small molecule inhibitors have been developed that are disruptors of this key complex. One of the first targets has been the direct interaction of transcript cap with the eIF4E protein. Binding protein eIF4E to N(7)-methylated guanosine capped mRNA has been found to be the rate-limiting step governing translation initiation, and therefore represents an attractive target for drug discovery. It has been found that 7-benzyl guanosine monophosphate (7Bn-GMP) is a potent antagonist of eIF4E cap binding [K(d) =0.8 µM] (19,20). Recent X-ray crystallographic studies have revealed that the cap-dependent pocket undergoes a unique structural change in order to accommodate the benzyl group. Unfortunately, 7Bn-GMP is not cell permeable. Recently, a synthetic tryptamine phosphoramidate prodrug of 7Bn-GMP was developed, 4Ei-1, and shown to be a substrate for human histidine triad nucleotide binding protein (hHINT1). This compound inhibits eIF4E initiated epithelial-mesenchymal transition (EMT) as assessed by Zebra fish embryo cells. Intracellular uptake of 4Ei-1 results in conversion to 7Bn-GMP in cancer cells (21).
4Ei-1 has been tested in vitro in mesothelioma cells resulting in cell killing and suppression of critical neoplasia related proteins. Treatment of mesothelioma cells with 4Ei-1 resulted in displacement of the eI4G docking protein effecting disruption of the cap-mediated translation in mesothelioma cells (22). Consequently, there is diminished translation and subsequent expression of ODC, cyclin D, and c-myc in mesothelioma cells following exposure to 4Ei-1. 4Ei-1 treatment of mesothelioma cells is also associated with a more global impact on the caner translatome (23). Genomic array analysis of polyribosomes isolated following 4Ei-1 treatment of both mesothelioma and breast cancer cells results in a high concordance with of transcripts negatively recruited for translation after treatment. Conversely, stimulation of mesothelioma cells with insulin like growth factor, a powerful stimulant of mesothelioma cell growth and activation of the eIF4 complex, reverses this array signature. Further attempts to modify 4Ei-1 are underway to increase it cell uptake following chemical modification. Such a molecule could have a powerful anti-cancer effect on translationally activated cells as well decreasing the apoptotic threshold of mesothelioma cells to conventional chemotherapy. This latter synergistic effect is already apparent with the parental 4Ei-1 compound, demonstrating increased cell killing in vitro with both pemetrexed and gemcitabine.
Cap-mediated translation mediates oncolytic virotherapy
Viral infection in non-transformed cells is highly associated with redirection of cap-mediated translation away from production of proteins associated with host cellular maintenance and towards viral replication. Moreover, viruses are fully dependent on the host cell translation machinery to produce the proteins that are crucial for viral replication (11). This is also likely true for viral infection of transformed cells. The hyper-activation of protein translation seen in the cancer phenotype may render transformed cells more sensitive to viral mediated oncolysis dependent upon the relative elevated levels of host cell protein synthesis.
Oncolytic virotherapy has been tested in a wide variety of cancers. Candidate therapeutic viruses include a wide number of vectors. Many, if not all, wild-type viruses possess the capacity to infect tumor cells. Among these, the parvoviruses, reovirus, mumps virus, Newcastle disease virus, and Moloney leukemia virus have a natural tendency to preferentially infect cancer cells, while measles virus, adenovirus, vaccinia, and herpes simplex virus can be genetically attenuated to infect cancer cells instead of human cells (24). Particular interest has been focused on the Edmonston strain of measles, a naturally occurring attenuated virus that interacts with cancers cells by the CD46 cell surface receptor. Measles virus has been shown to be highly effective in vitro for mesothelioma xenografts and has entered Phase 1 testing (25). In addition, trials using adenoviral therapies for mesothelioma have demonstrated early success (26). A key pathway for viral proliferation is the capacity following infection for the invading virion to utilize the cellular protein translation for expression of critical factors.
