
Stereotactic ablative body radiation for oligometastatic and oligoprogressive disease
Introduction
One area in which stereotactic ablative body radiation (SABR) has developed as a viable treatment modality over the past decade has been in metastatic disease. Several related categories of this malignancy have been defined. Oligometastasis is the setting of limited metastases, with the definition of “limited” varying by study. The most common classification is 3 or less metastatic lesions (1,2), though studies with one to eight sites of disease exist. Oligoprogression refers to malignancy that has progressed in a limited number of sites, while oligo-recurrence occurs after definitive treatment for locoregional disease, followed by the manifestation of metastases in a limited fashion. Retrospective and prospective trials have focused on all three of these scenarios. The aim of the current manuscript is to review the underlying biologic rationale and clinical data for SABR in the setting of oligometastases and oligoprogression to provide a sense of the current state of ablative radiation in these settings, with regard to which patients may be candidates for this approach, what high impact trials are currently addressing this issue, and where future directions may lie.
The role of SABR in the context of the oligometastatic biologic state
Emerging preclinical and clinical evidence over the past two decades support the existence of a distinct oligometastatic biologic state. First described in the landmark review of 1995 by Weichselbaum and Hellman (3) and updated in 2011 with more recent results (4), an underlying premise for this clinical entity is that multiple steps are required for metastases to occur (5). These include loss of cellular adhesion, increased motility, entrance and survival in the circulation, and entrance/survival into distant sites. If any of these steps are disrupted by the host, or if the tumor does not have the capacity to complete an individual step, the process of metastasis is disrupted. Furthermore, cellular and host factors may predispose a tumor to only invade and colonize in certain structures, thus leading to a full and almost continuous spectrum of disease that spans from completely localized to widely disseminated. Another approach to understanding the biologic state is to compare it to Darwinian evolution on a more macroscopic scale (5). Specifically, several host factors influence survival and progression of disease, such as hypoxemia, growth factors, external exposures, and the inherent immune response, which are then balanced with the “fitness” of an individual malignant cell to survive in any given structure. The balance that is achieved between the “environment” (e.g., host) and the “organism” (e.g., tumor cell) then dictates where on the spectrum of metastasis an individual tumor lies (6).
Several lines of evidence exist to support these broad hypotheses. In one study, autopsies and genomic profiling were performed on seven patients with pancreatic cancer who had dissemination to several metastatic sites. The investigators then compared the accumulation of mutations in individual lesions, to determine if patterns could be identified. They found that mutations accumulated in a manner that was dependent on the site of metastasis, thus suggesting a “hierarchy” of metastasis to the peritoneum, the liver, and finally the lung. They then used mathematical modeling to determine the time from transformation to a tumor cell, to the formation of a parental clone, to progression to subclones with metastatic capacity, and finally disseminated metastases (7). These findings thus support a “spectrum” theory of metastasis.
In another collection of studies, Weichselbaum and colleagues have identified microRNAs preclinically that appear to mediate an oligometastatic phenotype (8,9). In one study, the investigators identified three microRNAs in the 14q32 cluster that suppressed several steps of metastases, including cellular adhesion and invasion. Furthermore, these factors inhibited metastatic development in animal models of lung colonization through breast cancer (8). In another study, Weichselbaum and colleagues found that patients who failed to develop oligometastases could be identified through unique features of the microRNA-200 family. Through development of an oligometastatic-polymetastatic xenograft model, the authors found that enhancement of microRNA-200c resulted in polymetastatic progression (9). Data such as these support the mechanism of specific molecular factors determining the extent of progression along the metastatic spectrum in an individual patient. If they could ultimately be targeted, there is great potential for both limiting the extent of metastasis in an individual patient and identifying a predictive biomarker for oligometastasis that could be used to select patients for aggressive local therapy.
Indeed, there is an increasing rationale for utilizing SABR in the setting of oligometastasis and oligoprogression due to two major systemic therapy developments. First, the advent of targeted therapy has improved prognosis greatly for patients with metastatic disease, such that patients who initially present with polymetastases can then experience remarkable responses that result in a small number of active sites. The second scenario has arisen with the incorporation of immunotherapy into large populations of patients with metastatic disease. Indeed, several lines of preclinical data support the abscopal effect of radiation therapy (RT) in this context, and that RT can affect immune upregulation (10,11). Thus, the combination of radiation and immunotherapy could have synergistic local and synergistic distant effects in eradicating metastatic disease. These concepts are currently being explored in ongoing prospective trials.
