Rationale and early outcomes for the management of thymoma with proton therapy
Introduction
Thymic cancers, including thymoma and thymic carcinoma, are the most common primary malignancy in the anterior mediastinum (1). Thymoma is the dominant type with an indolent natural course of disease and a long survival often extending over 10 years (2). Despite its rarity, several large meta-analyses of cohort studies have been published demonstrating improved survival rates by the addition of postoperative external-beam radiotherapy (EBRT), especially for incompletely resected (3), locally advanced (3-5), or phenotypically aggressive thymic cancer (6). In a surgically inoperable situation, EBRT with chemotherapy also proved useful in producing a durable local control and, in some cases, converting the tumor into an operable one (7).
The primary challenge for using EBRT for thymic cancer is radiation toxicity to vital intrathoracic organs such as the heart, lungs, and esophagus. Furthermore, the younger age of onset of thymomas, 40–60 years of age, and excellent survival outcome under current multimodality treatment, means radiation-induced second malignancies have been of concern for survivors (8,9). Although few studies exist on radiation dose and toxicities among thymomas, much data can be extrapolated from studies of mediastinal irradiation in lymphoma, for which a wide range of acute and late pulmonary and cardiac toxicities have been described, including radiation pneumonitis, pericarditis, congestive heart failure, valvular disease, and myocardial infarction (10-13). Additionally, studies of locally advanced non-small cell lung cancer have demonstrated a correlation of heart dose to patient survival (14,15). In fact, the National Comprehensive Cancer Network (NCCN) guidelines for thymomas suggest the mean total dose to the heart should be as low as reasonably achievable to potentially maximize survival (16).
Conventional photon-based radiotherapy, including intensity-modulated radiotherapy (IMRT), is limited by its inability to spare most of the heart and lungs from radiation when targeting the anterior mediastinal compartment. In comparison, proton therapy (PT) has demonstrated dosimetric advantages compared to IMRT in case reports and small case series. Here we report dosimetric and clinical outcomes of six consecutive patients with thymic malignancies who were treated with PT at our institution under a prospective protocol.
Methods
Patient selection and follow-up
From January 2008 to March 2017, six patients with de novo thymic malignancies were treated with postoperative or definitive PT after consenting to a prospective institutional review board-approved outcomes tracking protocol at the University of Florida Health Proton Therapy Institute. The study was conducted in accordance with the Helsinki Declaration. Two patients treated early on received part of their treatment with photons, while the other four received just PT.
Clinical outcomes, pathology, treatment dose, acute toxicities, and follow-up information were analyzed. Baseline patient, disease, and treatment characteristics are listed in Table 1. Follow-up was weekly during PT treatment and then at the discretion of the treating physician, but recommended for every 3 months for 2 years, then every 6 months for an additional 3 years, and then annually. Patient follow-up time was calculated from the PT start date to the date of last follow-up.
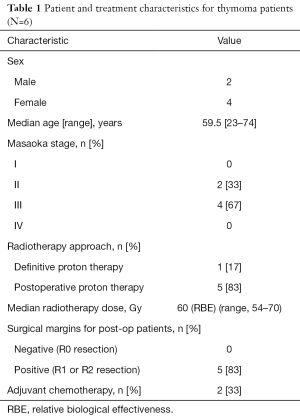
Full table
Radiation treatment planning
All patients underwent 4-dimensional computed tomography (CT)-based treatment simulation and planning, with the exception of one patient who was treated with the breath-hold technique. CT simulation was completed in the supine position with arms extended above the head. If available, diagnostic positron emission tomography (PET)-CT images were fused with simulation CT scans for preoperative tumor volume delineation. Gross tumor volume (GTV) was defined as radiographic disease at the time of diagnosis. Clinical target volume (CTV) was defined as the postoperative bed or GTV +5 mm for definitive cases. The internal target volume was defined as the CTV on the 10 phases of the 4-dimensional CT scan or for one patient who underwent treatment with breath-hold, was a 5-mm superior-inferior margin on the CTV. Finally, the internal target volume was uniformly expanded by 5 mm to create the planning target volume (PTV). Normal structures contoured included the heart, lungs, esophagus, and breasts for female patients.
