Radial endobronchial ultrasonography with distance measurement through a thin bronchoscope for the diagnosis of malignant peripheral pulmonary lesions
Introduction
With the widespread use of low-dose computed tomography (CT), increasing numbers of peripheral pulmonary lesions (PPLs) are being discovered. Determining whether such lesions are benign or malignant is of the utmost importance (1). Aside from surgery, the most common procedures for PPL diagnosis include CT-guided thoracic biopsy and transbronchial biopsy (TBB). Although the former procedure has relatively high diagnostic sensitivity and specificity (2), it has disadvantages of a high rate of pneumothorax (15–43%, with 4–18% requiring drainage) and a high risk of bleeding (1–27%) (3,4). TBB under X-ray fluoroscopy has a relatively low diagnostic rate and is no longer recommended (5). In 2002, the diagnosis of PPLs with radial probe endobronchial ultrasound (rEBUS) was reported with a diagnostic rate of 80% (6). In 2004, Kurimoto et al. (7) reported for the first time that EBUS with a guide sheath (EBUS-GS) can further increase diagnostic accuracy; however, there is the disadvantage that covering the ultrasound probe with the GS increases its diameter and the bronchoscope becomes stiffer after GS insertion into the bronchoscope channel. Thus, it is difficult to reach the openings of distal bronchioles, which have greater curvatures (8). In addition, a GS is expensive as a disposable supply. An alternative method is TBB guided by EBUS with distance measurement (EBUS-D) (9). Many studies have confirmed the effectiveness and safety of this method (8,10-13), and it has been shown that the use of this technique in fine bronchoscopy can further increase the diagnostic rate (53% vs. 73%) (10,11).
Therefore, during the diagnosis of PPLs, we use rEBUS-D and a thin bronchoscope to guide TBB. The diagnostic efficiency of this method has been previously reported (9,11). However, the present study used postoperative pathology as the gold standard for the diagnosis of lung cancer, which allows the study results to be confirmed more objectively and accurately.
Methods
Patients
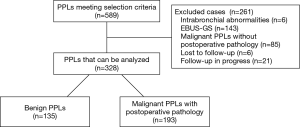
The characteristics of all patients with PPLs who underwent rEBUS examination at the Third Affiliated Hospital of Soochow University between October 28, 2013, and November 30, 2016, were retrospectively analyzed. The following two criteria were required for inclusion in the study: (I) PPLs on thoracic CT examination; and (II) at least one rEBUS examination. The exclusion criteria were: (I) bronchial lesions (bronchial stenosis or congestion and edema) on routine bronchoscopic examination by thin bronchoscope (outer diameter, 4.0 or 4.2 mm); (II) patients that underwent EBUS-GS-TBB; (III) patients with malignant lesions without postoperative pathology; and (IV) patients lost to follow-up or whose follow-up is in progress with the final diagnosis unknown. Figure 1 is a flowchart with the specific details regarding study inclusion.
This study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University (No. 2014077).
Procedures
The procedure employed a BF-P260F or BF-P290 flexible bronchoscope (outer diameter, 4 or 4.2 mm; biopsy channel, 2 mm), an EU-ME1 ultrasound processor, a MAJ-935 probe-driving unit, and a 1.4 or 1.7 mm outer diameter ultrasound probe (UM-S20-17S or UM-S20-20R, 20 MHz). The above equipment was manufactured by Olympus (Tokyo, Japan). The outer diameter of the single-use biopsy forceps was 1.8 mm. All procedures were performed by three pulmonary physicians familiar with the procedures.
