Targeting the metastatic niche through anti-angiogenic approaches in epidermal growth factor receptor mutant non-small cell lung cancer
Despite high response rates (RR) observed of up to 70% with epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) in treatment-naïve EGFR mutant non-small cell lung cancers (NSCLC), eliciting durable responses remains a perennial challenge. The median progression-free survival (PFS) achieved for 1st and 2nd generation EGFR TKIs is 9–13 months in the front line setting (1-7), with 50–60% of resistance due to the emergence of the gatekeeper T790M mutation in EGFR exon 20 (8). Preclinical modeling has shown that T790M mutations can be ‘acquired’ by initially T790M-negative drug-tolerant cells under selection pressure after TKI treatment, although improvement in molecular diagnostics has suggested that such resistant clones may have ‘pre-existed’ as a minor sub-population prior to TKI exposure. In the treatment-naïve setting, de novo T790M mutations have been reported across a range of frequencies depending on assay sensitivity—ranging from <1% with Sanger sequencing to 80% with ultra-sensitive methods (9). De novo T790M mutations have been associated with shorter PFS and poorer outcomes in patients with advanced NSCLC treated with EGFR TKIs (10,11), underscoring the need for novel combinatorial strategies to circumvent resistance.
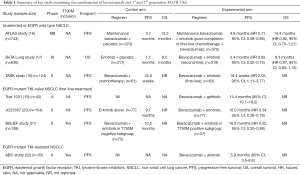
Bevacizumab is a recombinant humanized monoclonal antibody that selectively binds vascular endothelial growth factor (VEGF) and blocks its interaction with VEGF receptor, thereby preventing a cascade of molecular events that drives tumor angiogenesis, an essential hallmark in NSCLC (12). Phase III clinical trials of bevacizumab-chemotherapy combinations as first-line therapy in advanced non-squamous NSCLC have shown improved outcomes with platinum-based chemotherapy alone; and the pivotal E4599 trial, as well as the AVAIL study, demonstrated significantly improved RR and PFS (13,14). VEGF has also been implicated in resistance to EGFR TKI, with preclinical studies demonstrating EGFR inhibition can result in compensatory increase in stroma-derived VEGF levels that fosters disease progression (15). Thus, dual inhibition of EGFR and VEGF signaling pathways appears to be a synergistic strategy to abrogate EGFR TKI resistance. Bevacizumab and EGFR TKI combinations have been investigated widely, encompassing unselected NSCLC, as well as more specifically in the context of EGFR-mutant NSCLC in the TKI resistant and TKI naïve setting (Table 1) (16-22).
Full table
Notably, in the first-line setting, a Japanese randomized phase II trial (JO25567) demonstrated that the combination of erlotinib-bevacizumab showed a significantly prolonged PFS when compared with erlotinib alone (16.0 vs. 9.7 months respectively, HR 0.54, P=0.0015), meeting its primary endpoint albeit immature overall survival (OS) data at time of analysis, and primarily led to European Union (EU) approval of erlotinib-bevacizumab combination (20). This prolongation in PFS was also observed in other trials combining EGFR TKI plus bevacizumab, where a single arm phase II study in treatment naïve EGFR-mutant NSCLC, showed that the combination of gefitinib-bevacizumab was tolerable, with a median PFS of 14.4 months (19). In contrast, no significant difference in PFS was seen in the single-arm phase II ABC study of afatinib and bevacizumab in TKI-resistant EGFR-mutant, with a RR of 18.2%, modest regardless of T790M status (detected in 42% patients) (22), highlighting the importance of identifying appropriate therapeutic niches when designing trials.
The BELIEF trial by Rosell et al. adds to the burgeoning data supporting the role of adding bevacizumab to the current standard of care TKI in treatment naïve EGFR-mutant NSCLC to forestall resistance (21). This was an international, multicenter (across eight European countries), single-arm phase II trial involving 109 patients with advanced treatment-naive NSCLC and activating EGFR mutations (exon 19 deletions or L858R mutations) who received erlotinib (150 mg/day) plus bevacizumab (15 mg/kg every 3 weekly). In contrast to the JO25567 study, it included patients with brain metastasis (19%) whereas patients with T790M mutation and brain metastases were both excluded in the JO25567 study. Activating EGFR and T790M mutations were centrally tested with a highly sensitive peptide-nucleic acid (PNA) probe-based TaqMan assay (real-time PCR)—detecting pretreatment T790M resistance mutation in 34% (37/109) of patients.
For the total population (n=109), the overall PFS was 13.2 months (95% CI, 10.3–15.5) and median OS 28.2 months after a median follow-up of 21.4 months. In addition, patients were stratified according to pretreatment T790M status into two parallel sub-studies (T790M-positive, n=37; or T790M-negative, n=72 cohorts). Interestingly, the T790M-positive subgroup, traditionally associated with worse PFS and OS (11,23), demonstrated a median PFS of 16.0 months (12-month PFS 68%). Notwithstanding the caveat that the BELIEF trial was not a randomized controlled trial with a relatively small sample size, the impact of erlotinib-bevacizumab in overcoming the detrimental effect of pretreatment T790M mutation, was striking. This result has several implications on the application of rational combination strategies in the future, including patient selection and provides fresh insights into the biological basis of targeting the VEGFR axis in EGFR mutant NSCLC.
