
Treatment modes for EGFR mutations in patients with brain metastases from non-small cell lung cancer: controversy, causes, and solutions
Introduction
Over the past decade, targeted therapy for lung cancer has been a major breakthrough in the field of lung cancer treatment. Several significant achievements have been made in lung cancer research, and the survival of non-small cell lung cancer (NSCLC) patients with sensitive mutations has been significantly prolonged (1-7). However, patients with brain metastases from NSCLC with sensitive mutations have been difficult to manage and have become a hot topic in this field over the past decade (1-7). In the meantime, due to inconsistent results from various studies and different understandings across disciplines, there are many opinions on exact management of NSCLC brain metastases. The contention is most prominent between medical oncology and radiotherapy oncology, causing much confusion to clinicians in the field. In this article, the relevant literatures were summarized and combined with the authors’ own points of view to provide reference for clinical treatment.
What kind of treatment modes are currently available?
Treatment mode currently recommended by medical oncology
Since the first-generation tyrosine kinase inhibitors (TKIs), brain metastases of lung cancer have been the focus of treatment. It is undeniable that TKIs have the advantages of low molecular weight, lipid/water ratio and good permeability (8-11), achieving exciting results in brain metastases when compared to traditional drug treatment. It also has played a key role in promoting the treatment of brain metastases with sensitive mutation. There are several phases I–II studies conducted when TKI debuted (12-15), but the study that really laid the foundation for this treatment mode was the CTONG0803 study (16). In this study, patients with epidermal growth factor receptor (EGFR) mutations and those without mutations were treated with TKI, and the results showed that the progression-free survival (PFS) of patients with brain metastases from NSCLC with EGFR mutation was significantly longer than that of patients without EGFR mutation. This study suggested that TKI can be used as a standard treatment for patients with asymptomatic brain metastases from NSCLC with EGFR mutations. After that, Professor Wu published an article in lung cancer (17), suggesting that lung cancer patients with brain metastases with slow local progression should be considered for local treatment (including radiotherapy), and he formally raised the issue of treatment mode for lung cancer. After medical oncology initiated the discussions around the use and importance of radiotherapy for brain metastases from lung cancer in the era of targeted therapy, research on radiotherapy in medical oncology is still under progress. In 2016, an article on TKI alone or combined with whole brain radiotherapy (WBRT) was published in the Journal of Thoracic Oncology (18), and the results suggested that radiotherapy could not increase the local control of brain, and showed no improvement in the survival of patients. In 2017, the brain study (19) was published in the Lancet Oncology, and the results showed that compared with WBRT combined with chemotherapy, the efficacy of TKI was significantly superior. Although there are limitations in this study and the treatment modes of the two groups were not balanced, the efficacy of radiotherapy according to this study and other previous studies were put into questions again. In 2016, the conclusion of QUTARZ study (20) published in the Lancet Oncology suggested that WBRT did not benefit the local control and survival. Although this study did not involve TKI therapy, the status of radiotherapy was further weakened in the era of TKI-targeted therapy. Radiotherapy did not improve local control and the survival, and can even affect cognitive functions. Many studies (21,22) have suggested that WBRT may significantly increase the risk of cognitive impairment in patients, further casting doubts on brain radiotherapy in the medical oncology community.
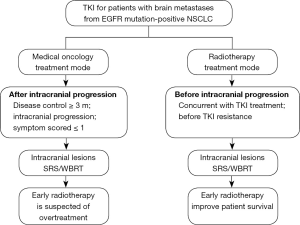
In conclusion, a series of research results have led to the formation of treatment mode in medical oncology. The viewpoint of medical oncology is that the symptoms and the progression are used as indications and criteria for local therapy, where in the absence of symptoms, local radiotherapy especially WBRT may increase the suffering of patients due to overtreatment. At the same time, some previous studies have shown that radiotherapy did not improve local control or quality of life of patients, and could not prolong survival of patients. Therefore, in the era of TKI, for patients with asymptomatic brain metastases, drug treatment should be used initially, followed by radiotherapy when symptoms present or disease progresses. This has become the mainstream opinion in medical oncology and the principle in clinical practice (Table 1).