Data supporting aggressive consolidation therapy in the oligometastatic state
Observational trials (retrospective and single-arm prospective studies)
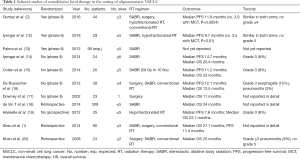
Most studies that have assessed the role of local consolidative therapy (LCT) in the setting of oligometastases have been retrospective and single-arm prospective trials (Table 1) (5). In comparative effectiveness analyses, multiple studies have demonstrated a benefit in progression-free survival (PFS) ± overall survival (OS) for patients with up to 5 sites of disease. For example, the previously cited meta-analysis of seven observational cohort trials demonstrated a 2-year survival rate of 52.1% in patients with up to 5 synchronous metastases receiving “aggressive thoracic therapy”, defined as surgery or radiotherapy >40 Gy, compared to those patients that did not receive this treatment (21). The MD Anderson propensity-matched retrospective trial comparing 90 patients between 1998 and 2012 with up to 3 metastatic lesions who received comprehensive local therapy vs. those that did not demonstrated an OS benefit, with a hazard ratio of 0.37 (1). And in a secondary analysis of two prospective studies assessing patients with both oligometastatic and polymetastatic disease, patients who received more aggressive radiation to the primary tumor, defined as a dose of ≥63 Gy, had better OS, with a 3-year OS rate of 17% vs. 2% in those patients that did not undergo this treatment (22). These findings were similar to another report demonstrating that both higher dose to the tumor, again defined as a threshold of ≥63 Gy, was associated with improved OS (23). Taken together, studies such as these suggest that oligometastases represents a unique disease entity for which aggressive treatment can improve OS. It was this rationale through an analysis of the 94,708 patients in the International Association for the Study of Lung Cancer (IASLC) database that informed the proposed revision for the 8th edition of the TNM staging system, in which oligometastatic disease is classified as a new “M1b” category, defined relatively narrowly as a single metastatic lesion (24).
Full table
Limitations of non-randomized prospective studies
While mounting clinical evidence has supplemented the biologic data for an oligometastatic state that should be classified and treated differently than polymetastatic disease, it is important to understand the unique limitations of the data within this context (5). These caveats have been described well in a recent review on this topic (25). The first is that on the spectrum of consensus levels of evidence, retrospective and single-arm prospective trials (with comparisons to historical controls) are low on this scale. Second, a specific issue to analyses of LCT in the setting of oligometastases is the issue of immortal time bias (5). Specifically, when comparing two groups of patients that have oligometastatic disease, one of which has received aggressive local therapy and one that has not, the group that has received surgery/radiation by definition needed to survive long enough to undergo this treatment to be included in this group. Therefore, patients that succumb early to the disease or progress early enough that local therapy is not an option will be excluded. This period in which patients cannot fail treatment is referred to as immortal time (5). It is thus unclear from examining observational data alone if the apparent superiority with aggressive local treatment is secondary to an actual causative effect in the setting of indolent disease, or whether the patients that are treated with aggressive therapy appear to fare better because in these cohorts of patients, examined retrospectively, the selection of patients for aggressive treatment occurred preferentially because of favorable prognostic factors. While some of these characteristics can be controlled in multivariate analysis (performance status, number of sites of disease) others, such as the treating physician’s general impression of a patient, are not as easily incorporated into statistical analyses (5).
Barriers to randomized trials and goals in designing effective randomized clinical studies
Over the past decade, there have been several randomized studies that have been designed with the purpose of comparing standard of care (e.g., maintenance therapy) with LCT in the setting of oligometastatic disease (5). Until recently, however, all of these trials had been closed due to poor accrual (26). The lack of accrual was likely due to several reasons. First, in the context of retrospective data suggesting a benefit there was a lack of equipoise among many physicians with regards to optimal treatment (5). Second, many patients may have been hesitant to be randomized to clinical trials of LCT vs. maintenance therapy due to the off-trial option of LCT. Prior analyses have demonstrated that if an off-trial option in the experimental arm is available, randomized studies become less efficient, with accrual time increasing substantially (27). Finally, many studies may have included relatively stringent inclusion criteria or treatment regimens, attempting to answer a more specific scientific question at the expense of accrual (5).
