Strategies for first-line immunotherapy in squamous cell lung cancer: are combinations a game changer?
The initial treatment for metastatic squamous cell non-small cell lung cancer (NSCLC) consists of platinum-based combination chemotherapy. The most commonly used regimens are carboplatin and paclitaxel, carboplatin and protein-bound paclitaxel (nab-paclitaxel), and cisplatin and gemcitabine. In November 2015, the U.S. Food and Drug Administration (FDA) approved necitumumab, a fully human IgG1 monoclonal antibody that targets epidermal growth factor receptor (EGFR), for the first-line treatment of metastatic squamous cell NSCLC, in combination with cisplatin and gemcitabine (1). Still, the clinical utility of necitumumab is limited due to the high cost of the drug, the added toxicity when combined with cisplatin and gemcitabine and the limited clinical benefit, with a 16% reduction of the risk of death in comparison to chemotherapy alone (1). Immunotherapy has now emerged as an approach to combat, among other tumors, squamous cell NSCLC (2).
Pembrolizumab, a fully human IgG4 anti-programmed cell death-1 (PD-1) monoclonal antibody, has shown clinical efficacy in lung cancer patients, particularly those with high PD-L1 expression (3-5). Pembrolizumab is approved for the first-line therapy of squamous and non-squamous cell NSCLC patients with programmed cell death ligand-1 (PD-L1) of at least 50% tumor proportion score. The drug is also approved for the treatment of patients after progression to first-line chemotherapy, if there is at least 1% PD-L1 expression on tumor cells (6). In most trials that compare anti-PD-1/L1 antibodies with chemotherapy (5,7-12), progression-free survival (PFS) and overall survival (OS) curves are overlapping at early time points (13). One biological explanation for this could be that immunotherapy needs some time to demonstrate its effect and, patients with rapidly progressive disease, lack an effective adaptive immune response. Cytotoxic chemotherapy can delay progression and allow immunotherapy to elicit its treatment effect (13). Indeed, we saw this in the PACIFIC study, in which the anti-PD-L1 antibody durvalumab significantly prolonged the PFS of stage III NSCLC patients who had previously received chemoradiotherapy (14). Now, combination strategies with chemotherapy and immunotherapy in NSCLC are ongoing or have been completed, with evidence that they may be the way to go ahead with the treatment of this disease. In the case of non-squamous cell NSCLC, the combination of pembrolizumab with platinum-pemetrexed has been tested in a phase II (KEYNOTE-021) (15) and a phase III (KEYNOTE-189) (16) clinical trial. In May 2017, FDA approved pembrolizumab in combination with pemetrexed-carboplatin for the first-line treatment of metastatic non-squamous cell NSCLC, irrespective of PD-L1 expression based on the tumor response rate and PFS results of the KEYNOTE-021 (6). Continued approval for this indication is contingent and FDA has now granted priority review for the results of the phase III KEYNOTE-189, which confirmed a PFS and OS benefit compared to chemotherapy alone in patients with non-squamous cell NSCLC, independent of PD-L1 expression (16).
In the 2018 ASCO Annual Meeting, the results of the KEYNOTE-407 (17) followed on the heels of the KEYNOTE-189 clinical trial. A total of 559 treatment naïve patients with stage IV squamous cell NSCLC were enrolled and randomized 1:1 to receive pembrolizumab with chemotherapy (carboplatin-paclitaxel/nab-paclitaxel) or chemotherapy alone (17). PD-L1 expression was not required for the entry in the study, but before randomization, patients were stratified based on three criteria: PD-L1 expression (<1% vs. ≥1%), the choice of the taxane (paclitaxel vs. nab-paclitaxel) and race (East Asia versus the rest of the world). The treatment consisted of four cycles of chemotherapy combined with either pembrolizumab or placebo followed by maintenance with the anti-PD-1 antibody or placebo. The patients in the placebo arm who developed disease progression were allowed to crossover to the pembrolizumab arm at any time (17).
