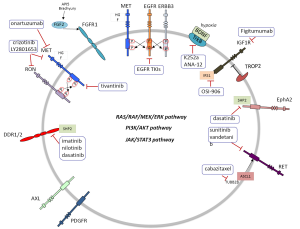
A critical question for cancer therapy: what new targets exist?
Lung cancer is still a common disease with dismal prognosis. However, several driver lesions have been identified which can permit the use of targeted therapy. Epidermal growth factor receptor (EGFR) mutations, ALK and ROS1 translocations have become part of the molecular diagnosis of patients, particularly in lung adenocarcinoma (1). Triple negative lung adenocarcinomas (EGFR, ALK and ROS1 negative) should be examined for other potentially druggable mutations such as HER2, BRAF, PIK3CA and NRAS among others (2). KRAS is also a common alteration for which no specific therapy yet exists. We still do not know how to take advantage of the fact that many non-small cell lung cancers (NSCLC) exhibit oncogenic kinase signaling through several receptor protein tyrosine kinases (RTKs), not only EGFR but also MET and RON, EPHA2, AXL, RET, TRKA and FGFR1 (3). In NSCLC, MET can be overexpressed along with hepatocyte growth factor (HGF). Several MET inhibitors (4) have been tested in combination with EGFR inhibitors. However, no difference in overall survival (OS) was observed either with the combination of tivantinib plus erlotinib vs. erlotinib alone (5). Neither was any benefit shown in OS with an anti-MET antibody (onartuzumab) in combination with erlotinib in a phase II randomized trial (6). The same authors did not find any difference in OS in the phase III randomized trial in MET-positive NSCLC (7). However, it is possible that a subset of MET expressing tumors can respond to anti-MET therapeutics as has been recently demonstrated in some NSCLC cell lines (8). Crosstalk of MET with its family member RON (3) has been observed (Table 1, Figure 1). MET/RON complexes are present on the cell surface and ligand-stimulated MET activation results in direct transphosphorylation of RON (21). A MET/RON dual kinase inhibitor (LY2801653) was more efficacious than crizotinib (a MET/ALK/RON/ROS inhibitor) in A549 (KRAS G12S), H1703 (PDGFRA amplified), and H1993 (MET amplified) NSCLC cell lines. Also LY2801653 was effective in in vivo models. Inhibition of MET and RON was associated with decreased phosphorylation of CBL, PI3K and STAT3 (8). Since NSCLC is a heterogeneous group of diseases it is important to understand which biomarkers can model the activity of MET and specific inhibition of MET and RON could become clinically relevant, even in tumors harboring KRAS mutations. It is important to highlight that co-activation of several RTKs occur in many tumors. For example, the A549 NSCLC cell line coexpresses EGFR, MET, ERBB3, EPHA2 and AXL. The 8988T pancreatic adenocarcinoma cell line coexpresses EGFR, MET, ERBB2, RON, INSR, EPHA2 and AXL (22). The type of ALK inhibitor also matters in the mechanism of resistance that can be developed in EML4-ALK NSCLC cells. Paracrine receptor activation by ligands from the microenvironment may trigger resistance to ALK inhibitors in EML4-ALK lung cancer cells. Fibroblasts produce HGF which activates MET/Gab1 and triggers resistance to TAE684 but not to crizotinib which also inhibits MET, as explained above). Conversely, endothelial cells which produce EGFR ligands decrease sensitivity to crizotinib (23).
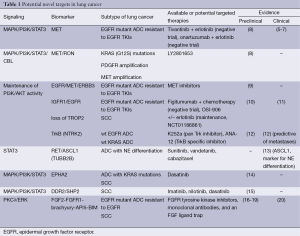
Full table
Intriguingly, in MET amplified cells, MET signaling through ERBB3 maintains PI3K/AKT cell survival signaling despite EGFR inhibition (24). In spite of the negative studies combining MET inhibitors with erlotinib, it has been shown that EGFR-MET signaling is critical for aggressive behavior of NSCLC and provides the basis for further investigations into therapeutic target combinations. It has been determined that EGFR activation by ligand or mutation is sufficient to induce MET phosphorylation. In addition, ERBB3 enhances EGFR-driven phosphorylation of MET and activates MET itself (9). A seminal study identified two pathways leading to PI3K/AKT signaling in A431 gefitinib resistant cells: the EGFR/ERBB3 and the IGF1R/IRS1 pathways. Combining therapeutic inhibition of EGFR and IGF1R abrogates this acquired mechanism of drug resistance (10) (Table 1, Figure 1). IGF1R inhibitors are reviewed in Gold et al. (11). TROP2 modulates IGF-1R signaling in lung adenocarcinoma and low levels of expression of TROP2 in NSCLC cells are related to resistance to EGFR TKIs (25). Intriguingly, IGF1R/PI3K signaling is enhanced in resistant melanomas and combined treatment with IGF1R/PI3K and MEK inhibitors induced death of BRAF inhibitor-resistant cells (26).
