
Brain metastases in oncogene-driven non-small cell lung cancer
Introduction
Molecular targeted therapy against mutated driver oncogenes such as epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) dramatically improved the outcome of patients with non-small cell lung cancer (NSCLC) (1,2). However, the prognosis is not yet satisfactory. One of the most significant causes for poor prognosis and quality of life (QOL) in advanced NSCLC is brain metastases (3). According to the literature, the major prognostic factors affecting the treatment outcome in patients with metastatic NSCLC include age, time from diagnosis, and the location and extension of the intracranial disease (4-7).
About 40–50% of the brain metastases originate from systemic lung cancers. Conversely, approximately 10–20% of NSCLC patients present with brain metastases at diagnosis (8,9). NSCLC with brain metastases has a poor overall survival (OS) (10). Two-thirds of brain metastases present with multiple lesions while remaining one-third present with solitary lesions. Major sites of NSCLC brain metastases are the cerebrum (80%), cerebellum (15%), and the brain stem (5%) (11). NSCLC patients with EGFR/ALK mutations appear to have a higher incidence (50–60%) of brain metastasis (12-15). In contrast, NSCLC with ROS1 translocations is reported to have a lower incidence (33%) of brain metastasis compared to that with EGFR/ALK (16).
The efficacy of platinum doublet chemotherapy (carboplatin and paclitaxel) which is the conventional treatment option is generally low (20%) (17). However, newer agents such as cisplatin + pemetrexed or carboplatin, paclitaxel plus bevacizumab showed better intracranial response rate [42% (18) and 61% (19), respectively] in the phase 2 trials. The standard of care for the treatment of NSCLC patients with a limited number of brain metastases has been local therapy either by surgical resection, whole-brain irradiation, and stereotactic radiosurgery (SRS). However, the newly developed tyrosine kinase inhibitors (TKIs) such as osimertinib or alectinib that can efficiently penetrate the blood-brain barrier (BBB) have demonstrated remarkable intracranial activity. Furthermore, the overall improvement of survival outcome for NSCLC patients increases the chances of developing cognitive dysfunctions induced by radiation. This further emphasizes the advantage of systemic treatment with targeted therapies over radiation therapies (12,13).
While developing new strategies to improve patient care in NSCLC, it is important to understand why oncogene-driven NSCLC have a high incidence of brain metastases. Further, the molecular mechanisms that lead to the development of brain metastases need to be identified. In addition, it is important to develop a treatment strategy that utilizes an ideal balance of targeted therapeutics that cannot penetrate the BBB and those that can cross the BBB for providing the best possible management of the disease without the risk of developing new brain lesions.
In this review, we describe the epidemiology and molecular background of brain metastases in driver-oncogene positive NSCLC. We also discuss what we have learned from the first-generation TKIs and how this has helped us develop the second and third-generation TKIs with improved BBB penetration capabilities for the management of brain metastases.
Mechanisms underlying brain metastasis
Development of clinical cancer metastases is a multistep process starting from an asymptomatic micrometastases initiating from single cancer cell colonization followed by invasion or extravasation leading to the development of symptomatic macro-metastases through proliferation, angiogenesis, and interaction with the microenvironment (20). Metastasis to the brain, unlike metastasis to other distal organ sites, involves the breach of the BBB, which is a physical, metabolic, and chemical separation of the blood and the cerebrospinal fluid in the central nervous system (CNS). The BBB is made up of endothelial cells connected via tight junctions, the basement membrane, pericytes, astrocytic foot process, and the transporter systems. The transporter systems consist of proteins, such as the ATP-binding cassette efflux-transporters (ABC-transporter), including the breast cancer resistance protein (BRCP) and the multidrug-resistant proteins [MDR; MDR-1 also known as P glycoprotein (P-gp)] (21-29). The BBB restricts the diffusion of microorganisms, pathogens, and toxins, as it obstructs the entry of particles which are over 500 Daltons. Interestingly, some cancer cells can cross the BBB through specific mediators.
In most brain metastases, the BBB is disrupted and appears to be different from the normal healthy BBB (30-33). The extent of BBB disruption is a key factor that affects the entry of anti-cancer agents into the CNS. Efficient treatment requires attaining targetable drug concentrations in the CNS. Therefore, effective control of brain lesions requires efficient drug delivery across the BBB.