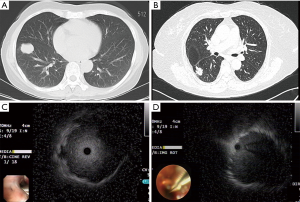
CT images were thoroughly read before the procedure to confirm the location of the target bronchus. The lesion size, region and lobe in which the lesion was present, and whether the bronchus sign was positive on CT were recorded. The regions of the lungs were divided as previously reported in the literature (14). Because there were relatively few peripheral lesions located in the central region, central region lesions were included statistically as intermediate region lesions in this study. The positive bronchus sign is shown in Figure 2A and the negative bronchus sign is shown in Figure 2B. After inhalation of 2% lidocaine, patients underwent routine bronchoscopic examination for observation of the central airway. If no intrabronchial abnormalities were found, the bronchoscope was extended into the target bronchial sub-segment or sub-sub-segment, and the ultrasound probe was inserted via the bronchoscope biopsy channel until the operator felt resistance, at which point the ultrasound was turned on. Next, screen images were observed as the probe was slowly withdrawn. After the discovery of typical lesions on imaging, the probe needed to be inserted and withdrawn several times to confirm that the probe had been inserted into the lesion and that the best position was reached. The relationship between the probe and lesion was recorded (Figure 2C shows a probe located at the center of a lesion and Figure 2D shows a probe adjacent to a lesion). The distance between the lesion and target bronchiole was measured according to methods described in the literature (9); the specific details of the procedure have been described previously (12). Chest radiographs were taken 2–4 hours after biopsy to determine the presence or absence of pneumothorax. If no typical lesion was found after searching for 30 min with rEBUS, the patient’s diagnosis was deferred and the procedure was terminated. Intraoperative and postoperative adverse events, such as chest pain, bleeding, pneumothorax, fever, and postoperative lung infection, were documented.
Diagnosis
TBB samples were diagnosed by pathology as obviously malignant (various types of malignant tumors or malignant tumors that cannot be classified) or as having benign characteristics (large amounts of inflammatory cell infiltration, granulomatous inflammation, and so on); these were diagnosed as rEBUS-D-TBB-positive. Heterotypic cells, chronic mucosal inflammation, and fibrous hyperplasia were diagnosed as negative. The gold standards for final diagnosis were as follows. For malignant tumors, the pathology report for the surgically resected specimen was the gold standard; for benign lesions, the gold standard was the pathology report for the surgically resected specimen or the lesion must have shrunk by over 2/3 after treatment or during follow-up.
Study endpoints
The primary endpoints were the sensitivity, specificity, positive predictive value, negative predictive value, and diagnostic accuracy of EBUS-D-TBB in the diagnosis of malignant PPLs. Secondary endpoints were factors affecting the diagnosis rate (location and size of lesions, bronchus sign, and relationship between the ultrasound probe and lesion) and adverse events.
Statistical methods
SPSS 22.0 software was used for the statistical analysis. All measurement data are shown as the mean ± standard deviation, and count data are shown as rates. A logistic regression analysis was used to determine single-factor and multi-factor effects on the diagnosis. The least significant difference (LSD) method was used for pairwise comparisons. P<0.05 was considered statistically significant.
Results
General characteristics
EBUS examination was conducted on 589 PPLs. Six cases were eliminated because of intrabronchial abnormalities on bronchoscopic examination, 143 cases were eliminated because they underwent EBUS-GS-TBB for a clinical study, 85 cases were eliminated because of the presence of un-operated malignant tumors, six cases were lost to follow-up, and follow-up was still in progress in 21 cases. The remaining 328 cases met the gold standard for diagnosis and were included in the study. Among the 193 cases of malignant tumors, the patients were aged 62.83±8.88 years on average. There were 103 male patients and 90 female patients and the mean lesion diameter was 29.04±11.69 mm. Among the 135 cases of benign tumors, the patients were aged 59.87±9.38 years on average. There were 97 male patients and 38 female patients and the mean lesion diameter was 33.62±15.64 mm. Malignant tumors accounted for 58.84% of the total number of samples.
Diagnosis status
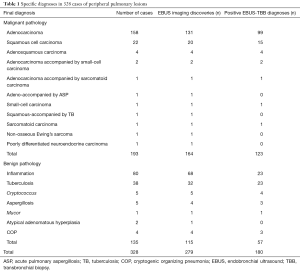
The discovery rate with radial ultrasound was 85.06% (279/328) and the overall diagnosis rate with EBUS-D-TBB was 54.88% (180/328). Of these, the diagnosis rate of benign lesions was 42.22% (57/135) and the diagnosis rate of malignant lesions was 63.73% (123/193). The sensitivity of EBUS-D-TBB in diagnosing malignant PPLs was 63.73% [95% confidence interval (CI), 56.88–70.6%], the specificity was 100%, the positive predictive value was 100%, the negative predictive value was 65.85%, and the diagnostic accuracy was 78.40%. Specific details of the diagnoses are shown in Table 1.