With a median follow-up of 7.8 months (range, 0.1–19.1), median OS was 15.9 months for the pembrolizumab plus chemotherapy arm compared to 11.3 months for the chemotherapy arm [hazard ratio (HR) 0.64, 95% confidence interval (CI): 0.49–0.85; P=0.0008] (17) (Table 1). The survival benefit of the combination remained in all subgroups of patients with the biggest benefit for female vs. male (HR 0.42, 95% CI: 0.22–0.81 vs. HR 0.69, 95% CI: 0.51–0.94), and East Asian population vs. patients from the rest of the world (HR 0.44, 95% CI: 0.22–0.89 vs. HR 0.69, 95% CI: 0.51–0.93). As far as PD-L1 expression concerns, the OS benefit of the combination was consistent among patients with low (<1%; HR 0.61, 95% CI: 0.38–0.98), intermediate (1–49%; HR 0.57, 95% CI: 0.36–0.90) or high (≥50%; HR 0.64, 95% CI: 0.37–1.10) PD-L1 expression (17) (Table 1). The investigators reported a statistically significant 1.6 months improvement in PFS with pembrolizumab plus chemotherapy vs. chemotherapy alone (6.4 vs. 4.8 months; HR 0.56, 95% CI: 0.45–0.70; P<0.0001), across all PD-L1 expression subgroups (17) (Table 1). There was a higher objective response rate (58.4% vs. 35.0%; P=0.0004) and more durable responses (median, 7.7 vs. 4.8 months) with pembrolizumab plus chemotherapy versus chemotherapy alone (17). Adverse events occurred with a similar frequency between the two arms (overall adverse events, 98.2% vs. 97.9% and grade 3–5 adverse events, 69.8% vs. 68.2%), but immune-related adverse events (like hypothyroidism, hyperthyroidism and pneumonitis) were more frequent when pembrolizumab was added to chemotherapy (overall immune-related adverse events, 28.8% vs. 8.6% and grade 3–5 immune-related adverse events 10.8% vs. 3.2%) (17).
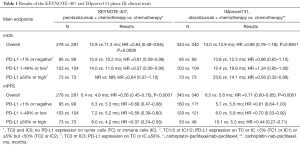
Full table
In the same meeting, the interim OS results of the IMpower131 phase III clinical trial, did not look favorable for the anti-PD-L1 antibody, atezolizumab plus carboplatin-paclitaxel/nab-paclitaxel in patients with newly diagnosed stage IV squamous cell NSCLC (18). The IMpower131 has a different design from the KEYNOTE-407, with 1021 patients randomly assigned to one of three arms: atezolizumab plus carboplatin/paclitaxel (Arm A), atezolizumab plus carboplatin/nab-paclitaxel (Arm B), or carboplatin/nab-paclitaxel (Arm C) (18). In the first analysis of investigator-assessed PFS, there was a PFS benefit with the addition of atezolizumab to chemotherapy that, similar to the KEYNOTE-407, emerged across all patient subgroups evaluated, including all PD-L1 expressing subgroups (18) (Table 1). However, the IMpower131 did not show a difference in median OS between Arm B and Arm C (14.0 vs. 13.9 months). Although the median OS trended favorably for Arm B in the high PD-L1 expressing subgroup (23.6 vs. 14.1 months; HR 0.56, 95% CI: 0.32–0.99) there was an unexpected worse median OS for the low PD-L1 expressing subgroup with the addition of atezolizumab to chemotherapy (12.4 vs. 16.6 months; HR 1.34, 95% CI: 0.95–1.90) (18) (Table 1). In summary, atezolizumab combined with chemotherapy for the first-line therapy of squamous cell NSCLC reduces the risk of disease progression by 29% compared to chemotherapy alone (18), when at the same time pembrolizumab in the same setting reduces the risk of disease progression by 44% and the risk of death by 36% compared to chemotherapy alone (17).