First, it is noteworthy that the reported prevalence of 30% of patients with de novo T790M mutations is significantly higher than the usual reported prevalence of <1% with standard sequencing platforms. In the BELIEF study, post hoc analysis confirmed sensitivity of the PNA TaqMan assay with concordance testing using standard COBAS EGFR assay and ddPCR. While the COBAS test revealed high concordance with the TaqMan assay for the activating EGFR mutations, it detected only 1/47 samples with T790M mutations. Conversely, concordance rates for pretreatment T790M detection between ddPCR and TaqMan was 62.3%, as ddPCR detected 25/50 (50%) evaluable samples for T790M positive cases compared to 21/50 (42%) by the TaqMan assay. Taken together, these findings suggest that a subset of patients likely harbor pre-existing T790M-positive clones before TKI exposure, although at levels below the detection thresholds of many standard lab assays like Sanger sequencing and COBAS EGFR assays on FFPE tissue samples. With multiple platforms now available for high sensitive detection in both tissue and plasma, prevalence rates of pretreatment T790M mutations have been shown to be wide-ranging (from <1% to 80%), primarily due to variations in performance characteristics and detection limits of various assays (24). Thus, in order to enable accurate interpretation of future confirmatory clinical studies, definitions of “T790M-positive (T790M+)” patient populations will need to be accompanied by technical specifications of the detection method.
Second, the studies to date consistently show no difference in RR, but improvement in PFS when combining EGFR TKI with bevacizumab in EGFR mutant NSCLC (Table 1). This highlights the potential for VEGFR targeting approaches to augment the metastatic niche and forestall the emergence of systemic disease progression, regardless of the presence of T790M+ clones. On the other hand, the shorter PFS of 10.5 months for T790M-negative patients in BELIEF, was numerically less compared to 16.0 months for patients in the erlotinib-bevacizumab group in the J025567 study. This shorter duration of response may be influenced by the proportion of patients with brain metastases (19%) in BELIEF (25), particularly since the absence of brain metastasis was significantly associated with longer PFS by multivariable analysis. Further follow up on the sites of initial disease progression will be illuminating, with definitive data on clinical benefit awaited from ongoing randomized phase III trials.
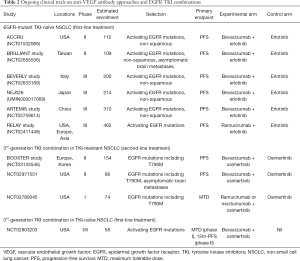
Currently, there are several ongoing phase III randomized trials comparing erlotinib-bevacizumab against erlotinib – such as the ACCRU study (NCT01532089) and the BEVERLY study (NCT02633189). Combination of 3rd generation EGFR TKI osimertinib plus bevacizumab in T790M-positive EGFR-mutant NSCLC compared with osimertinib alone is now being explored both in the second line (BOOSTER, NCT03133546) as well as in the first-line setting (NCT02803203), as a strategy to circumvent resistance to third generation TKIs. With a reported PFS of 18.9 months (95% CI, 12.5–21.4) with front-line osimertinib in EGFR-mutant NSCLC from the FLAURA study (26), further extension of PFS with osimertinib-bevacizumab combination will be anticipated. Other ongoing trials involve the combination of erlotinib with other VEGFR antibodies such as ramucirumab (RELAY, NCT02411448), as well as those targeting specific metastatic niches such as brain metastases (summarized in Table 2).
Full table
In conclusion, dual targeting of EGFR and VEGF pathways remains a promising approach to circumvent EGFR TKI resistance and warrants further evaluation. The hypothesis-generating results from BELIEF provide timely and intriguing efficacy data in “poor risk” patients with de novo T790M mutations. Furthermore, the recent advent of cell-free DNA detection methods may also yield a higher prevalence of pretreatment T790M mutations (27), highlighting the need for additional stringent concordance studies to better define this evolving patient subgroup. A key priority in the eagerly anticipated phase III bevacizumab-EGFR TKI trials, remains how best to stratify EGFR mutant NSCLC patients, so as to facilitate individualized combinatorial approaches for maximal therapeutic gain.
Acknowledgements
Funding: DS Tan acknowledges funding support from the National Medical Research Council (Singapore) Clinician-Scientist Award (NMRC/CSA/007/2016) as well as the Lung Cancer Translational & Clinical Research Flagship Program (NMRC/TCR/007-NCC/2013)
Footnote
Conflicts of Interest: DS Tan has received honoraria from and has been an advisory board member for Novartis, Merck, Pfizer, Boehringer Ingelheim, AstraZeneca, Roche and Loxo Oncology. He has also received research funding from Novartis, GSK, Bayer and AstraZeneca. WL Tan has no conflicts of interest to declare.