Two main strategies used for efficient drug delivery across the BBB are chemical modifications of drugs to inhibit efflux-transporters and allow BBB penetration. It was reported that an mTOR/PI3K inhibitor (GNE-317) modified to bypass P-gp and BRCP activation improved treatment outcome in brain metastasis. In addition, it was also shown that agents that can penetrate the BBB controlled brain dormant cancer cells, other distal metastases, and brain lesions, while agents that cannot penetrate the BBB were not able to control brain lesions (34-37).
EGFR-driven NSCLCs
EGFR is a receptor tyrosine kinase receptor that normally activates several downstream pathways upon binding to the ligands such as EGF, or TGF-α. In NSCLC with mutated EGFR, the pathway is activated without ligand binding, and this activation facilitates survival and proliferation of cancer cells (38). Based on the results from the IPASS trial and several other clinical trials that selected patients based on the presence of EGFR mutations such as NEJ002 or WJTOG3405 (39-41), EGFR-TKI monotherapy has been established as the standard first-line treatment for these patients. However, life-time incidence of brain metastases in NSCLC patients with EGFR mutation is reported to be higher compared to those with wild-type EGFR (70% in EGFR+, 38% in EGFR−) (42). It is also noteworthy that 1 out of 3 EGFR+ NSCLC patients develops brain metastasis during their clinical course (43). The secondary mutation of the EGFR gene resulting in the substitution of threonine 790 to methionine (T790M) that has lower affinity to gefitinib/erlotinib and higher affinity to ATP (44) is responsible for acquired resistance in about 50% of the cases. However, brain metastases usually do nor harbor T790M, and the emergence of the cancer cells in the CNS is due to an insufficient concentration of EGFR-TKI, often referred to as pharmacokinetic resistance.
Among the 1st generation EGFR-TKIs, erlotinib has relatively better BBB penetration capabilities compared to gefitinib (Table 1) (14,43-51). In some patients who develop brain metastases/leptomeningeal disease after gefitinib treatment, switching to erlotinib results in intracranial tumor shrinkage or symptom alleviation. However, the effect is usually transient (52-60). Pulsatile high-dosing and dose-escalation of erlotinib were also shown to achieve more effective control of brain metastases (59), with limited efficacy.
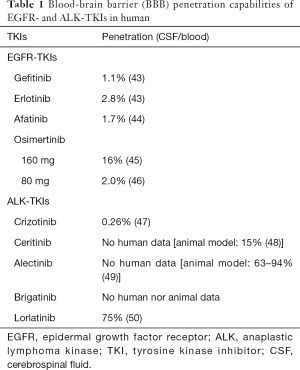
Full table
In contrast, the third-generation EGFR-TKI, osimertinib much more efficiently penetrated the BBB (58) (Table 1). A subset analysis of the results from the FLAURA trials that compared osimertinib with gefitinib or erlotinib as the first-line treatment of EGFR+ patients showed that CNS progression-free survival with osimertinib was significantly better [hazard ratio (HR) 0.48; 95% CI: 0.26–0.86] with manageable adverse effects (59,60).
ALK fusion-positive NSCLC
Patients with gene rearrangement in the ALK gene are also known to have a higher risk of brain metastases—23.8% at initial evaluation. The cumulative incidence of brain metastasis after diagnosis will sum up to 58.4% 3 years later (61).
Currently, there are five ALK-TKIs that are approved by the FDA for ALK-positive NSCLC, namely crizotinib (1st generation), alectinib, ceritinib, brigatinib (2nd generation), and lorlatinib (3rd generation). It is noteworthy that up to 74% of those who were treated with crizotinib develop brain metastases (62). The 2nd generation TKIs have a better ability to penetrate the BBB and control brain metastases compared to crizotinib (Table 1). The ALEX trial revealed alectinib had 81% of intracranial response toward previously untreated brain metastases, while the response rate of crizotinib was 50%. High intracranial responses were also obtained either with ceritinib (45%) (63) and brigatinib (42–67%) (61,64), in patients with recurrence after first-line treatment with crizotinib. Among the TKIs, the 2nd generation ALK-TKIs showed better survival at the front-line compared to crizotinib (65-67). The 2nd generation ALK-TKI intracranial ORR was also reported to be almost 2 to 3 times higher than that of the 1st generation TKI, crizotinib (68) and are now positioned as front-line drugs in NSCLC with brain metastases. Similar to that with the erlotinib, alectinib dose-escalation therapy achieved ALK inhibition and is awaiting clinical approval (69). The 3rd generation ALK-TKI, lorlatinib also demonstrated 42–48% intracranial response in patients with recurrence after first-line crizotinib (51). The sequence in which ALK-TKIs are to be used for effective disease control needs further evaluation. Further studies on the effectiveness of the ALK-TKIs in controlling oligo-recurrence or oligo-progression (one or a few lesions) in the brain should be conducted.