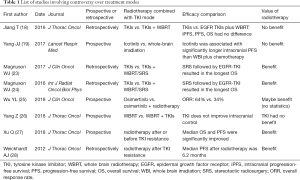
Full table
Treatment mode currently recommended by radiotherapy
Different from the viewpoint of medical oncology, the discipline of radiotherapy has also performed some research on this topic. There has been no prospective study result on this issue from the discipline of radiotherapy so far. Although lacking results from randomized clinical trials (RCTs), clinical retrospective study based on the real world can still provide important evidence. Moreover, radiotherapy and TKI may have combined effects, which is supported by theoretical basis for improving efficacy (29). Meanwhile, previous studies also showed that the toxicity due to the combination treatment can be well tolerated (30,31). In 2016, an article that compared TKI alone to TKI combined with brain radiotherapy was published in the International Journal of Radiation Oncology Biology and Physics (23), and the results showed that radiotherapy combined with TKI significantly prolonged patient survival. Similarly, Professor Magnuson WJ conducted a multicenter analysis with six centers in 2017, and the results showed that TKI combined with either WBRT or stereotactic radiosurgery (SRS) significantly improved patient survival (24). Doherty et al. (32) and Robin et al. (33) also showed that brain radiotherapy combined with TKI could significantly improve patient survival and local control.
Interestingly, Professor Wu’s AURA3 study on brain metastases published in the Journal of Clinical Oncology in 2018 (25) showed that in the osimertinib group, brain radiotherapy treatment within six months before osimetinib was associated with longer survival compared to no brain radiotherapy. At the World Congress on Lung Cancer 2018 (26), a clinical study compared TKI with brain radiotherapy, and the results showed that TKI combined with WBRT did not benefit patients when compared with WBRT alone. The negative results poured cold water on targeted therapy combined with brain radiotherapy in the era of TKI. In 2018, American Society for Radiation Oncology (ASTRO) (34) reported that for patients with advanced cancer, local radiotherapy including radiotherapy for brain metastases also achieved encouraging results. Therefore, theoretically, no matter the site of local treatment, local treatment can increase local control, alleviate local symptoms, and potentially increase the depth of systemic treatment, which in turn leads to prolonged patient survival (35). This has also been confirmed in the discipline of medical oncology (Table 1).
Although different studies have different results and opinions on the efficacy of radiotherapy (36,37), overall, contrary to a series of studies on medical oncology, the perspective of the discipline of radiotherapy is to treat the local lesions as early as possible, to kill them as soon as possible, to increase the depth of treatment, and to achieve the purpose of prolonged patient survival. This viewpoint and mode have widely influenced the discipline of oncology radiotherapy and has been implemented in clinical practice (Figure 1).
Currently, why different treatment modes have been developed?
Similar situations can be found in the history of controversy over treatment modes
Controversy over treatment modes has occurred based on different understandings of the different disciplines (Figure 1). But there is only one truth in clinical practice, i.e., the clinical treatment development is to seek the best outcome for patients rather than status of disciplines. In fact, there are always similarities in the history of medical development. Looking back to the history of the small cell lung cancer (SCLC) treatment (38,39), when platinum drugs achieved nearly breakthroughs in treatment effects, controversy over the status of radiotherapy developed. However, further studies found that even on the basis of platinum-based treatment, radiotherapy was also needed to further improve patient survival, and the results resolved the controversy. Therefore, brain metastases from NSCLC with EGFR mutations also need to be further studied and more clinical data are needed to obtain a more optimal mode for disease treatment.
Radiotherapy research is about to disappear
Over the past decade, clinical research on targeted therapy has been emerging more and more rapidly, but at the same time, the discipline of radiotherapy has been dwindling (40). The number of clinical studies does not match the status of radiotherapy in cancer treatment, and there are many reasons for this. Firstly, radiotherapy is a downstream discipline, and it is usually not the first to diagnose and treat patients. Therefore, pharmaceutical companies mainly promoted clinical trials in medical oncology and surgical oncology, and thus radiotherapy gained less data. Secondly, compared with tremendous progression in drug therapy, the multi-disciplinary collaboration in cancer therapy has not developed synchronously in the past decade, affecting the participation of radiotherapy in development of new treatment modes. Thirdly, in the process of collecting data of clinical trials, having multiple treatment modalities can complicate patient follow-up and data analysis. For this reason, the discipline of radiotherapy was divorced from the other disciplines. Therefore, over the past decade of targeted therapy with great breakthroughs in medical oncology treatment, radiotherapy research has relatively decreased in proportion. However, disease treatment in real world cannot be the same as clinical trials restricted and tailored to show treatment effects. The bias of clinical trials may also lead to bias in treatment. Drug therapy has brought tremendous progress in cancer treatment as well as imbalance in the development of disciplines. However, clinical practice should maximize the benefits of patients.