From these previous studies, several features can be incorporated into future randomized studies to enhance accrual. These include limiting off-trial availability options in the experimental arm when a randomized study is open, broadening inclusion criteria to take into account varying practices among physicians and institutions, presuming that the overall scientific question is not compromised, and educating both participating physicians and potential patients about the current level of evidence supporting the experimental regimen (5). In addition, in a secondary analysis of three prospective studies, it was found that the earlier a patient is approached for participation into a clinical trial, the more likely they are to agree to randomization (28). This finding is likely secondary to patients anticipating randomization early in treatment, versus having a preconception about the optimal approach based on initial discussions such that there is increased resistance to potentially being randomized to an alternative arm.
One important question when designing randomized trials in oligometastatic non-small cell lung cancer (NSCLC) is what endpoints are clinically relevant? While OS is certainly the “gold standard,” other endpoints could certainly have utility in defining the role of LCT, though with reduced time to accrual. The primary alternative to OS has been PFS, and certainly many oncologic therapeutic regimens has received Food and Drug Administration approval and have been incorporated into national guidelines as a result of meeting this endpoint (5). One limitation to PFS in the context of surgery/RT for metastatic disease is that it could be argued that the impact is attenuated because patients treated with an aggressive regimen will inherently have less failure at the sites that have been ablated. That is, patients in the alternative systemic therapy arm will be at risk for recurrence in all sites of disease, whereby patients in the LCT arm will have a minimal risk of failure in the sites that have undergone ablation (29). Therefore, alternative endpoints may be useful in strengthening PFS data. These include time to failure in a new lesion, quality of life (QOL) endpoints, and cost-effectiveness data. Incorporation of these endpoints as secondary aims will potentially provide stronger evidence than PFS alone that LCT is a high-quality treatment option (5).
Randomized trials
To the best of our knowledge, the first multi-institutional, randomized, phase II study was recently completed comparing the role of aggressive LCT in patients with ≤3 metastases who did not progress on standard front-line systemic therapy (FLST)/observation (Table 1) (5). The hypothesis was that consolidative therapy would improve PFS in this setting (2). Eligible patients required: (I) histologic confirmation of NSCLC; (II) stage IV; (III) ≤3 sites of disease at post-FLST; and (IV) no RECIST progression during FLST or before randomization. Appropriate FLST was defined as either ≥4 cycles of platinum doublet therapy or ≥3 months of epidermal growth factor receptor (EGFR)/anaplastic lymphoma kinase (ALK) inhibitor for patients with EGFR mutations/ALK rearrangements, respectively. Patients were randomized to either LCT (chemo-radiation or surgical resection of all sites) ± ongoing maintenance therapy/observation (MT/O) vs. MT/O alone. The MT/O was physician choice (from predefined standard-of-care options). Randomization was balanced dynamically on five prognostic factors: number of metastases, response to FLST, central nervous system (CNS) metastases, intrathoracic nodal status, and EGFR/ALK status. The primary endpoint was PFS, powered to detect an increase from 4 to 7 months (HR =0.57) using intention-to-treat (ITT) analysis (5). The planned study size was 94 randomized patients, with interim analysis planned at 44 patients. PFS and OS were assessed, and in an exploratory analysis, the time to developing a new lesion was compared between the two arms with a log-rank test [time to new site failure (TNSF)].
On the recommendation of the Data Safety Monitoring Board, the study was terminated early due to efficacy with LCT. Forty-nine patients were randomized from 2/2013–1/2016. Of the patients that underwent randomization to the LCT arm, 20% of them underwent surgery as part of their LCT regimen. Eight patients had EGFR/ALK alterations. The median follow-up time for PFS was 18.7 months for all patients. The median PFS time in the LCT arm was 11.9 (90% CI: 5.72–20.90) months, compared to 3.9 (90% CI: 0.18–0.66) months in the no LCT arm (HR =0.35; P=0.005) (2). The LCT arm also had improved TNSF, with 11.9 months compared to 5.7 months in the no-LCT arm (P=0.0497). Toxicity was similar between the two arms, with no grade 4–5 toxicity. OS data is immature, with only 14 deaths recorded. However, the investigators concluded that in patients with oligometastatic NSCLC without progression after FLST, immediate LCT improved PFS compared to standard treatment alone (5).