ROS1 and beyond
For NSCLC patients with ROS1-rearrangement (1–2% of all NSCLC), the standard first-line treatment is crizotinib (70-72). As the pivotal trial did not capture CNS metastasis in the database, there is no separate analysis of intracranial-overall response rate. Several early phase studies have suggested the potentially improved intracranial activity of next-generation ROS1-targeted therapies, including ceritinib, entrectinib, and lorlatinib, although only a small number of patients were included because of the rarity of this type of NSCLC. Among these TKIs, lorlatinib appears to have the most promising treatment effects in both crizotinib-naïve and -resistant ROS1-positive patients (73,74).
BRAF mutation and NTRK fusion are emerging molecular targets in NSCLC. The combination of dabrafenib, BRAF inhibitor, and trametinib, a MEK inhibitor, was approved for the treatment of NSCLC with BRAF mutations and larotrectinib, a TRK inhibitor, was approved for use in NSCLC with NTRK fusion. However, information about brain metastases in these tumors is lacking because of the rarity of these tumors (75-77).
Radiotherapy
Irradiation to tumor cell triggers mitotic cell death, apoptosis, autophagy, and senescence (78,79). Brain metastases are traditionally treated by whole-brain radiation therapy (WBRT) (a total dose of 30 Gy in 10 daily fractions of 3 Gy). WBRT may improve neurological symptoms from brain metastasis (with approximately 70–90%), and its intracranial control rate is known to be approximately 40–60%. There is a continuing discussion on whether WBRT improves QOL, and survival (80-83). On the other hand, SRS or stereotactic radiotherapy (SRT) use scattered γ rays or high-energy X-ray, respectively, converging on the target to effectively kill tumor cells, induce apoptosis of endothelial cells and lead to tumor radio-sensitization, maximizing the protection of tumor peripheral tissues to increase local control and microscopic tumor infiltration, while reducing the risks of neurocognitive side effects compared to WBRT. Radionecrosis is still a challenging complication to manage (19,84,85). SRS/SRT is now considered as a standard treatment for patients with brain metastasis when the total volume is low enough, and the number is limited (86). Combination of WBRT and SRS/SRT is not recommended because it does not improve survival benefits but increases neurocognitive deficits (87-89). In order to prevent and reduce neurocognitive decline, the use of memantine (90,91) and Hippocampal-sparing radiation (92) is under investigation.
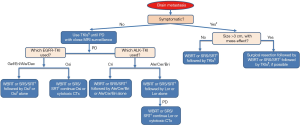
A meta-analysis on 12 observational studies that evaluated CNS response rate and 2-year OS in patients with EGFR mutation-positive NSCLC with brain metastases revealed that radiotherapy (SRS and WBRT) improved the OS by 2 years. Furthermore, it showed similar CNS response rate as that of the 1st generation EGFR-TKIs for the initial intervention but also resulted in more frequent adverse effects (93). On the other hand, a couple of retrospective studies have suggested that postponing radiotherapy for brain metastasis in EGFR mutation-positive NSCLC results in a poor outcome (94,95). In cases with disease progression in CNS after treatment with 1st or 2nd generation EGFR-TKIs, consider switching to osimertinib if T790M mutation is detected in any other site or lesion. If there are no extracranial progressive lesions for re-biopsy to prove T790M mutation, and if there is no need for neurosurgical intervention, local radiation therapy by SRS/SRT or WBRT for oligo or multiple metastases, respectively, to control brain metastases (holding TKI until radiation is completed) with continued treatment with EGFR-TKIs is recommended (Figure 1). Moreover, EGFR-TKIs and concurrent WBRT seems to have good tumor control ability (96) but increase the risk of potential cognitive complications (97).
Neurosurgical resection of NSCLC brain metastases
Surgical resection of the metastatic brain tumors has been another effective local treatment. Surgery is especially indicated when the brain lesion is large, and a patient is symptomatic due to elevated intracranial hypertension, and the tumor is preferably located in a non-functional region. Postoperative WBRT has shown to prolong OS from 16 to 19 months and is usually recommended (98,99).