The above results raise several thoughts and concerns. If it is a matter of chemotherapy plus immunotherapy induced immunogenic cell death that leads to immune memory and a sustained long-term response (19,20), then why we do not have similar results from the KEYNOTE-407 and IMpower131? As it was discussed during the 2018 ASCO Annual Meeting, maybe with a longer follow-up, by the time of the final analysis, a difference may emerge for the atezolizumab combination in the IMpower131 study (18). At least in breast cancer models, it has been shown that chemotherapy may have an immunotherapy countertherapeutic effect by inducing hypoxia and the expression of proteins like CD47, CD73 and PD-L1, that ultimately cause T-cell anergy and increase the intratumoral ratio of regulatory/effector T-cells (21). In the squamous cell NSCLC subgroup of the KEYNOTE-024 trial, pembrolizumab alone reduced the risk of death by 65% compared to chemotherapy, for patients with high (≥50%) PD-L1 expression (5). In both the KEYNOTE-407 (17) and IMpower131 (18) trials, in the same group of patients, the combination of pembrolizumab or atezolizumab with chemotherapy cut that same risk by 44%. Caution should be taken, considering the recent restriction of pembrolizumab and atezolizumab by the European Medicines Agency as first-line therapy only for locally advanced or metastatic urothelial cancer patients with high PD-L1 expression (22). This is a surprise, considering that, initially, PD-L1 expression was not correlated with response for both pembrolizumab (23) and atezolizumab (24). Both drugs showed reduced survival compared to chemotherapy for treatment-naïve patients with low PD-L1 expression KEYNOTE-361 (NCT02853305) and IMvigor130 (NCT02807636) trials (22). Finally, the cost effectiveness vs. the affordability of the treatments should be also taken into account.
Acknowledgements
Funding: Work in Dr Rosell’s laboratory is partially supported by a grant from La Caixa Foundation, an Instituto de Salud Carlos III grant (RESPONSE, PIE16/00011) and a Marie Skłodowska-Curie Innovative Training Networks European Grant (ELBA No. 765492).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Thatcher N, Hirsch FR, Luft AV, et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): an open-label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763-74. [Crossref] [PubMed]
- Karachaliou N, Rosell R. Science and biology drives the immune system to cure lung cancer patients: a revolution but not without challenges. Ther Adv Med Oncol 2018;10. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Doroshow DB, Herbst RS. Treatment of Advanced Non-Small Cell Lung Cancer in 2018. JAMA Oncol 2018;4:569-70. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373:1803-13. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med 2017;376:1015-26. [Crossref] [PubMed]
- Alexander BM, Schoenfeld JD, Trippa L. Hazards of Hazard Ratios - Deviations from Model Assumptions in Immunotherapy. N Engl J Med 2018;378:1158-9. [Crossref] [PubMed]
- Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:1919-29. [Crossref] [PubMed]
- Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016;17:1497-508. [Crossref] [PubMed]
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Paz-Ares LG, Luft A, Tafreshi A, et al. Phase 3 study of carboplatin-paclitaxel/nab-paclitaxel (Chemo) with or without pembrolizumab (Pembro) for patients (Pts) with metastatic squamous (Sq) non-small cell lung cancer (NSCLC). J Clin Oncol 2018;36:105. [Crossref]
- Jotte RM, Cappuzzo F, Vynnychenko I, et al. IMpower131: Primary PFS and safety analysis of a randomized phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel as 1L therapy in advanced squamous NSCLC. J Clin Oncol 2018;36. [Crossref]
- Gebremeskel S, Johnston B. Concepts and mechanisms underlying chemotherapy induced immunogenic cell death: impact on clinical studies and considerations for combined therapies. Oncotarget 2015;6:41600-19. [Crossref] [PubMed]
- Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014;11:24-37. [Crossref] [PubMed]
- Samanta D, Park Y, Ni X, et al. Chemotherapy induces enrichment of CD47(+)/CD73(+)/PDL1(+) immune evasive triple-negative breast cancer cells. Proc Natl Acad Sci U S A 2018;115:E1239-48. [Crossref] [PubMed]
- Gourd E. EMA restricts use of anti-PD-1 drugs for bladder cancer. Lancet Oncol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Balar AV, Castellano D, O'Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18:1483-92. [Crossref] [PubMed]
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017;389:67-76. [Crossref] [PubMed]