Full table
Factors influencing EBUS-D-TBB diagnoses of PPLs
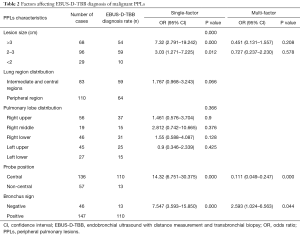
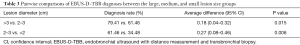
The effects of lesion location and size, the bronchus sign, and relationship between the ultrasound probe and lesion on the diagnosis are shown in Table 2. The single-factor analysis showed that the region and lobar distribution of the lesions had no statistically significant effect on the diagnosis (P>0.05). The diagnostic rate when the ultrasound probe was located at the center of the lesion was higher than that when the probe was adjacent to the lesion or not near the lesion at all (80.88% vs. 22.81%, P=0.000). The diagnostic rate when the bronchus sign was positive on thoracic CT was higher than that when the bronchus sign was negative (74.83% vs. 28.26%, P=0.000). The effect of lesion size on the diagnosis rate was also statistically significant (P=0.000). Table 3 compares the diagnosis rates between the >3, 2–3, and <2 cm lesion diameter groups, and shows that the diagnosis rate of the >3 cm group was higher than that of the 2–3 cm group (79.41% vs. 61.46%, P=0.015) and the diagnosis rate of the 2–3 cm group was higher than that of the <2 cm group (61.46% vs. 34.48%, P=0.006). Finally, the three statistically significant factors from the single-factor analysis were included in the multi-factor analysis, which showed that ultrasound probe position and the bronchus sign on thoracic CT were independent influencing factors with P values of 0.000 and 0.044, respectively.
Full table
Full table
Complications
Statistics on complications included the benign and malignant pathology groups. There was 50–100 mL of blood loss after lesion biopsy in a total of 13 cases 3.96% (13/328). The patients improved after intravenous pituitrin and a local injection of epinephrine and iced saline. Postoperative hemoptysis, all as blood in the sputum, occurred in 43 cases for which no special management was needed. Intraoperative chest pain occurred in three cases and the pain disappeared after the procedure. Pneumothorax, infection, and hypoxia were not observed.
Other conditions
In two TBB cases, there was clear evidence of benign lesions with granulomatous lesions in one case and inflammatory activity in the other case. Postoperatively, these lesions were found to be squamous cell carcinoma accompanied by tuberculosis and adenocarcinoma accompanied by aspergillosis, respectively. In addition, postoperative pathology showed a total of seven cases of mixed carcinomas, among which there were four cases of adenosquamous carcinoma, two cases of adenocarcinoma accompanied by small-cell carcinoma, and one case of adenocarcinoma accompanied by sarcomatoid carcinoma. TBB pathology did not suggest the presence of two types of malignant tumors.
Discussion
Recently, to improve the diagnosis of PPLs, an increasing number of new techniques related to bronchoscopy have been employed in the clinic, including ultrathin bronchoscope, rEBUS, virtual bronchoscopy, and electromagnetic navigation bronchoscopy (15). Among these, radial ultrasound has obvious roles in discovering and locating PPLs. This technique has a sensitivity of 73% and a specificity of 100% for the diagnosis of malignant pulmonary tumors (16). In addition, the rEBUS-GS technique can improve the biopsy accuracy rate (7). To reduce costs, we employed rEBUS-D as a substitute for rEBUS-GS diagnoses of PPLs.