All patients had PT plans generated with a passive-scattering system. Both PT and IMRT plans were generated for all patients. Both treatment plans were designed to optimize tumor (PTV) coverage and minimize exposure to normal structures. All IMRT and PT plans were required to have at least 95% of the PTV covered by the prescription dose and 99% of the PTV receiving at least 93% of the prescription dose while no hot spot received over 120% of the prescription doses. The following normal tissue toxicity constraints were used when generating the treatment plans: the hard constraint was 0.1 cm3 of the spinal cord receiving a maximum of 50 Gy; other relative constraints included a mean dose to the bilateral lungs receiving a maximum of 20 Gy, bilateral lung V20 maximum of 30%, mean heart
Dosimetric analysis and statistical analysis
Dose-volume histograms (DVH) were generated from each IMRT and proton plan for target volumes and normal structures, including the heart, esophagus, bilateral breasts, and lungs. The mean doses for these structures and the bilateral lungs V20 were calculated using Eclipse Treatment Planning System (Varian Medical Systems, Palo Alto, CA, USA). These dosimetric datasets (one from the IMRT plan and the other from a proton plan for each patient) were compared using a two-tailed paired t-test; a P value ≤0.05 being significant.
Toxicities
All acute toxicities were graded per the Common Terminology Criteria for Adverse Events (CTCAE) version appropriate at diagnosis and treatment, and then retrospectively re-graded using CTCAE, v4, for the present study.
Results
Treatments
Among the six patients treated with PT for thymoma, two received part of their treatment with photon radiation and four received just PT. The median age at diagnosis was 59.5 years (range, 23–74 years), two patients had Masaoka stage II disease, and four had stage III. One patient was treated with definitive PT and five were treated postoperatively. All patients who underwent a thymoma resection had positive surgical margins (R1 or R2 resection) on final pathology. The median PT dose was 60 Gy (RBE) (range, 54–70 Gy).
Clinical outcomes
The median follow-up was 2.6 years (range, 0.8–4.9 years) and the local control rate was 100%. At last follow-up, two patients developed recurrences out of field, including one in the lung and liver and the other within the lung. Both of these patients died, one of disease 44 months following treatment, while the other died of pre-existing co-morbidities 58 months following treatment. These were also the two patients who received combination photons and protons.
No patients with de novo disease experienced grade ≥3 toxicities after PT. Five patients developed grade II radiation dermatitis. One patient developed grade II esophagitis.
Dosimetric comparison
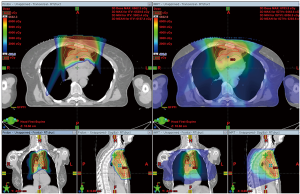
Representative colorwash dose distributions for PT and IMRT are depicted in Figure 1. Compared with the IMRT plans, the PT plans on average reduced the mean heart dose by 36.5%, mean lung dose by 33.5%, and lung V20 by 27.7%, all of which were significant (Table 2). The mean doses to the bilateral breast tissue were calculated in four female patients. The PT plan was superior in sparing breast tissue doses in only two patients, while the IMRT plan was better in the other two patients.

Full table
Discussion
Over the last few decades, while the role of EBRT in treating anterior mediastinal malignancies like lymphoma, thyroid cancer, and germ cell tumors has been diminishing (17), EBRT remains a critical part of the standard of care for advanced stage thymoma and thymic carcinoma (6). However, using EBRT to treat thymoma requires great care with consideration of the risks of acute and late side effects. In a prospective phase II study on induction chemoradiation for unresectable thymic cancers, 77% of patients achieved R0 resection after induction therapies at the expense of 41% experiencing acute grade 3 and 4 toxicities, including cardiac arrest (7).