These results were further supported in another phase II randomized study examining the role of consolidative RT in the setting of oligometastases (primary + up to 5 metastatic sites). In this single-institution trial, patients received front-line systemic therapy and then were randomized to hypofractionated consolidative RT ± maintenance chemotherapy vs. maintenance chemotherapy alone (5). While this study was similarly terminated early, in the 29 patients that were randomized, there was found to be a substantial improvement and similar tripling in PFS for patients in the consolidation arm (12). Taken together, these studies support a PFS benefit with aggressive consolidation in the setting of limited metastatic NSCLC (5).
In addition to the trials above, the recently completed COMET study compares the efficacy of standard of care RT vs. ablative radiation (SABR) in the setting of multiple oligometastatic tumor types, defined as up to 5 lesions (5). In this Canadian study, patients are randomized to one of these two arms with a 2:1 ratio favoring the experimental arm, and a primary endpoint of OS. Secondary endpoints include QOL, toxicity, and PFS (13).
Ongoing randomized trials in the setting of oligometastatic NSCLC
Several current trials are examining the role of aggressive therapy in the setting of oligometastatic NSCLC. The SARON trial from the United Kingdom compares the treatment regimens of chemotherapy + RT vs. chemotherapy alone in the setting of oligometastatic NSCLC (NCT02417662). Planned enrollment for the trial is 340 patients, with a primary outcome of OS. In a phase II/III intergroup study being performed in the United States (NRG-LU002), patients with up to 3 sites of disease receive frontline systemic therapy (5). Patients that do not progress are then randomized in a 2:1 fashion to local consolidation therapy and maintenance systemic therapy vs. maintenance systemic therapy alone. In addition, other ongoing trials demonstrate the extrapolation of the indication and definition of oligometastatic NSCLC to determine which patient subsets may benefit from aggressive local therapy. An example of these are the LONESTAR and NORTHSTAR studies. In LONESTAR, patients with metastatic disease (no limit to number of sites) are treated with front-line nivolumab and ipilimumab and are then randomized to further immunotherapy vs. LCT followed by immunotherapy. The primary endpoint is OS, and as many sites can be treated as are clinically feasible. In the partner trial NORTHSTAR, a similar paradigm is utilized, though patients receive frontline osimertinib and are then randomized to consolidation to as many sites as are feasible vs. continued osimertinib, with a primary endpoint of PFS (5).
The choice between surgery and RT as consolidation is likely to be physician and patient dependent. None of the current data compares the two approaches, and the MD Anderson phase II study implies that either treatment can be used. Given that the phase III study in the United States also will include both modalities, if LCT is shown to provide a benefit, the likely conclusion will be that each patient should be evaluated by a multidisciplinary team and a tailored approach made for both the primary tumor and the metastatic site. Certainly, the recent influx of studies demonstrating a benefit to aggressive therapy in oligometastases has the potential to dramatically broaden the indications for surgical resection, not just in lung cancer but across a spectrum of primary tumor sites (5).
Selection of patients for treatment with oligometastases
Number of sites/lesions
The most common method used to stratify patients with oligometastatic disease utilizes the number of total metastatic sites or lesions. And indeed, much observational and single arm data suggests that a lower number of lesions is associated with improved prognosis (5). A phase II dose-escalation radiation trial from the University of Chicago assessed outcomes in multiple histologic types and found that the number of sites was the primary prognostic factor for survival (30). In a recent systematic review of seven eligible retrospective observational cohort studies, which included 668 patients with oligometastatic disease (defined by up to 5 synchronous metastases), the authors found that those patients with a single organ involvement had substantially improved OS, with a HR of 0.49 (95% CI: 0.31–0.75) (21). And in another study reporting on 156 patients with 228 brain metastases, the number of brain metastases was noted to be an independent prognostic factor for survival (31). Indeed, the threshold of oligometastatic disease is currently under debate, with some arguing that 4–5 metastases do not fit the criteria of oligometastases (5). However, this cohort of patients continues to be included in prospective clinical trials addressing this disease.