The present study showed that rEBUS-D-TBB through a thin bronchoscope for the diagnosis of malignant PPLs had a sensitivity of 63.73%, specificity of 100%, positive predictive value of 100%, negative predictive value of 65.80%, and diagnostic accuracy of 78.40%. The diagnostic sensitivity was lower than that reported previously in the literature (73%) (16) and in our previous report (75%) (12), which may be because cases in which lesions were not operated on were eliminated from the present study. However, it was significantly higher than that of EBUS-D-TBB through a therapeutic bronchoscope (17) (malignant lesion diagnosis rate of 26.5%, EBUS discovery rate of 63.9%, and diagnosis rate of 60% after EBUS). The reason for this may be that a thin bronchoscope can enter soft level 5 and level 6 bronchioles and approaches peripheral lesions more closely, which increases the chance of the ultrasound discovery of peripheral lesions. In addition, extending a thin bronchoscope to distal bronchioles partially substitutes for the function of a GS and reduces the chance of inserting the biopsy forceps into the wrong bronchiole.
Because postoperative pathology served as the diagnostic gold standard for malignant tumors in the present study, comparisons of TBB sample pathology with postoperative sample pathology revealed some notable situations. Malignant tumors can be combined with benign lesions, such as squamous cell carcinoma combined with tuberculosis and adenocarcinoma combined with aspergillosis in the present study; and the present study had a mixed carcinoma rate of 3.63% (7/193). Bronchoscopic biopsy samples are small and may suggest a diagnosis of only one disorder. Therefore, small samples are a limitation of TBB that must be fully recognized. If the TBB pathological diagnosis and clinical presentation or treatment outcome do not match, further samples should be obtained.
Factors influencing the diagnosis rate included lesion size, the presence or absence of the bronchus sign on CT, and the relationship between the ultrasound probe and lesion. The diagnosis rates for lesions with diameters >30, 20–30, and <20 mm were 77.94%, 62.50%, and 34.48%, respectively. The differences in pairwise comparisons of the diagnostic rates between the three groups were statistically significant. This suggests that lesions with diameters of <20 mm had low positive rates on EBUS-D-TBB and other diagnostic methods should be considered. The presence of the bronchus sign on thoracic CT (74.83% vs. 28.26%) and positioning of the ultrasound probe at the center of the lesion (80.88% vs. 46.43%) increased the diagnosis rate, consistent with reports in the literature (18,19). The present study showed that lesions in the central and intermediate regions of the lung and lesions in different lobes of the lung did not have statistically significantly different diagnosis rates. There have been reports that the diagnosis rate in the left upper lobe was 40%, which was significantly lower than the diagnosis rate of 76% in other locations (7). Previous studies may have used therapeutic bronchoscopes that were difficult to insert into the distal segments of the bronchioles in the upper lobes of the lung.
The primary adverse event in the present study was bleeding after biopsy. There was blood loss of 50–100 mL in 13 cases (3.96%), which improved after local treatment combined with intravenous pituitrin. Although there was chest pain in three cases, postoperative chest radiographs did not reveal complications such as pneumothorax or infection, and we suspect that the pleura were pulled during the biopsy. These results suggest that TBB with the measurement method after rEBUS positioning is safe.
In summary, TBB using rEBUS-D through a thin bronchoscope for the diagnosis of malignant PPLs had high sensitivity, 100% specificity, and a high level of safety. Lesions over 2 cm in size, a positive bronchus sign on thoracic CT, and ultrasound probe positioning at the center of the lesion increased the positive rate. The medical costs are far lower compared with those of EBUS-GS, the rate of pneumothorax is lower compared with that of CT-guided thoracic biopsy, and the status of the airway can still be clearly determined. Therefore, in the current environment of a relative lack of medical funding in China, EBUS-D-TBB examination with a thin bronchoscope is a relatively good choice for the diagnosis of malignant PPLs in appropriate cases, including lesions size >2 cm, clear bronchus sign on CT scan or CT-guided thoracic biopsy not possible (20). However, the TBB sample size was small, the biopsy margins were limited, and a diagnosis may be missed in cases of mixed disease, which warrants attention.