Conclusions: general principles of current management of brain metastases
For those NSCLC patients with driver-oncogene mutations, including EGFR and ALK mutations, systemic therapy with the newest targeted therapy is preferred as the initial intervention rather than old generation TKIs. This is because the new-generation TKIs, such as osimertinib and alectinib, are designed to penetrate the BBB, and possess significantly higher intracranial activities compared to other chemotherapies. Local radiotherapy followed by TKI is generally preferred, except when brain metastases have the risk of herniation or possess severe mass effect that needs neurosurgical intervention.
Acknowledgments
None.
Footnote
Conflicts of Interest: M Nishino has received lecture fees from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical Co. Ltd., Pfizer Japan Inc., and Taiho Pharmaceutical Co. Ltd. K Soejima has received personal fees as honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co. Ltd., Ono Pharmaceutical Co. Ltd., Bristol-Myers Squibb Japan, MSD Oncology, Eli Lilly Japan K.K. and Novartis Pharma K.K and has received research funding from Boehringer Ingelheim Japan Inc. and Taiho Pharmaceutical Co. Ltd. T Mitsudomi has received lecture fees from AstraZeneca K.K., Pfizer Japan Inc., Chugai Pharmaceutical Co. Ltd., Boehringer Ingelheim Japan Inc., MSD K.K., Ono Pharmaceutical Co. Ltd., Bristol Myers Squibb, Eli Lilly Japan K.K., and Taiho Pharmaceutical Co. Ltd.; research funding from Boehringer Ingelheim Japan Inc., Chugai Pharmaceutical Co. Ltd., Pfizer Japan Inc., Ono Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd.; as well as advisory fees from AstraZeneca K.K., Boehringer Ingelheim Japan Inc., Bristol Myers Squibb, MSD K.K., Chugai Pharmaceutical Co. Ltd., Pfizer Japan Inc., Ono Pharmaceutical Co. Ltd., and Taiho Pharmaceutical Co. Ltd.
References
- Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497-500. [Crossref] [PubMed]
- Li T, Kung HJ, Mack PC, Gandara DR. Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies. J Clin Oncol 2013;31:1039-49. [Crossref] [PubMed]
- Peters S, Bexelius C, Munk V, et al. The impact of brain metastasis on quality of life, resource utilization and survival in patients with non-small-cell lung cancer. Cancer Treat Rev 2016;45:139-62. [Crossref] [PubMed]
- Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky performance status. Cancer 1980;45:2220-4. [Crossref] [PubMed]
- Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys 1997;37:745-51. [Crossref] [PubMed]
- Lagerwaard FJ, Levendag PC, Nowak PJ, et al. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys 1999;43:795-803. [Crossref] [PubMed]
- Fife KM, Colman MH, Stevens GN, et al. Determinants of outcome in melanoma patients with cerebral metastases. J Clin Oncol 2004;22:1293-300. [Crossref] [PubMed]
- Schouten LJ, Rutten J, Huveneers HA, et al. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002;94:2698-705. [Crossref] [PubMed]
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol 2004;22:2865-72. [Crossref] [PubMed]
- Morgensztern D, Ng SH, Gao F, et al. Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 2010;5:29-33. [Crossref] [PubMed]
- Norden AD, Wen PY, Kesari S. Brain metastases. Curr Opin Neurol 2005;18:654-61. [PubMed]
- Schuette W. Treatment of brain metastases from lung cancer: chemotherapy. Lung Cancer 2004;45 Suppl 2:S253-7. [Crossref] [PubMed]
- Shin DY, Na II, Kim CH, et al. EGFR mutation and brain metastasis in pulmonary adenocarcinomas. J Thorac Oncol 2014;9:195-9. [Crossref] [PubMed]
- Zhang I, Zaorsky NG, Palmer JD, et al. Targeting brain metastases in ALK-rearranged non-small-cell lung cancer. Lancet Oncol 2015;16:e510-21. [Crossref] [PubMed]
- Johung KL, Yeh N, Desai NB, et al. Extended Survival and Prognostic Factors for Patients With ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastasis. J Clin Oncol 2016;34:123-9. [Crossref] [PubMed]
- Gainor JF, Tseng D, Yoda S, et al. Patterns of Metastatic Spread and Mechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell Lung Cancer. JCO Precis Oncol 2017. doi: 10.1200/PO.17.00063. [Crossref]
- Ou SI, Zhu VW. CNS metastasis in ROS1+ NSCLC: An urgent call to action, to understand, and to overcome. Lung Cancer 2019;130:201-7. [Crossref] [PubMed]
- Lee JS, Pisters KM, Komaki R, et al. Paclitaxel/carboplatin chemotherapy as primary treatment of brain metastases in non-small cell lung cancer: a preliminary report. Semin Oncol 1997;24:S12-52-S12-55.