Recent studies have demonstrated blunting cap-mediated translation in mesothelioma cells will result in a non-permissive state for viral invasion and replication. This appears to largely be mediated at the level of disruption of the eIF4E complex with virally infected mesothelioma and lung cancer cells incapable of the replication or oncolysis (27). The density of the CD46 receptor, the portal of entry for oncolytic measles virus, is not effected by inhibition of cap-mediated translation, and mesothelioma cells remain permissive to initial infection following eIF4E targeted therapy (28). Conversely, activation of cap-mediated translation leads to improved infectivity for oncolytic therapy in mesothelioma. IGF mediated activation of mTOR directly resulting in enhanced eIF4E binding and displacement of the 4EBP repressor proteins.
Whereas blunting of cap-mediated translation leads to lowering the apoptotic potential of cancer cells, including mesothelioma, the manipulation of translation results in lowering the efficacy of oncolytic therapies. Although this may be limited interest in the absence of an approved oncolytic therapy for mesothelioma, it does suggest additional therapeutic approaches. Viral vectors have been developed that exploit the over-expression of the IGF axis in tumors such as mesothelioma (29). Such a ligand targeted oncolytic therapy will potentially prime cap-mediated translation thus making the mesothelioma cell more permissive for infection and lysis. This approach exploiting ligand targeted oncolytic therapy is currently under investigation in the laboratory.
Summary
Activation of the 5' cap mediated translation complex is a hallmark of malignant transformation. This is true in many cancers, and mesothelioma has been demonstrated to be highly susceptible to interventions disrupting this mechanism. Current research to target the eIF4 complex in mesothelioma has focused on both small molecule and silencing approaches. This promises to not only lead to direct cell killing, but modulation of the apoptotic threshold rendering standard cytotoxic therapy more effective. In addition, with the introduction of viral oncolytics to mesothelioma clinical trials, efforts to modulate translational activation to increase the benefits to this promising therapy should lead to a better appreciation of their efficacy. Ongoing efforts to further modify small molecule disruptors of the eIF4 complex and to better comprehend the role translational control plays in oncolytics stand to be the area of greatest research interest in the coming years.
Acknowledgements
The author would like to acknowledge the assistance of Ahad Siddiq, Manish Patel, and Blake Jacobson in assembling this review.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [Crossref] [PubMed]
- Jacobson BA, Alter MD, Kratzke MG, et al. Repression of cap-dependent translation attenuates the transformed phenotype in non-small cell lung cancer both in vitro and in vivo. Cancer Res 2006;66:4256-62. [Crossref] [PubMed]
- Pelletier J, Graff J, Ruggero D, et al. Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res 2015;75:250-63. [Crossref] [PubMed]
- De Benedetti A, Graff JR. eIF-4E expression and its role in malignancies and metastases. Oncogene 2004;23:3189-99. [Crossref] [PubMed]
- Avdulov S, Li S, Michalek V, et al. Activation of translation complex eIF4F is essential for the Genesis and maintenance of the malignant phenotype in human mammary epithelial cells. Cancer Cell 2004;5:553-63. [Crossref] [PubMed]
- Graff JR, Zimmer SG. Translational control and metastatic progression: enhanced activity of the mRNA cap-binding protein eIF-4E selectively enhances translation of metastasis-related mRNAs. Clin Exp Metastasis 2003;20:265-73. [Crossref] [PubMed]
- Thumma SC, Kratzke RA. Translational control: a target for cancer therapy. Cancer Lett 2007;258:1-8. [Crossref] [PubMed]
- She QB, Halilovic E, Ye Q, et al. 4E-BP1 is a key effector of the oncogenic activation of the AKT and ERK signaling pathways that integrates their function in tumors. Cancer Cell 2010;18:39-51. [Crossref] [PubMed]