Acknowledgements
We thank all the physicians and endoscopy suite personnel at the Third Affiliated Hospital of Soochow University for their assistance and the recruitment of subjects.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the ethics committee of the Third Affiliated Hospital of Soochow University (No. 2014077).
References
- National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Rivera MP, Mehta AC, Wahidi MM. Establishing the diagnosis of lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e142S-65S.
- Khan MF, Straub R, Moghaddam SR, et al. Variables affecting the risk of pneumothorax and intrapulmonal hemorrhage in CT-guided transthoracic biopsy. Eur Radiol 2008;18:1356-63. [Crossref] [PubMed]
- Wiener RS, Schwartz LM, Woloshin S, et al. Population-based risk for complications after transthoracic needle lung biopsy of a pulmonary nodule: an analysis of discharge records. Ann Intern Med 2011;155:137-44. [Crossref] [PubMed]
- Rivera MP, Mehta AC; American College of Chest Physicians. Initial diagnosis of lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:131S-48S.
- Herth FJ, Ernst A, Becker HD. Endobronchial ultrasound-guided transbronchial lung biopsy in solitary pulmonary nodules and peripheral lesions. Eur Respir J 2002;20:972-4. [Crossref] [PubMed]
- Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004;126:959-65. [Crossref] [PubMed]
- Fuso L, Varone F, Magnini D, et al. Role of ultrasound-guided transbronchial biopsy in the diagnosis of peripheral pulmonary lesions. Lung Cancer 2013;81:60-4. [Crossref] [PubMed]
- Chung YH, Lie CH, Chao TY, et al. Endobronchial ultrasonography with distance for peripheral pulmonary lesions. Respir Med 2007;101:738-45. [Crossref] [PubMed]
- Huang CT, Ho CC, Tsai YJ, et al. Factors influencing visibility and diagnostic yield of transbronchial biopsy using endobronchial ultrasound in peripheral pulmonary lesions. Respirology 2009;14:859-64. [Crossref] [PubMed]
- Huang CT, Tsai YJ, Liao WY, et al. Endobronchial ultrasound-guided transbronchial biopsy of peripheral pulmonary lesions: how many specimens are necessary? Respiration 2012;84:128-34. [Crossref] [PubMed]
- Zhang S, Zhou J, Zhang Q, et al. Endobronchial ultrasonography with distance by thin bronchoscopy in diagnosing peripheral pulmonary lesions. Zhonghua Jie He He Hu Xi Za Zhi 2015;38:566-9. [PubMed]
- Zhang SJ, Zhang M, Zhou J, et al. Comparison of radial endobronchial ultrasound with a guide sheath and with distance by thin bronchoscopy for the diagnosis of peripheral pulmonary lesions: a prospective randomized crossover trial. J Thorac Dis 2016;8:3112-8. [Crossref] [PubMed]
- Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000;117:1049-54. [Crossref] [PubMed]
- Asano F. Advanced bronchoscopy for the diagnosis of peripheral pulmonary lesions. Respir Investig 2016;54:224-9. [Crossref] [PubMed]
- Steinfort DP, Khor YH, Manser RL, et al. Radial probe endobronchial ultrasound for the diagnosis of peripheral lung cancer: systematic review and meta-analysis. Eur Respir J 2011;37:902-10. [Crossref] [PubMed]
- Durakovic A, Andersen H, Christiansen A, et al. Retrospective analysis of radial EBUS outcome for the diagnosis of peripheral pulmonary lesion: sensitivity and complications. Eur Clin Respir J 2015;2:28947. [Crossref] [PubMed]
- Guvenc C, Yserbyt J, Testelmans D, et al. Computed tomography characteristics predictive for radial EBUS-miniprobe-guided diagnosis of pulmonary lesions. J Thorac Oncol 2015;10:472-8. [Crossref] [PubMed]
- Okachi S, Imai N, Imaizumi K, et al. Factors affecting the diagnostic yield of transbronchial biopsy using endobronchial ultrasonography with a guide sheath in peripheral lung cancer. Intern Med 2016;55:1705-12. [Crossref] [PubMed]
- Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017;6:23-34. [Crossref] [PubMed]