Let disciplines do their own work
When analyzing the clinical trials from medical oncology, some important studies on brain radiotherapy are not done by radiotherapists (18,19,25). Different disciplines have different understandings regarding the same problem, and thus the understanding of radiotherapy from medical or surgical oncology is not comprehensive, which may in turn affect patient enrollment and follow-up observations. The conclusions may not be consistent with clinical practice. We emphasize the comprehensive treatment of cancer, but when we analyze treatment effects in research, it is better to include expertise in the field for the field under study. Therefore, studies on radiotherapy should be designed and conducted by radiotherapists to draw more valuable and realistic conclusions.
More personalized enrollment is needed in clinical research of radiotherapy
Brain metastases patients are usually divided into groups according to the number of metastases, regardless of disease or pathology (41-46). When combined with the development of molecular oncology of lung cancer, clinical trials need to study brain metastases not only from the perspective of radiotherapy, but also should combine with drug therapy to understand brain metastases and the rapid development of molecular pathology. Meanwhile, according to comprehensive factors such as gene mutation, general condition and graded prognostic assessment (GPA) score (GPA and Lung-Mol GPA) (36,47), more personalized enrollment should be conducted to draw valuable conclusions that are more consistent with clinical practice, which better guides the clinical treatment.
How to solve the current controversy over treatment modes, and how should we choose in clinical practice in the current situation?
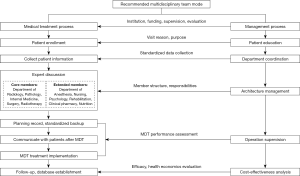
The best solution is to carry out prospective clinical trials with multi-disciplinary efforts, and the truth needs time to explore and verify. But limitations regarding the disciplinary knowledge in reality can be avoided by a relatively reasonable treatment mode. Based on the current situation, multi-disciplinary participation is required to optimize for patients. The development of multi-disciplinary team (MDT) mode is considered to be the most reasonable choice (48,49). MDT needs to formulate the guiding ideology and detailed treatment plan, as well as give feedback to patients, resulting in multi-disciplinary collaboration that benefits patients (Figure 2). Efforts need to be made to avoid disciplinary biases, institutional barriers and lack of executability and persistence on the development of MDT mode, thereby improving patient outcome.
What is the future of mode controversy?
The controversy over treatment modes of a disease between disciplines is not a new problem. With the breakthrough in treatment methods, the controversy over treatment modes often arises. In fact, controversy is beneficial for the development of disciplines and also for patients. The controversy of any discipline is periodic, and the imbalance of the development of any discipline is temporary. The controversy over clinical treatments is a part of the developmental process. It is the ultimate goal of every clinical trial and clinician to focus on the ultimate goal of solving problems and benefiting patients when the controversy between disciplines arises (27,28,50). At present, we should adopt a reasonable interdisciplinary collaboration mode of disease treatment and carry out further clinical trials in the long run to seek the truth. The controversy over treatment modes would certainly avoid the limitations of current disciplines and promote the development of disciplines in the future to better benefit patients. We also look forward to the bright future in the development of disciplines.
Conclusions
Brain metastasis from NSCLC with EGFR mutations is a hot and difficult research topic, and is also raising controversy in the field of lung cancer treatment. Controversies between disciplines have brought much confusion to the treatment choices of clinicians. We should adopt a reasonable interdisciplinary collaboration mode of disease treatment and avoid the limitations of current disciplines and promote the development to better benefit patients. Seeking truth, not status of a discipline, should be the real focus of development of disciplines.
Acknowledgments
The authors thank the groups of the Cyberknife center.