Synchronous vs. metachronous oligometastatic disease
Virtually all comparisons of synchronous vs. metachronous metastatic disease have demonstrated improved outcomes with metachronous lesions (5). One study assessed outcomes of 72 patients with synchronous vs. metachronous solitary brain metastases in NSCLC and found that patients with metachronous metastases had a median survival of 33.3 months, compared to 8.6 months with a synchronous lesion (P=0.001) (32). In a review of surgical outcomes in the setting of oligometastatic lung cancer, it was also found that most studies observed improved survival when surgery was performed for metachronous vs. synchronous disease (33). And in a systematic review of 757 patients in several institutions with stage IV NSCLC and 1–5 metastases, only metachronous metastases and lower N-stage were associated with an improved survival group in recursive partitioning analysis (34). These data highlight that, while the focus of many trials is that of synchronous disease, it is also important to explore optimal patient selection for aggressive treatment in the setting of oligorecurrence (5).
Intracranial vs. extracranial disease
Historically, intracranial oligometastases have been thought to represent a special subset of metastatic disease amenable to local therapy due to improved prognosis. Indeed, the highest-level data supporting aggressive treatment of oligometastatic disease is the randomized study comparing surgery + RT vs. RT alone in the setting of a single brain metastasis (5). Patients randomized to surgery + radiation had an improvement in OS of 40 vs. 15 weeks with radiation alone. Notably, of the 48 patients in this trial, 37 had lung cancer (35). The cooperative group trial RTOG 9508 also demonstrated an OS benefit of stereotactic body radiosurgery (SRS) + whole brain radiation therapy (WBRT) vs. WBRT alone in patients with 1–3 brain metastases, thus also suggesting that patients with brain metastases can have improved prognosis with more aggressive therapy. It is notable that 64% of patients in RTOG 9508 had a diagnosis of lung cancer (36). Other observational studies have also supported the potential role of aggressive therapy in the setting of a solitary brain metastasis, including a report from MD Anderson Cancer Center that found that patients with a single brain metastasis and N0 disease had a 3-year survival rate that approximated that of patients without metastasis (50%) (37).
It is notable that, while several lines of data have suggested that patients with intracranial metastases have improved prognosis with aggressive local therapy, there is not high-level data demonstrating that, comparing outcomes site by site and controlling for treatment given, the natural history of oligometastatic disease with an intracranial metastasis is more indolent than that of a single site elsewhere (5). In a propensity score-matched analysis of comprehensive local therapy in limited metastatic NSCLC, no difference was observed among the various metastatic sites (1). In fact, some of the basis behind the National Comprehensive Cancer Network (NCCN) guidelines advocating surgery or RT in the context of a solitary metastasis to the adrenal gland or brain (www.nccn.org) may be due to the relatively high incidence of a single metastasis to these sites and the data demonstrating that a subset of these patients can achieve long-term survival. Similar outcomes may be demonstrated in the future with a solitary metastasis in other locations such as the bone, liver, or abdominal lymph nodes (5).
Regional nodal involvement
Several studies have suggested that decreased regional extent of tumor improves prognosis in the setting of oligometastatic disease (5). Two of these studies have been cited above, that being the MD Anderson study examining aggressive treatment in the setting of a single brain metastasis demonstrating worse outcomes with N1 or N2 involvement (37), and the systematic review of 757 patients with oligometastatic disease (34). In the latter study, recursive partitioning analysis (RPA) demonstrated a 3-year OS of 36% with synchronous metastases and N0 disease vs. 13% in synchronous metastases and N1/N2 involvement (34). This correlation has also been demonstrated through at least one surgical series in which patients with synchronous metastatic disease underwent surgery to both sites of disease (38). Patients with regional disease may have worse outcomes both because of the more advanced nature of their malignancy as well as the lower likelihood of controlling intrathoracic disease (surgery/stereotactic body radiation therapy (SBRT) in early stage disease vs. chemoradiation with N2 involvement) (5).