- Hsieh AC, Costa M, Zollo O, et al. Genetic dissection of the oncogenic mTOR pathway reveals druggable addiction to translational control via 4EBP-eIF4E. Cancer Cell 2010;17:249-61. [Crossref] [PubMed]
- Jacobson BA, De A, Kratzke MG, et al. Activated 4E-BP1 represses tumourigenesis and IGF-I-mediated activation of the eIF4F complex in mesothelioma. Br J Cancer 2009;101:424-31. [Crossref] [PubMed]
- Hoang CD, Zhang X, Scott PD, et al. Selective activation of insulin receptor substrate-1 and -2 in pleural mesothelioma cells: association with distinct malignant phenotypes. Cancer Res 2004;64:7479-85. [Crossref] [PubMed]
- Whitson BA, Jacobson BA, Frizelle S, et al. Effects of insulin-like growth factor-1 receptor inhibition in mesothelioma. Thoracic Surgery Directors Association Resident Research Award. Ann Thorac Surg 2006;82:996-1001; discussion 1001-2. [Crossref] [PubMed]
- Svitkin YV, Pause A, Haghighat A, et al. The requirement for eukaryotic initiation factor 4A (elF4A) in translation is in direct proportion to the degree of mRNA 5' secondary structure. RNA 2001;7:382-94. [Crossref] [PubMed]
- Svitkin YV, Herdy B, Costa-Mattioli M, et al. Eukaryotic translation initiation factor 4E availability controls the Switch between cap-dependent and internal ribosomal entry site-mediated translation. Mol Cell Biol 2005;25:10556-65. [Crossref] [PubMed]
- Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5' cap. Nature 1990;345:544-7. [Crossref] [PubMed]
- Graff JR, Konicek BW, Vincent TM, et al. Therapeutic suppression of translation initiation factor eIF4E expression reduces tumor growth without toxicity. J Clin Invest 2007;117:2638-48. [Crossref] [PubMed]
- Rudin CM, Salgia R, Wang X, et al. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J Clin Oncol 2008;26:870-6. [Crossref] [PubMed]
- Jacobson BA, Thumma SC, Jay-Dixon J, et al. Targeting eukaryotic translation in mesothelioma cells with an eIF4E-specific antisense. PLoS One 2013;8:e81669. [Crossref] [PubMed]
- Jia Y, Chiu TL, Amin EA, et al. Design, synthesis and evaluation of analogs of initiation factor 4E (eIF4E) cap-binding antagonist Bn7-GMP. Eur J Med Chem 2010;45:1304-13. [Crossref] [PubMed]
- Li S, Jia Y, Jacobson B, et al. Treatment of breast and lung cancer cells with a N-7 benzyl guanosine monophosphate tryptamine phosphoramidate pronucleotide (4Ei-1) results in chemosensitization to gemcitabine and induced eIF4E proteasomal degradation. Mol Pharm 2013;10:523-31. [Crossref] [PubMed]
- Smith KA, Zhou B, Avdulov S, et al. Transforming Growth Factor-β1 Induced Epithelial Mesenchymal Transition is blocked by a chemical antagonist of translation factor eIF4E. Sci Rep 2015;5:18233. [Crossref] [PubMed]
- Chen EZ, Jacobson BA, Patel MR, et al. Small-molecule inhibition of oncogenic eukaryotic protein translation in mesothelioma cells. Invest New Drugs 2014;32:598-603. [Crossref] [PubMed]
- De A, Jacobson B, Peterson M, et al. 4EGI-1 represses cap-dependent translation in malignant pleural mesothelioma. AACR Annual Meeting; Apr 12-16, 2008; San Diego, CA.
- Ahmad Z, Kratzke RA. Novel oncolytic viral therapies in patients with thoracic malignancies. Oncolytic Virother 2016;6:1-9. [Crossref] [PubMed]
- Li H, Peng KW, Dingli D, et al. Oncolytic measles viruses encoding interferon beta and the thyroidal Sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther 2010;17:550-8. [Crossref] [PubMed]
- Sterman DH, Haas A, Moon E, et al. A trial of intrapleural adenoviral-mediated Interferon-α2b gene transfer for malignant pleural mesothelioma. Am J Respir Crit Care Med 2011;184:1395-9. [Crossref] [PubMed]
- Patel MR, Jacobson BA, Belgum H, et al. Measles vaccine strains for virotherapy of non-small-cell lung carcinoma. J Thorac Oncol 2014;9:1101-10. [Crossref] [PubMed]
- Jacobson BA, Sadiq AA, Shaogeng T, et al. Cap-dependent translational control of oncolytic measles virus infection in malignant mesothelioma. Oncotarget 2017. [Epub ahead of print].
- Schneider U, Bullough F, Vongpunsawad S, et al. Recombinant measles viruses efficiently entering cells through targeted receptors. J Virol 2000;74:9928-36. [Crossref] [PubMed]