- Sawrie SM, Guthrie BL, Spencer SA, et al. Predictors of distant brain recurrence for patients with newly diagnosed brain metastases treated with stereotactic radiosurgery alone. Int J Radiat Oncol Biol Phys 2008;70:181-6. [Crossref] [PubMed]
- Cheng H, Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet Oncol 2018;19:e43-e55. [Crossref] [PubMed]
- Serlin Y, Shelef I, Knyazer B, et al. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol 2015;38:2-6. [Crossref] [PubMed]
- Sevenich L, Bowman RL, Mason SD, et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat Cell Biol 2014;16:876-88. [Crossref] [PubMed]
- Jilaveanu LB, Parisi F, Barr ML, et al. PLEKHA5 as a Biomarker and Potential Mediator of Melanoma Brain Metastasis. Clin Cancer Res 2015;21:2138-47. [Crossref] [PubMed]
- Miller DS. Regulation of ABC transporters blood-brain barrier: the good, the bad, and the ugly. Adv Cancer Res 2015;125:43-70. [Crossref] [PubMed]
- Chamberlain MC, Baik CS, Gadi VK, et al. Systemic therapy of brain metastases: non-small cell lung cancer, breast cancer, and melanoma. Neuro Oncol 2017;19:i1-i24. [Crossref] [PubMed]
- Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol 2011;8:344-56. [Crossref] [PubMed]
- Patel MM, Patel BM. Crossing the Blood-Brain Barrier: Recent Advances in Drug Delivery to the Brain. CNS Drugs 2017;31:109-33. [Crossref] [PubMed]
- Miller DS. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol Sci 2010;31:246-54. [Crossref] [PubMed]
- Fidler IJ, Yano S, Zhang RD, et al. The seed and soil hypothesis: vascularisation and brain metastases. Lancet Oncol 2002;3:53-7. [Crossref] [PubMed]
- Ningaraj NS, Rao M, Hashizume K, et al. Regulation of blood-brain tumor barrier permeability by calcium-activated potassium channels. J Pharmacol Exp Ther 2002;301:838-51. [Crossref] [PubMed]
- Percy DB, Ribot EJ, Chen Y, et al. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol 2011;46:718-25. [Crossref] [PubMed]
- Gaudino S, Di Lella GM, Russo R, et al. Magnetic resonance imaging of solitary brain metastases: main findings of nonmorphological sequences. Radiol Med 2012;117:1225-41. [Crossref] [PubMed]
- Daneman R. The blood-brain barrier in health and disease. Ann Neurol 2012;72:648-72. [Crossref] [PubMed]
- Salphati L, Heffron TP, Alicke B, et al. Targeting the PI3K pathway in the brain--efficacy of a PI3K inhibitor optimized to cross the blood-brain barrier. Clin Cancer Res 2012;18:6239-48. [Crossref] [PubMed]
- Agarwal S, Hartz AM, Elmquist WF, et al. Breast cancer resistance protein and P-glycoprotein in brain cancer: two gatekeepers team up. Curr Pharm Des 2011;17:2793-802. [Crossref] [PubMed]
- Sordella R, Bell DW, Haber DA, et al. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 2004;305:1163-7. [Crossref] [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [Crossref] [PubMed]
- Mitsudomi T, Kosaka T, Endoh H, et al. Mutations of the epidermal growth factor receptor gene predict prolonged survival after gefitinib treatment in patients with non-small-cell lung cancer with postoperative recurrence. J Clin Oncol 2005;23:2513-20. [Crossref] [PubMed]
- Ge M, Zhuang Y, Zhou X, et al. High probability and frequency of EGFR mutations in non-small cell lung cancer with brain metastases. J Neurooncol 2017;135:413-8. [Crossref] [PubMed]
- Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res 2010;16:5873-82. [Crossref] [PubMed]
- Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008;105:2070-5. [Crossref] [PubMed]
- Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol 2012;70:399-405. [Crossref] [PubMed]
- Katayama T, Shimizu J, Suda K, et al. Efficacy of erlotinib for brain and leptomeningeal metastases in patients with lung adenocarcinoma who showed initial good response to gefitinib. J Thorac Oncol 2009;4:1415-9. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Tamiya A, Tamiya M, Nishihara T, et al. OA08.05 Efficacy and Cerebrospinal Fluid Concentration of Afatinib in NSCLC Patients with EGFR Mutation Developing Leptomeningeal Carcinomatosis. J Thorac Oncol 2017;12:S273. [Crossref]
- Yang JC, Cho BC, Kim DW, et al. Osimertinib for patients (pts) with leptomeningeal metastases (LM) from EGFR-mutant non-small cell lung cancer (NSCLC): Updated results from the BLOOM study. J Clin Oncol 2017;35:abstr 2020.