Tropomyosin-related kinase B (TrkB) (3) expression is regulated by hypoxia-inducible factor 1 (HIF-1) and TrkB is required for AKT activation during lung tumor cell migration. Importantly, TrkB expression is more frequent in NSCLC wild-type for KRAS and EGFR. These observations suggest that TrkB could be an alternative way for tumors to enhance PI3K signaling. Therefore targeting TrkB could be a useful strategy in patient subsets for whom there is no currently available targeted therapy (12). Moreover, ASCL1 and RET expression define a clinically relevant subgroup of 10% of lung adenocarcinomas characterized by neuroendocrine differentiation. ASCL1 acts upstream of RET. Also, STAT3 levels are reduced in ASCL1 depleted cells, suggestion potential activation of the JAK/STAT3 pathway. Currently available drugs targeting RET, such as sunitinib or vandetinib, could be appropriate for this subgroup of patients, as well as drugs targeting TUBB2B such as cabazitaxel since TUBB2B is also associated with high levels of ASCL1 (13) (Table 1, Figure 1).
Also of great clinical relevance is the fact that EPHA2 expression (3) is increased in patients harboring KRAS mutations (27). EPHA2 expression also positively correlates with history of smoking and poor survival (27). Therefore, EPHA2 is a therapeutic target for NSCLC and dasatinib is a multi-target kinase inhibitor with significant activity against EPHA2 (14). EPHA2 mutations have been reported in squamous cell lung carcinoma (SCC) (28). Discoidin domain receptors, particularly DDR2 (3), are activated in lung cancer and DDR2 mutations have been reported in lung SCC (29). Inhibition of DDR1 and 2 can be achieved with different multi-target kinase inhibitors such as imatinib, nilotinib and dasatinib (15). SHP2 is a key signaling node downstream of the DDR2 receptor which leads to activation of multiple signaling pathways (29) (Table 1, Figure 1).
FGFR expression also matters in lung cancer (3). An important new finding is that FGFR1 mRNA levels may serve as a better biomarker of FGFR1 TKI response in lung cancer than FGFR1 gene copy number (30). Also, activation of FGF2-FGFR1 was described as a mechanism of acquired resistance to gefitinib in NSCLC (16). Brachyury, described as a driver of epithelial to mesenchymal transition, was reported to be overexpressed in NSCLC and suggested to offer an opportunity for novel therapeutic interventions (17). More recently, it has been shown that FGFR phosphorylation activates MEK/ERK resulting in increased Brachyury expression. Brachyury in turn promotes secretion of FGF and enhances again FGF-FGFR signaling (18). Brachyury levels could be a new biomarker for therapeutic interventions with FGF pathway inhibitors. The list of inhibitors is reviewed in Corn et al. (20). Also the anti-apoptotic gene AP15 mediates resistance by upregulating FGF2 signaling through FGFR1/PKCδ/ERK effector pathway which triggers degradation of the pro-apoptotic molecule BIM (19) (Table 1, Figure 1).
In summary, in NSCLC patients pan-negative for druggable driver genetic alterations, selection for multi-target kinase inhibitors also warrants selection based on the expression of one or more than one RTKs (3,22). In addition, several new biomarkers could be candidates for incorporation in customizing treatment for NSCLC patients negative for the most common driver mutations.
Acknowledgements
Work in Dr. Rosell’s laboratory is partially supported by a grant from Fundació La Caixa.
Disclosure: The authors declare no conflict of interest.
References
- Rosell R, Bivona TG, Karachaliou N. Genetics and biomarkers in personalisation of lung cancer treatment. Lancet 2013;382:720-31. [PubMed]
- Pillai RN, Ramalingam SS. Advances in the diagnosis and treatment of non-small cell lung cancer. Mol Cancer Ther 2014;13:557-64. [PubMed]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature 2001;411:355-65. [PubMed]
- Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res 2013;19:2310-8. [PubMed]
- Scagliotti GV, Novello S, Schiller JH, et al. Rationale and design of MARQUEE: a phase III, randomized, double-blind study of tivantinib plus erlotinib versus placebo plus erlotinib in previously treated patients with locally advanced or metastatic, nonsquamous, non-small-cell lung cancer. Clin Lung Cancer 2012;13:391-5. [PubMed]
- Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105-14. [PubMed]
- Spigel DR, Edelman MJ, O’Byrne K, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. ASCO Annual Meeting. Abstract 8000. Presented June 2, 2014.