References
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:213-22. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Yu HA, Arcila ME, Rekhtman N, et al. Analysis of Mechanisms of Acquired Resistance to EGFR TKI therapy in 155 patients with EGFR-mutant Lung Cancers. Clin Cancer Res 2013;19:2240-7. [Crossref] [PubMed]
- Watanabe M, Kawaguchi T, Isa SI, et al. Ultra-Sensitive Detection of the Pretreatment EGFR T790M Mutation in Non–Small Cell Lung Cancer Patients with an EGFR-Activating Mutation Using Droplet Digital PCR. Clin Cancer Res 2015;21:3552-60. [Crossref] [PubMed]
- Yu HA, Arcila M, Hellmann M, et al. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol 2014;25:423-8. [Crossref] [PubMed]
- Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med 2008;359:366-77. [Crossref] [PubMed]
- Tan WL, Jain A, Takano A, et al. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol 2016;17:e347-62. [Crossref] [PubMed]
- Sandler A, Gray R, Perry MC, et al. Paclitaxel–carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med 2006;355:2542-50. [Crossref] [PubMed]
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer: AVAiL. J Clin Oncol 2009;27:1227-34. [Crossref] [PubMed]
- Naumov GN, Nilsson MB, Cascone T, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res 2009;15:3484-94. [Crossref] [PubMed]
- Johnson BE, Kabbinavar F, Fehrenbacher L, et al. ATLAS: randomized, double-blind, placebo-controlled, phase IIIB trial comparing bevacizumab therapy with or without erlotinib, after completion of chemotherapy, with bevacizumab for first-line treatment of advanced non–small-cell lung cancer. J Clin Oncol 2013;31:3926-34. [Crossref] [PubMed]
- Herbst RS, Ansari R, Bustin F, et al. Efficacy of bevacizumab plus erlotinib versus erlotinib alone in advanced non-small-cell lung cancer after failure of standard first-line chemotherapy (BeTa): a double-blind, placebo-controlled, phase 3 trial. Lancet 2011;377:1846-54. [Crossref] [PubMed]
- Ciuleanu T, Tsai CM, Tsao CJ, et al. A phase II study of erlotinib in combination with bevacizumab versus chemotherapy plus bevacizumab in the first-line treatment of advanced non-squamous non-small cell lung cancer. Lung Cancer 2013;82:276-81. [Crossref] [PubMed]
- Ichihara E, Hotta K, Nogami N, et al. Phase II Trial of Gefitinib in Combination with Bevacizumab as First-Line Therapy for Advanced Non–Small Cell Lung Cancer with Activating EGFR Gene Mutations: The Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol 2015;10:486-91. [Crossref] [PubMed]
- Seto T, Kato T, Nishio M, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): an open-label, randomised, multicentre, phase 2 study. Lancet Oncol 2014;15:1236-44. [Crossref] [PubMed]
- Rosell R, Dafni U, Felip E, et al. Erlotinib and bevacizumab in patients with advanced non-small-cell lung cancer and activating EGFR mutations (BELIEF): an international, multicentre, single-arm, phase 2 trial. Lancet Respir Med 2017;5:435-44. [Crossref] [PubMed]
- Hata A, Katakami N, Kaji R, et al. Afatinib (Afa) plus bevacizumab (Bev) combination after acquired resistance (AR) to EGFR-tyrosine kinase inhibitors (TKIs) in EGFR-mutant non-small cell lung cancer (NSCLC): Multicenter single arm phase II trial (ABC-study). J Clin Oncol 2017;35:9034.
- Liu Y, Sun L, Xiong ZC, et al. Meta-analysis of the impact of de novo and acquired EGFR T790M mutations on the prognosis of patients with non-small cell lung cancer receiving egFr-TKis. Onco Targets Ther 2017;10:2267. [Crossref] [PubMed]
- Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer Consensus Statement on Optimizing Management of EGFR Mutation–Positive Non–Small Cell Lung Cancer: Status in 2016. J Thorac Oncol 2016;11:946-63. [Crossref] [PubMed]
- Jain A, Lim C, Gan EM, et al. Impact of smoking and brain metastasis on outcomes of advanced EGFR mutation lung adenocarcinoma patients treated with first line epidermal growth factor receptor tyrosine kinase inhibitors. PloS One 2015;10:e0123587. [Crossref] [PubMed]
- Ramalingam S, Reungwetwattana T, Chewaskulyong B, et al. LBA2_PROsimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with EGFRm advanced NSCLC: FLAURA. Ann Oncol 2017;28:v605-49. [Crossref]
- Sorber L, Zwaenepoel K, Deschoolmeester V, et al. Circulating cell-free nucleic acids and platelets as a liquid biopsy in the provision of personalized therapy for lung cancer patients. Lung Cancer 2017;107:100-7. [Crossref] [PubMed]