- Nanjo S, Hata A, Okuda C, et al. Standard-dose osimertinib for refractory leptomeningeal metastases in T790M-positive EGFR-mutant non-small cell lung cancer. Br J Cancer 2018;118:32-7. [Crossref] [PubMed]
- Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol 2011;29:e443-5. [Crossref] [PubMed]
- Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol 2014;74:1023-8. [Crossref] [PubMed]
- Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol 2017;18:1590-9. [Crossref] [PubMed]
- Gerber NK, Yamada Y, Rimner A, et al. Erlotinib versus radiation therapy for brain metastases in patients with EGFR-mutant lung adenocarcinoma. Int J Radiat Oncol Biol Phys 2014;89:322-9. [Crossref] [PubMed]
- Porta R, Sánchez-Torres JM, Paz-Ares L, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur Respir J 2011;37:624-31. [Crossref] [PubMed]
- Ceresoli GL, Cappuzzo F, Gregorc V, et al. Gefitinib in patients with brain metastases from non-small-cell lung cancer: a prospective trial. Ann Oncol 2004;15:1042-7. [Crossref] [PubMed]
- Wu C, Li YL, Wang ZM, et al. Gefitinib as palliative therapy for lung adenocarcinoma metastatic to the brain. Lung Cancer 2007;57:359-64. [Crossref] [PubMed]
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:113-25. [Crossref] [PubMed]
- Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018.JCO2018783118. [Epub ahead of print]. [PubMed]
- Ballard P, Yates JW, Yang Z, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res 2016;22:5130-40. [Crossref] [PubMed]
- Grommes C, Oxnard GR, Kris MG, et al. "Pulsatile" high-dose weekly erlotinib for CNS metastases from EGFR mutant non-small cell lung cancer. Neuro Oncol 2011;13:1364-9. [Crossref] [PubMed]
- Rangachari D, Yamaguchi N, VanderLaan PA, et al. Brain metastases in patients with EGFR-mutated or ALK-rearranged non-small-cell lung cancers. Lung Cancer 2015;88:108-11. [Crossref] [PubMed]
- Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in Patients With Crizotinib-Refractory Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer: A Randomized, Multicenter Phase II Trial. J Clin Oncol 2017;35:2490-8. [Crossref] [PubMed]
- Petrelli F, Lazzari C, Ardito R, et al. Efficacy of ALK inhibitors on NSCLC brain metastases: A systematic review and pooled analysis of 21 studies. PLoS One 2018;13:e0201425. [Crossref] [PubMed]
- Mok T, Spigel D, Felip E, et al. ASCEND-2: A single-arm, open-label, multicenter phase II study of ceritinib in adult patients (pts) with ALK-rearranged (ALK+) non-small cell lung cancer (NSCLC) previously treated with chemotherapy and crizotinib (CRZ). J Clin Oncol 2015;33:abstr 8059.