Safety and technical considerations for SABR in oligometastatic disease
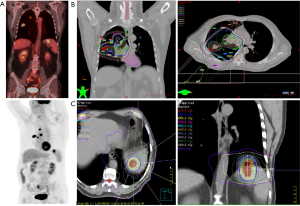
Most studies examining the role of radiation in oligometastases have utilized SABR. Dose/fractionation regimens have varied, but overall the results support adequate safety profiles in treating multiple sites of disease. In one of the first reports SABR for oligometastases, Salama and colleagues examined 29 patients with metastatic lesions treated to 56 sites between November 2004 and March 2007. Two patients experienced Grade 3 or higher toxicity, including one patient with radiation pneumonitis and one with persistent nausea/vomiting. Notably, though with small numbers, the authors found a dose response in the control of targeted tumors, 57% in those treated to 24 Gy (n=12) to 83% if treated to 36 Gy (n=5) (30). In a comprehensive review of SBRT for oligometastases in multiple disease sites, including lung cancer, Tree and colleagues demonstrated a low rate of high-grade toxicity (5% or less for most studies) (39). In general, we recommend that treating clinicians adhere to standard, fractionation-dependent radiation constraints (www.nccn.org), but that current data support treating multiple sites in appropriate clinical circumstances (Figure 1).
Conclusions
In summary, oligometastatic NSCLC may reflect a more indolent phenotype of disease amenable to definitive therapy. LCT has been shown to improve outcomes for these patients, as demonstrated by the growing body of supportive evidence. In particular, SABR is an effective and tolerable treatment modality for ablation of metastatic sites and should be considered a feasible tool in the management of oligometastatic NSCLC. We recommend that SABR be further utilized in the setting of randomized controlled trials in order to elucidate which subgroups of patients are most likely to benefit from local ablative therapy in the oligometastatic setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: Note that portions of this review have been previously published in the textbook Adult Chest Surgery, 3rd Edition, “The Role of Surgery in Oligometastatic Disease.”
References
- Sheu T, Heymach JV, Swisher SG, et al. Propensity score-matched analysis of comprehensive local therapy for oligometastatic non-small cell lung cancer that did not progress after front-line chemotherapy. Int J Radiat Oncol Biol Phys 2014;90:850-7. [Crossref] [PubMed]
- Gomez DR, Blumenschein GR Jr, Lee JJ, et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol 2016;17:1672-82. [Crossref] [PubMed]
- Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8-10. [Crossref] [PubMed]
- Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol 2011;8:378-82. [Crossref] [PubMed]
- Gomez D, Heymach J, Swisher S. The role of surgery in oligometastatic NSCLC. In: Sugarbaker DJ. Adult Chest Surgery, 3rd Edition. New York: McGraw-Hill Professional, 2018.
- Greaves M, Maley CC. Clonal evolution in cancer. Nature 2012;481:306-13. [Crossref] [PubMed]
- Yachida S, Jones S, Bozic I, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 2010;467:1114-7. [Crossref] [PubMed]
- Uppal A, Wightman SC, Mallon S, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget 2015;6:3540-52. [Crossref] [PubMed]
- Lussier YA, Xing HR, Salama JK, et al. MicroRNA expression characterizes oligometastasis(es). PLoS One 2011;6:e28650. [Crossref] [PubMed]
- Vanpouille-Box C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nature communications 2017;8:15618. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Iyengar P, Wardak Z, Gerber DE, et al. Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol 2018;4:e173501. [Crossref] [PubMed]
- Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): study protocol for a randomized phase II trial. BMC Cancer 2012;12:305. [Crossref] [PubMed]
- Iyengar P, Kavanagh BD, Wardak Z, et al. Phase II trial of stereotactic body radiation therapy combined with erlotinib for patients with limited but progressive metastatic non-small-cell lung cancer. J Clin Oncol 2014;32:3824-30. [Crossref] [PubMed]