Funding: This paper was supported by Clinical key project of Peking University Third Hospital (BYSY2017030).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [Crossref] [PubMed]
- Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011;12:735-42. [Crossref] [PubMed]
- Shi Y, Zhang L, Liu X, et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol 2013;14:953-61. [Crossref] [PubMed]
- Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol 2017;28:2443-50. [Crossref] [PubMed]
- Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327-34. [Crossref] [PubMed]
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol 2017;18:1454-66. [Crossref] [PubMed]
- Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med 2017;376:629-40. [Crossref] [PubMed]
- Kitazaki T, Oka M, Nakamura Y, et al. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer 2005;49:337-43. [Crossref] [PubMed]
- Deng Y, Feng W, Wu J, et al. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol Clin Oncol 2014;2:116-20. [Crossref] [PubMed]
- Masuda T, Hattori N, Hamada A, et al. Erlotinib efficacy and cerebrospinal fluid concentration in patients with lung adenocarcinoma developing leptomeningeal metastases during gefitinib therapy. Cancer Chemother Pharmacol 2011;67:1465-9. [Crossref] [PubMed]
- Togashi Y, Masago K, Fukudo M, et al. Efficacy of increased-dose erlotinib for central nervous system metastases in non-small cell lung cancer patients with epidermal growth factor receptor mutation. Cancer Chemother Pharmacol 2011;68:1089-92. [Crossref] [PubMed]
- Hotta K, Kiura K, Ueoka H, et al. Effect of gefitinib ('Iressa', ZD1839) on brain metastases in patients with advanced non-small-cell lung cancer. Lung Cancer 2004;46:255-61. [Crossref] [PubMed]
- Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer 2009;65:351-4. [Crossref] [PubMed]
- Chonan M, Narita N, Tominaga T. Total regression of brain metastases in non-small cell lung cancer patients harboring EGFR mutations treated with gefitinib without radiotherapy: two case reports. BMC Res Notes 2016;9:2. [Crossref] [PubMed]
- Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer 2013;82:282-7. [Crossref] [PubMed]
- Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol 2013;24:993-9. [Crossref] [PubMed]
- Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung Cancer 2013;79:33-9. [Crossref] [PubMed]
- Jiang T, Su C, Li X, et al. EGFR TKIs plus WBRT Demonstrated No Survival Benefit Other Than That of TKIs Alone in Patients with NSCLC and EGFR Mutation and Brain Metastases. J Thorac Oncol 2016;11:1718-28. [Crossref] [PubMed]
- Yang JJ, Zhou C, Huang Y, et al. Icotinib versus whole-brain irradiation in patients with EGFR-mutant non-small-cell lung cancer and multiple brain metastases (BRAIN): a multicentre, phase 3, open-label, parallel, randomised controlled trial. Lancet Respir Med 2017;5:707-16. [Crossref] [PubMed]
- Mulvenna P, Nankivell M, Barton R, et al. Dexameth as one and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomized trial Lancet 2016;388:2004-14. [Crossref] [PubMed]
- Gondi V, Paulus R, Bruner DW, et al. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys 2013;86:656-64. [Crossref] [PubMed]
- Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol 2013;31:65-72. [Crossref] [PubMed]
- Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of Brain Metastases in Tyrosine Kinase Inhibitor-Naïve Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer: A Retrospective Multi-Institutional Analysis. J Clin Oncol 2017;35:1070-7. [Crossref] [PubMed]
- Magnuson WJ, Yeung JT, Guillod PD, et al. Impact of Deferring Radiation Therapy in Patients With Epidermal Growth Factor Receptor-Mutant Non-Small Cell Lung Cancer Who Develop Brain Metastases. Int J Radiat Oncol Biol Phys 2016;95:673-9. [Crossref] [PubMed]
- Wu YL, Ahn MJ, Garassino MC, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (AURA3). J Clin Oncol 2018;36:2702-9. [Crossref] [PubMed]
- Yang Z, Zhang Y, Li R, et al. MA08.01 Phase 3 Trial of Whole Brain Radiotherapy with Concurrent Erlotinib Versus WBRT Alone for NSCLC with Brain Metastases (ENTER). J Thorac Oncol 2018;13:S381-2. [Crossref]
- Xu Q, Zhou F, Liu H, et al. Consolidative Local Ablative Therapy Improves the Survival of Patients with Synchronous Oligometastatic NSCLC Harboring EGFR Activating Mutation Treated With First-Line EGFR-TKIs. J Thorac Oncol 2018;13:1383-92. [Crossref] [PubMed]