- Camidge DR, Kim DW, Tiseo M, et al. Exploratory Analysis of Brigatinib Activity in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer and Brain Metastases in Two Clinical Trials. J Clin Oncol 2018;36:2693-701. [Crossref] [PubMed]
- Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017;389:917-29. [Crossref] [PubMed]
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet 2017;390:29-39. [Crossref] [PubMed]
- Peters S, Camidge DR, Shaw AT, et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2017;377:829-38. [Crossref] [PubMed]
- Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol 2018;29:2214-22. [PubMed]
- Gainor JF, Chi AS, Logan J, et al. Alectinib Dose Escalation Reinduces Central Nervous System Responses in Patients with Anaplastic Lymphoma Kinase-Positive Non-Small Cell Lung Cancer Relapsing on Standard Dose Alectinib. J Thorac Oncol 2016;11:256-60. [Crossref] [PubMed]
- Takeuchi K, Soda M, Togashi Y, et al. RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012;18:378-81. [Crossref] [PubMed]
- Shaw AT, Ou SH, Bang YJ, et al. Crizotinib in ROS1-rearranged non-small-cell lung cancer. N Engl J Med 2014;371:1963-71. [Crossref] [PubMed]
- Dugay F, Llamas-Gutierrez F, Gournay M, et al. Clinicopathological characteristics of ROS1- and RET-rearranged NSCLC in caucasian patients: Data from a cohort of 713 non-squamous NSCLC lacking KRAS/EGFR/HER2/BRAF/PIK3CA/ALK alterations. Oncotarget 2017;8:53336-51. [Crossref] [PubMed]
- Lim SM, Kim HR, Lee JS, et al. Open-Label, Multicenter, Phase II Study of Ceritinib in Patients With Non-Small-Cell Lung Cancer Harboring ROS1 Rearrangement. J Clin Oncol 2017;35:2613-8. [Crossref] [PubMed]
- Wu YL, Yang JC, Kim DW, et al. Phase II Study of Crizotinib in East Asian Patients With ROS1-Positive Advanced Non-Small-Cell Lung Cancer. J Clin Oncol 2018;36:1405-11. [Crossref] [PubMed]
- Paik PK, Arcila ME, Fara M, et al. Clinical characteristics of patients with lung adenocarcinomas harboring BRAF mutations. J Clin Oncol 2011;29:2046-51. [Crossref] [PubMed]
- Planchard D, Smit EF, Groen HJM, et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: an open-label, phase 2 trial. Lancet Oncol 2017;18:1307-16. [Crossref] [PubMed]
- Drilon A, Laetsch TW, Kummar S, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 2018;378:731-9. [Crossref] [PubMed]
- Coia LR. The role of radiation therapy in the treatment of brain metastases. Int J Radiat Oncol Biol Phys 1992;23:229-38. [Crossref] [PubMed]
- Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science 2003;300:1155-9. [Crossref] [PubMed]
- Borgelt B, Gelber R, Kramer S, et al. The palliation of brain metastases: final results of the first two studies by the Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys 1980;6:1-9. [Crossref] [PubMed]
- Mehta MP, Rodrigus P, Terhaard CH, et al. Survival and neurologic outcomes in a randomized trial of motexafin gadolinium and whole-brain radiation therapy in brain metastases. J Clin Oncol 2003;21:2529-36. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Sheehan JP, Sun MH, Kondziolka D, et al. Radiosurgery for non-small cell lung carcinoma metastatic to the brain: long-term outcomes and prognostic factors influencing patient survival time and local tumor control. J Neurosurg 2002;97:1276-81. [Crossref] [PubMed]
- Gerosa M, Nicolato A, Foroni R, et al. Analysis of long-term outcomes and prognostic factors in patients with non-small cell lung cancer brain metastases treated by gamma knife radiosurgery. J Neurosurg 2005;102 Suppl:75-80. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery With Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol 2013;15:1429-37. [Crossref] [PubMed]
- Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol 2014;15:387-95. [Crossref] [PubMed]
- Day J, Zienius K, Gehring K, et al. Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation. Cochrane Database Syst Rev 2014.CD011335. [PubMed]
- Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol 2014;32:3810-6. [Crossref] [PubMed]
- Soon YY, Leong CN, Koh WY, et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol 2015;114:167-72. [Crossref] [PubMed]
- Magnuson WJ, Yeung JT, Guillod PD, et al. Impact of Deferring Radiation Therapy in Patients With Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Develop Brain Metastases. Int J Radiat Oncol Biol Phys 2016;95:673-9. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Olmez I, Donahue BR, Butler JS, et al. Clinical outcomes in extracranial tumor sites and unusual toxicities with concurrent whole brain radiation (WBRT) and Erlotinib treatment in patients with non-small cell lung cancer (NSCLC) with brain metastasis. Lung Cancer 2010;70:174-9. [Crossref] [PubMed]
- Auchter RM, Lamond JP, Alexander E, et al. A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys 1996;35:27-35. [Crossref] [PubMed]
- Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery 1998;42:1044-55. [Crossref] [PubMed]