EBRT for anterior mediastinal malignancies has been technically challenging due to its close proximity to vital organs such as the breasts, lungs, heart, and esophagus, damage to which can result in numerous acute and late radiation-related side effects. PT has long been investigated in treating mediastinal disease such as lung cancer and lymphoma. The dosimetric advantages and favorable toxicity profiles of PT treatment have been reported (18-20) and, in the case of lymphoma, collectively summarized in a recent review from the Particle Therapy Cooperative Group (20). Yet, owing to the rarity of thymic malignancies, only a few case reports and small series have been published on the use of PT for thymic tumors (21-23). Parikh et al. evaluated the dosimetric differences between a 3-dimensional conformal PT plan and a volume-modulated arc therapy photon plan for four thymoma cases treated with curative intent. The proton plans consistently delivered lower mean doses to the lung, esophagus, and heart. In particular, the mean heart dose was 6 Gy for the proton plans, a relative 42% reduction compared to the volume modulated arc therapy plans. Similarly, a 43% reduction in mean lung dose and 74% reduction in mean esophageal dose were reported using PT (22). Vogel et al. reported similar dosimetric advantages using PT (23). In the present study, we found 36.5% reduction of the mean heart dose, 60% reduction of the mean esophagus dose, 33.5% reduction in the mean lung dose, and 27.5% improvement in lung V20. Doses to the heart, lung, and esophagus from the PT plans observed in the present study were similar to other studies, as demonstrated in Table 3.
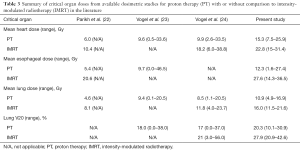
Full table
Given the sparse data on thymomas and thymic cancers, we must look to studies analyzing more common thoracic malignancies to inform our understanding of how PT can reduce the radiation dose to the organs at risk in patients with thymoma. Several dosimetric studies on historic radiotherapy trials for mediastinal Hodgkin lymphoma suggest that mean heart dose is correlated with an increased risk of late adverse cardiovascular events (13,25). Mean heart dose was also found to be significantly associated with increased cardiotoxicity among breast cancer patients (26) and lung cancer survivors with locally advanced disease (14). According to these studies, the average relative risk of cardiac events increases by 1.5% to 7.4% for every 1 Gy increase in mean heart dose; more importantly, there appears to be no minimally safe mean cardiac dose for breast or intrathoracic irradiation involving the mediastinum (14,26,27). For example, in a combined analysis of prospective multicenter trials for locally advanced non-small cell lung cancer, a rate of 15% for grade 3 cardiotoxicities was reported among 125 enrolled patients. The study found a 7% increase of grade 3 cardiotoxicities within 2 years of radiotherapy for every 1 Gy increase in mean heart dose, corresponding to an absolute risk of 10% for grade 3 cardiotoxicity with 23 Gy of mean heart dose among patients without prior cardiac history (14). Interestingly, had the patients in this study been treated with IMRT, the average mean heart dose would have been around 23 Gy, which could have resulted in a 10% grade 3 cardiotoxicity rate (21). By comparison, since the patients on average had a mean heart dose of 15 Gy with PT, the absolute risk would be roughly 5%, or reduced by about 50% based on the predictive model of that report. Similarly, Vogel et al. estimated a relative 45% reduction in the risk of major cardiac events with PT compared to IMRT based on a predictive model developed for breast cancer patients (24).
Long-term thymoma survivors also face an increased risk of second malignancies, such as intrathoracic lymphoma and lung cancer (28,29). Secondary cancer from radiotherapy was best demonstrated in Hodgkin lymphoma patients treated before the 1990s where mediastinal irradiation employed a dose close to that for thymoma (40–50 Gy). For example, an early study revealed a 17% risk of second malignancies 20 years after radiotherapy, with a 77% rate of second solid cancer within or adjacent to the radiation fields, most commonly in the lung and breast (30). Since PT can reduce the radiation dose to the different organs, we would expect a reduction in risk in second cancers. In fact, Vogel et al. reported an estimated five fewer second malignancies per 100 thymoma survivors by treating them with protons instead of IMRT (23).
Clinical outcomes of patients with thymic malignancies treated with PT are sparse in the literature. However, early clinical outcomes with PT demonstrate an acceptable rate of disease control. At 2.6 years, our local control was excellent, and comparable to a single-institution prospective study by Vogel et al. reporting clinical outcomes of thymoma patients treated with PT at the University of Pennsylvania (Philadelphia). Their 3-year regional control and overall survival rates were 96% and 94%, respectively.
Similar to our patients treated for de novo disease, Vogel et al. reported no grade 3 or greater acute toxicity. Only one patient with a history of two prior thoracic radiotherapy courses experienced grade 2 radiation pneumonitis. Other grade 2 toxicities included dermatitis (37%), fatigue (11%), and esophagitis (7%). Favorable acute toxicity profiles from PT were also reported by Parikh et al. Acute clinical toxicity outcomes for their four patients treated with PT for thymoma illustrated no grade 3 or greater toxicities. Radiation dermatitis was the only grade 2 toxicity reported.