- Weickhardt AJ, Scheier B, Burke JM, et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene-addicted non-small-cell lung cancer. J Thorac Oncol 2012;7:1807-14. [Crossref] [PubMed]
- Zhuang H, Wang J, Zhao L, et al. The theoretical foundation and research progress for WBRT combined with erlotinib for the treatment of multiple brain metastases in patients with lung adenocarcinoma. Int J Cancer 2013;133:2277-83. [Crossref] [PubMed]
- Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol 2013;31:895-902. [Crossref] [PubMed]
- Zhuang H, Yuan Z, Wang J, et al. Phase II study of whole brain radiotherapy with or without erlotinib in patients with multiple brain metastases from lung adenocarcinoma. Drug Des Devel Ther 2013;7:1179-86. [Crossref] [PubMed]
- Doherty MK, Korpanty GJ, Tomasini P, et al. Treatment options for patients with brain metastases from EGFR/ALK-driven lung cancer. Radiother Oncol 2017;123:195-202. [Crossref] [PubMed]
- Robin TP, Camidge DR, Stuhr K, et al. Excellent Outcomes with Radiosurgery for Multiple Brain Metastases in ALK and EGFR Driven Non-Small Cell Lung Cancer J Thorac Oncol 2018;13:715-20. [Crossref] [PubMed]
- Palma DA, Haasbeek CJ, Rodrigues GB, et al. Stereotactic ablative radiotherapy for comprehensive treatment of oligometastatic tumors (SABR-COMET): study protocol for a randomized phase II trial. BMC Cancer 2012;12:305. [Crossref] [PubMed]
- McCoach CE, Blumenthal GM, Zhang L, et al. Exploratory analysis of the association of depth of response and survival in patients with metastatic non-small-cell lung cancer treated with a targeted therapy or immunotherapy. Ann Oncol 2017;28:2707-14. [Crossref] [PubMed]
- Soon YY, Leong CN, Koh WY, et al. EGFR tyrosine kinase inhibitors versus cranial radiation therapy for EGFR mutant non-small cell lung cancer with brain metastases: a systematic review and meta-analysis. Radiother Oncol 2015;114:167-72. [Crossref] [PubMed]
- Zheng H, Liu QX, Hou B, et al. Clinical outcomes of WBRT plus EGFR-TKIs versus WBRT or TKIs alone for the treatment of cerebral metastatic NSCLC patients: a meta-analysis. Oncotarget 2017;8:57356-64. [PubMed]
- Haddadin S, Perry MC. History of small-cell lung cancer. Clin Lung Cancer 2011;12:87-93. [Crossref] [PubMed]
- Pietanza MC, Byers LA, Minna JD, et al. Small cell lung cancer: will recent progress lead to improved outcomes? Clin Cancer Res 2015;21:2244-55. [Crossref] [PubMed]
- Liu X, Zhang Y, Tang LL, et al. Characteristics of Radiotherapy Trials Compared With Other Oncological Clinical Trials in the Past 10 Years. JAMA Oncol 2018;4:1073-9. [Crossref] [PubMed]
- Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665-72. [Crossref] [PubMed]
- Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA 2006;295:2483-91. [Crossref] [PubMed]
- Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol 2011;29:134-41. [Crossref] [PubMed]
- Sperduto PW, Shanley R, Luo X, et al. Secondary analysis of RTOG 9508, a phase 3 randomized trial of whole-brain radiation therapy versus WBRT plus stereotactic radiosurgery in patients with 1-3 brain metastases; poststratified by the graded prognostic assessment (GPA). Int J Radiat Oncol Biol Phys 2014;90:526-31. [Crossref] [PubMed]
- Aoyama H, Tago M, Shirato H, et al. Stereotactic Radiosurgery with or Without Whole-Brain Radiotherapy for Brain Metastases: Secondary Analysis of the JROSG 99-1 Randomized Clinical Trial. JAMA Oncol 2015;1:457-64. [Crossref] [PubMed]
- Brown PD, Jaeckle K, Ballman KV, et al. Effect of Radiosurgery Alone vs Radiosurgery with Whole Brain Radiation Therapy on Cognitive Function in Patients With 1 to 3 Brain Metastases: A Randomized Clinical Trial. JAMA 2016;316:401-9. [Crossref] [PubMed]
- Wrona A, Dziadziuszko R, Jassem J. Management of brain metastases in non-small cell lung cancer in the era of tyrosine kinase inhibitors. Cancer Treat Rev 2018;71:59-67. [Crossref] [PubMed]
- Crawford SM. Multidisciplinary team working contributes to lung cancer survival. BMJ 2018;361:k1904. [Crossref] [PubMed]
- Denton E, Conron M. Improving outcomes in lung cancer: the value of the multidisciplinary health care team. J Multidiscip Healthc 2016;9:137-44. [PubMed]
- Corbin KS, Hellman S, Weichselbaum RR. Extracranial oligometastases: a subset of metastases curable with stereotactic radiotherapy. J Clin Oncol 2013;31:1384-90. [Crossref] [PubMed]