- Collen C, Christian N, Schallier D, et al. Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 2014;25:1954-9. [Crossref] [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [Crossref] [PubMed]
- Downey RJ, Ng KK, Kris MG, et al. A phase II trial of chemotherapy and surgery for non-small cell lung cancer patients with a synchronous solitary metastasis. Lung Cancer 2002;38:193-7. [Crossref] [PubMed]
- de Vin T, Engels B, Gevaert T, et al. Stereotactic radiotherapy for oligometastatic cancer: a prognostic model for survival. Ann Oncol 2014;25:467-71. [Crossref] [PubMed]
- Hasselle MD, Haraf DJ, Rusthoven KE, et al. Hypofractionated image-guided radiation therapy for patients with limited volume metastatic non-small cell lung cancer. J Thorac Oncol 2012;7:376-81. [Crossref] [PubMed]
- Khan AJ, Mehta PS, Zusag TW, et al. Long term disease-free survival resulting from combined modality management of patients presenting with oligometastatic, non-small cell lung carcinoma (NSCLC). Radiother Oncol 2006;81:163-7. [Crossref] [PubMed]
- Li D, Zhu X, Wang H, et al. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. J Thorac Dis 2017;9:310-7. [Crossref] [PubMed]
- Su S, Hu Y, Ouyang W, et al. Might radiation therapy in addition to chemotherapy improve overall survival of patients with non-oligometastatic Stage IV non-small cell lung cancer?: Secondary analysis of two prospective studies. BMC Cancer 2016;16:908. [Crossref] [PubMed]
- Lopez Guerra JL, Gomez D, Zhuang Y, et al. Prognostic impact of radiation therapy to the primary tumor in patients with non-small cell lung cancer and oligometastasis at diagnosis. Int J Radiat Oncol Biol Phys 2012;84:e61-7. [Crossref] [PubMed]
- Eberhardt WE, Mitchell A, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revision of the M Descriptors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2015;10:1515-22.
- Palma DA, Salama JK, Lo SS, et al. The oligometastatic state - separating truth from wishful thinking. Nat Rev Clin Oncol 2014;11:549-57. [Crossref] [PubMed]
- Patel PR, Yoo DS, Niibe Y, et al. A call for the aggressive treatment of oligometastatic and oligo-recurrent non-small cell lung cancer. Pulm Med 2012;2012:480961. [Crossref] [PubMed]
- Hamilton EP, Lyman GH, Peppercorn J. Availability of experimental therapy outside oncology randomized clinical trials in the United States. J Clin Oncol 2010;28:5067-73. [Crossref] [PubMed]
- Logan JK, Tang C, Liao Z, et al. Analysis of Factors Affecting Successful Clinical Trial Enrollment in the Context of Three Prospective, Randomized, Controlled Trials. Int J Radiat Oncol Biol Phys 2017;97:770-7. [Crossref] [PubMed]
- Malik N, Palma D. Oligometastatic non-small cell lung cancer: Where do we go next? Lung Cancer 2017;106:145-7. [Crossref] [PubMed]
- Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res 2008;14:5255-9. [Crossref] [PubMed]
- Pessina F, Navarria P, Cozzi L, et al. Outcome appraisal of patients with limited brain metastases (BMs) from non small cell lung cancer (NSCLC) treated with different local therapeutic strategies: a single institute evaluation. Br J Radiol 2017;90:20170022. [Crossref] [PubMed]
- Flannery TW, Suntharalingam M, Kwok Y, et al. Gamma knife stereotactic radiosurgery for synchronous versus metachronous solitary brain metastases from non-small cell lung cancer. Lung Cancer 2003;42:327-33. [Crossref] [PubMed]
- Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [Crossref] [PubMed]
- Ashworth AB, Senan S, Palma DA, et al. An individual patient data metaanalysis of outcomes and prognostic factors after treatment of oligometastatic non-small-cell lung cancer. Clin Lung Cancer 2014;15:346-55. [Crossref] [PubMed]
- Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Hu C, Chang EL, Hassenbusch SJ 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer 2006;106:1998-2004. [Crossref] [PubMed]
- Billing PS, Miller DL, Allen MS, et al. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg 2001;122:548-53. [Crossref] [PubMed]
- Tree AC, Khoo VS, Eeles RA, et al. Stereotactic body radiotherapy for oligometastases. Lancet Oncol 2013;14:e28-37. [Crossref] [PubMed]