The present study has several limitations. First, the PT technique used in this study was 3-dimensional conformal double-scattered PT, which might be slightly inferior to the pencil-beam scanning (PBS) technique (31). PBS allows for modulated individual proton beams to improve normal-tissue sparing and reduce neutron contamination to a patient’s entire body (32). As the pencil-beam technique becomes more widely available, it will be possible to reduce the dose to breast tissue in future patients. Also, breast dose comparisons between IMRT and PT were difficult owing to the few number of female patients in the analysis (n=4) as well as their advanced age, which influenced the prioritization of heart dose over breast dose. Furthermore, in some cases, we saw huge reductions in mean breast dose >10 Gy when breast tissue wasn’t prioritized, while in other cases in which breast dose was prioritized the breast dose was a bit higher with protons. The findings of our study were also limited by the small number of enrolled patients and the short follow-up. While dosimetric studies and early clinical data have shown advantages with PT to treat intrathoracic malignancies, including thymoma and thymic carcinoma, individual proton centers have faced difficulty in accruing patients for single-institution studies (33). Therefore, future proton studies, especially those studying rare malignancies like thymoma, should involve multiple institutions.
In conclusion, when comparing PT to IMRT, we found significant dose-sparing to the heart, lung, and esophagus while achieving good local control with PT. Newer techniques such as PBS may help further reduce the breast dose.
Acknowledgements
We would like to acknowledge Keri Hopper (RN) and Lana Cook for excellent patient care for the thymoma patients on this study, Robin Cacchio (RN) for her administrative research assistance, as well as Jessica Kirwan and Chris Stich for excellent editorial assistance.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was conducted in accordance with the Helsinki Declaration. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Davis RD Jr, Oldham HN Jr, Sabiston DC Jr. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987;44:229-37. [Crossref] [PubMed]
- de Jong WK, Blaauwgeers JL, Schaapveld M, et al. Thymic epithelial tumours: a population-based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008;44:123-30. [Crossref] [PubMed]
- Jackson MW, Palma DA, Camidge DR, et al. The Impact of Postoperative Radiotherapy for Thymoma and Thymic Carcinoma. J Thorac Oncol 2017;12:734-44. [Crossref] [PubMed]
- Lim YJ, Kim E, Kim HJ, et al. Survival Impact of Adjuvant Radiation Therapy in Masaoka Stage II to IV Thymomas: A Systematic Review and Meta-analysis. Int J Radiat Oncol Biol Phys 2016;94:1129-36. [Crossref] [PubMed]
- Zhou D, Deng XF, Liu QX, et al. The Effectiveness of Postoperative Radiotherapy in Patients With Completely Resected Thymoma: A Meta-Analysis. Ann Thorac Surg 2016;101:305-10. [Crossref] [PubMed]
- Komaki R, Gomez DR. Radiotherapy for thymic carcinoma: adjuvant, inductive, and definitive. Front Oncol 2014;3:330. [Crossref] [PubMed]
- Korst RJ, Bezjak A, Blackmon S, et al. Neoadjuvant chemoradiotherapy for locally advanced thymic tumors: a phase II, multi-institutional clinical trial. J Thorac Cardiovasc Surg 2014;147:36-44, 46.e1.
- Myojin M, Choi NC, Wright CD, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. Int J Radiat Oncol Biol Phys 2000;46:927-33. [Crossref] [PubMed]
- Berman AT, Litzky L, Livolsi V, et al. Adjuvant radiotherapy for completely resected stage 2 thymoma. Cancer 2011;117:3502-8. [Crossref] [PubMed]
- Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA 2003;290:2831-7. [Crossref] [PubMed]
- Aleman BM, van den Belt-Dusebout AW, De Bruin ML, et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878-86. [Crossref] [PubMed]
- van Nimwegen FA, Ntentas G, Darby SC, et al. Risk of heart failure in survivors of Hodgkin lymphoma: effects of cardiac exposure to radiation and anthracyclines. Blood 2017;129:2257-65. [Crossref] [PubMed]
- Maraldo MV, Giusti F, Vogelius IR, et al. Cardiovascular disease after treatment for Hodgkin's lymphoma: an analysis of nine collaborative EORTC-LYSA trials. Lancet Haematol 2015;2:e492-502. [Crossref] [PubMed]
- Dess RT, Sun Y, Matuszak MM, et al. Cardiac Events After Radiation Therapy: Combined Analysis of Prospective Multicenter Trials for Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2017;35:1395-402. [Crossref] [PubMed]
- Faivre-Finn C. Dose escalation in lung cancer: have we gone full circle? Lancet Oncol 2015;16:125-7. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Thymomas and Thymic Carcinomas, Version 1.2017. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines).