- Kawada I, Hasina R, Arif Q, et al. Dramatic antitumor effects of the dual MET/RON small-molecule inhibitor LY2801653 in non-small cell lung cancer. Cancer Res 2014;74:884-95. [PubMed]
- Breindel JL, Haskins JW, Cowell EP, et al. EGF receptor activates MET through MAPK to enhance non-small cell lung carcinoma invasion and brain metastasis. Cancer Res 2013;73:5053-65. [PubMed]
- Guix M, Faber AC, Wang SE, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest 2008;118:2609-19. [PubMed]
- Gold KA, Wistuba II, Kim ES. New strategies in squamous cell carcinoma of the lung: identification of tumor drivers to personalize therapy. Clin Cancer Res 2012;18:3002-7. [PubMed]
- Sinkevicius KW, Kriegel C, Bellaria KJ, et al. Neurotrophin receptor TrkB promotes lung adenocarcinoma metastasis. Proc Natl Acad Sci U S A 2014;111:10299-304. [PubMed]
- Kosari F, Ida CM, Aubry MC, et al. ASCL1 and RET expression defines a clinically relevant subgroup of lung adenocarcinoma characterized by neuroendocrine differentiation. Oncogene 2014;33:3776-83. [PubMed]
- Chang Q, Jorgensen C, Pawson T, et al. Effects of dasatinib on EphA2 receptor tyrosine kinase activity and downstream signalling in pancreatic cancer. Br J Cancer 2008;99:1074-82. [PubMed]
- Day E, Waters B, Spiegel K, et al. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol 2008;599:44-53. [PubMed]
- Terai H, Soejima K, Yasuda H, et al. Activation of the FGF2-FGFR1 autocrine pathway: a novel mechanism of acquired resistance to gefitinib in NSCLC. Mol Cancer Res 2013;11:759-67. [PubMed]
- Roselli M, Fernando RI, Guadagni F, et al. Brachyury, a driver of the epithelial-mesenchymal transition, is overexpressed in human lung tumors: an opportunity for novel interventions against lung cancer. Clin Cancer Res 2012;18:3868-79. [PubMed]
- Hu Y, Mintz A, Shah SR, et al. The FGFR/MEK/ERK/brachyury pathway is critical for chordoma cell growth and survival. Carcinogenesis 2014;35:1491-9. [PubMed]
- Noh KH, Kim SH, Kim JH, et al. API5 confers tumoral immune escape through FGF2-dependent cell survival pathway. Cancer Res 2014;74:3556-66. [PubMed]
- Corn PG, Wang F, McKeehan WL, et al. Targeting fibroblast growth factor pathways in prostate cancer. Clin Cancer Res 2013;19:5856-66. [PubMed]
- Follenzi A, Bakovic S, Gual P, et al. Cross-talk between the proto-oncogenes Met and Ron. Oncogene 2000;19:3041-9. [PubMed]
- Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 2007;318:287-90. [PubMed]
- Yamada T, Takeuchi S, Nakade J, et al. Paracrine receptor activation by microenvironment triggers bypass survival signals and ALK inhibitor resistance in EML4-ALK lung cancer cells. Clin Cancer Res 2012;18:3592-602. [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [PubMed]
- Lin JC, Wu YY, Wu JY, et al. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med 2012;4:472-85. [PubMed]
- Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010;18:683-95. [PubMed]
- Brannan JM, Dong W, Prudkin L, et al. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res 2009;15:4423-30. [PubMed]
- Faoro L, Singleton PA, Cervantes GM, et al. EphA2 mutation in lung squamous cell carcinoma promotes increased cell survival, cell invasion, focal adhesions, and mammalian target of rapamycin activation. J Biol Chem 2010;285:18575-85. [PubMed]
- Iwai LK, Payne LS, Luczynski MT, et al. Phosphoproteomics of collagen receptor networks reveals SHP-2 phosphorylation downstream of wild-type DDR2 and its lung cancer mutants. Biochem J 2013;454:501-13. [PubMed]
- Wynes MW, Hinz TK, Gao D, et al. FGFR1 mRNA and protein expression, not gene copy number, predict FGFR TKI sensitivity across all lung cancer histologies. Clin Cancer Res 2014;20:3299-309. [PubMed]