- Bokemeyer C, Nichols CR, Droz JP, et al. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol 2002;20:1864-73. [Crossref] [PubMed]
- Hoppe BS, Hill-Kayser CE, Tseng YD, et al. Consolidative proton therapy after chemotherapy for patients with Hodgkin lymphoma. Ann Oncol 2017;28:2179-84. [Crossref] [PubMed]
- Chang JY, Verma V, Li M, et al. Proton Beam Radiotherapy and Concurrent Chemotherapy for Unresectable Stage III Non-Small Cell Lung Cancer: Final Results of a Phase 2 Study. JAMA Oncol 2017;3:e172032. [Crossref] [PubMed]
- Tseng YD, Cutter DJ, Plastaras JP, et al. Evidence-based Review on the Use of Proton Therapy in Lymphoma From the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee. Int J Radiat Oncol Biol Phys 2017;99:825-42. [Crossref] [PubMed]
- Figura N, Hoppe BS, Flampouri S, et al. Postoperative proton therapy in the management of stage III thymoma. J Thorac Oncol 2013;8:e38-40. [Crossref] [PubMed]
- Parikh RR, Rhome R, Hug E, et al. Adjuvant Proton Beam Therapy in the Management of Thymoma: A Dosimetric Comparison and Acute Toxicities. Clin Lung Cancer 2016;17:362-6. [Crossref] [PubMed]
- Vogel J, Lin L, Litzky LA, et al. Predicted Rate of Secondary Malignancies Following Adjuvant Proton Versus Photon Radiation Therapy for Thymoma. Int J Radiat Oncol Biol Phys 2017;99:427-33. [Crossref] [PubMed]
- Vogel J, Lin L, Simone CB 2nd, et al. Risk of major cardiac events following adjuvant proton versus photon radiation therapy for patients with thymic malignancies. Acta Oncol 2017;56:1060-4. [Crossref] [PubMed]
- Hahn E, Jiang H, Ng A, et al. Late Cardiac Toxicity After Mediastinal Radiation Therapy for Hodgkin Lymphoma: Contributions of Coronary Artery and Whole Heart Dose-Volume Variables to Risk Prediction. Int J Radiat Oncol Biol Phys 2017;98:1116-23. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation Dose-Response Relationship for Risk of Coronary Heart Disease in Survivors of Hodgkin Lymphoma. J Clin Oncol 2016;34:235-43. [Crossref] [PubMed]
- Parzen JS, Bates JE, Milano MT, et al. Survival after subsequent non-Hodgkin's lymphoma and non-small cell lung cancer in patients with malignant thymoma. J Thorac Dis 2016;8:3605-13. [Crossref] [PubMed]
- Pan CC, Chen PC, Wang LS, et al. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406-11. [Crossref] [PubMed]
- Nyandoto P, Muhonen T, Joensuu H. Second cancer among long-term survivors from Hodgkin's disease. Int J Radiat Oncol Biol Phys 1998;42:373-8. [Crossref] [PubMed]
- Zeng C, Plastaras JP, James P, et al. Proton pencil beam scanning for mediastinal lymphoma: treatment planning and robustness assessment. Acta Oncol 2016;55:1132-8. [Crossref] [PubMed]
- Hall EJ. Intensity-modulated radiation therapy, protons, and the risk of second cancers. Int J Radiat Oncol Biol Phys 2006;65:1-7. [Crossref] [PubMed]
- Zhu HJ, Nichols RC Jr, Henderson RH, et al. Proton therapy in stage II-IV non-small cell lung cancer: pattern of care and impact on trial accrual. Acta Oncol 2018;57:692-3. [Crossref] [PubMed]


